Development in Additive Methods in Aramid Fiber Surface Modification to Increase Fiber-Matrix Adhesion: A Review
Abstract
:1. Introduction
2. Structure and Adhesion in Aramid Reinforced Composites
2.1. Structure of Aramid Fiber
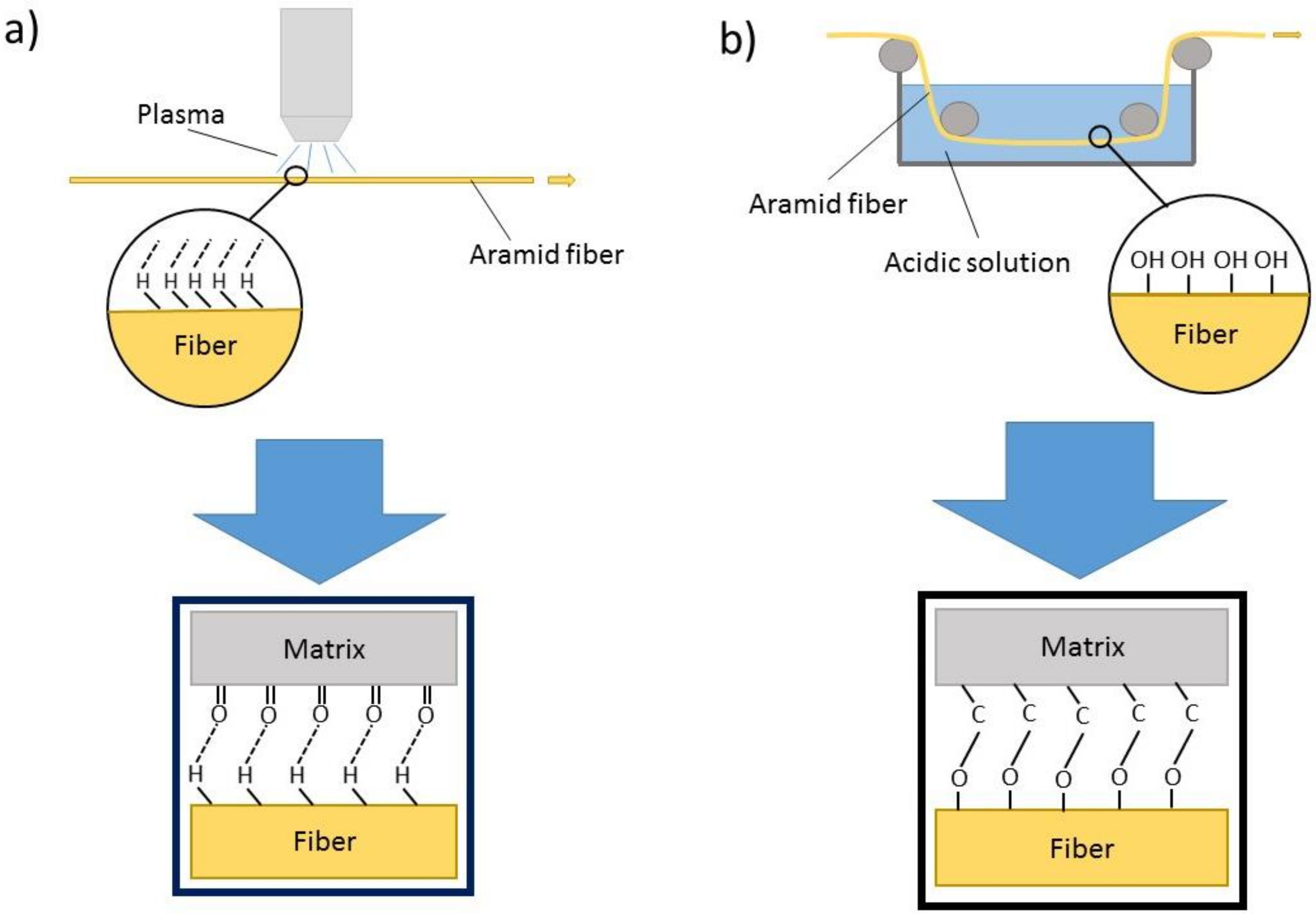
2.2. Chemical and Physical Bonding of Aramid
2.3. Mechanical Bonding with Multifunctional Fibers
3. Novel Coating Methods
3.1. Multifunctional Hybrid Coatings
3.2. Polymeric Coatings
3.3. Surface Coupling with Silanes
4. Creating Hierarchical Structures
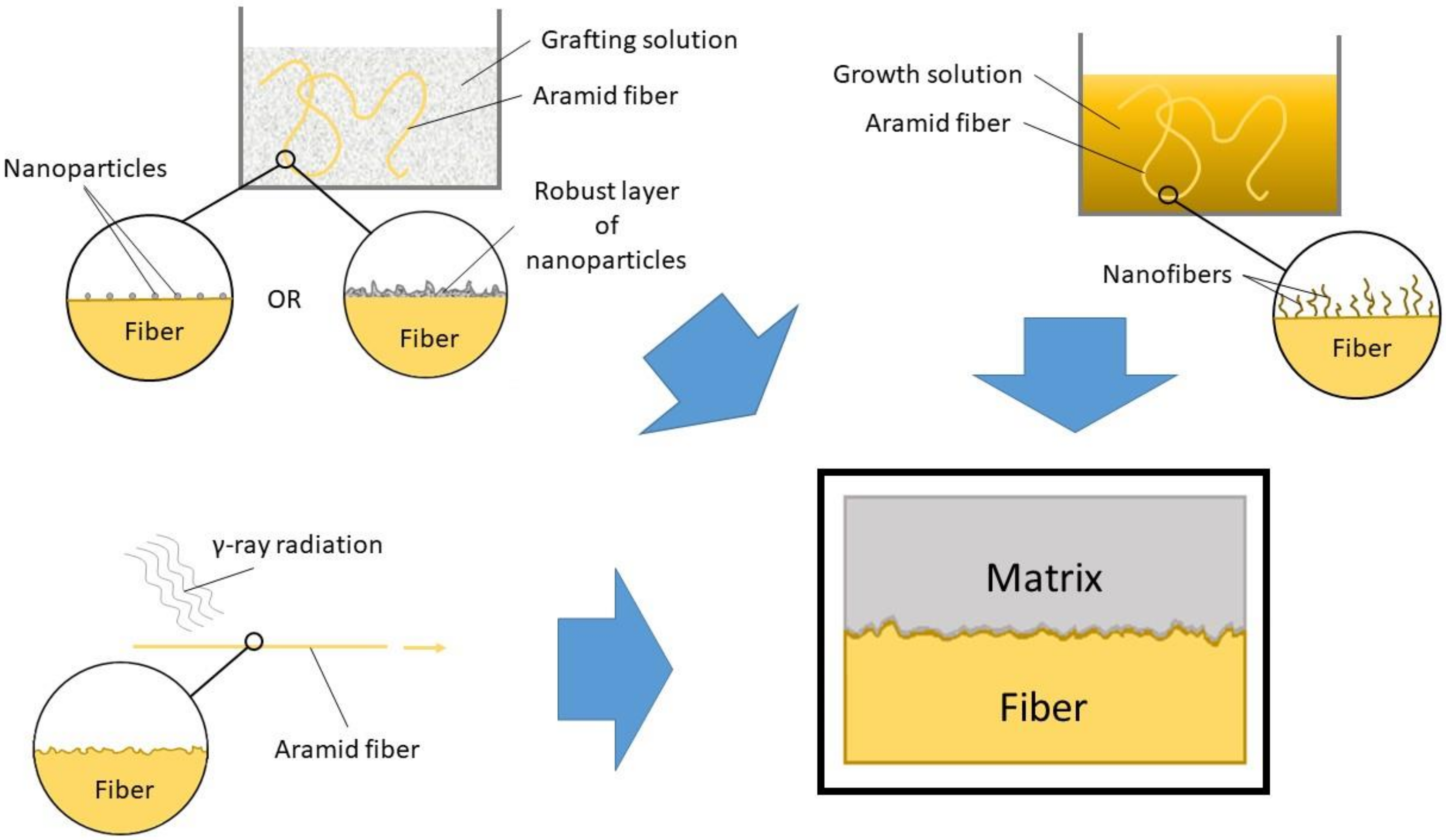
4.1. Grafting onto the Fiber Surface
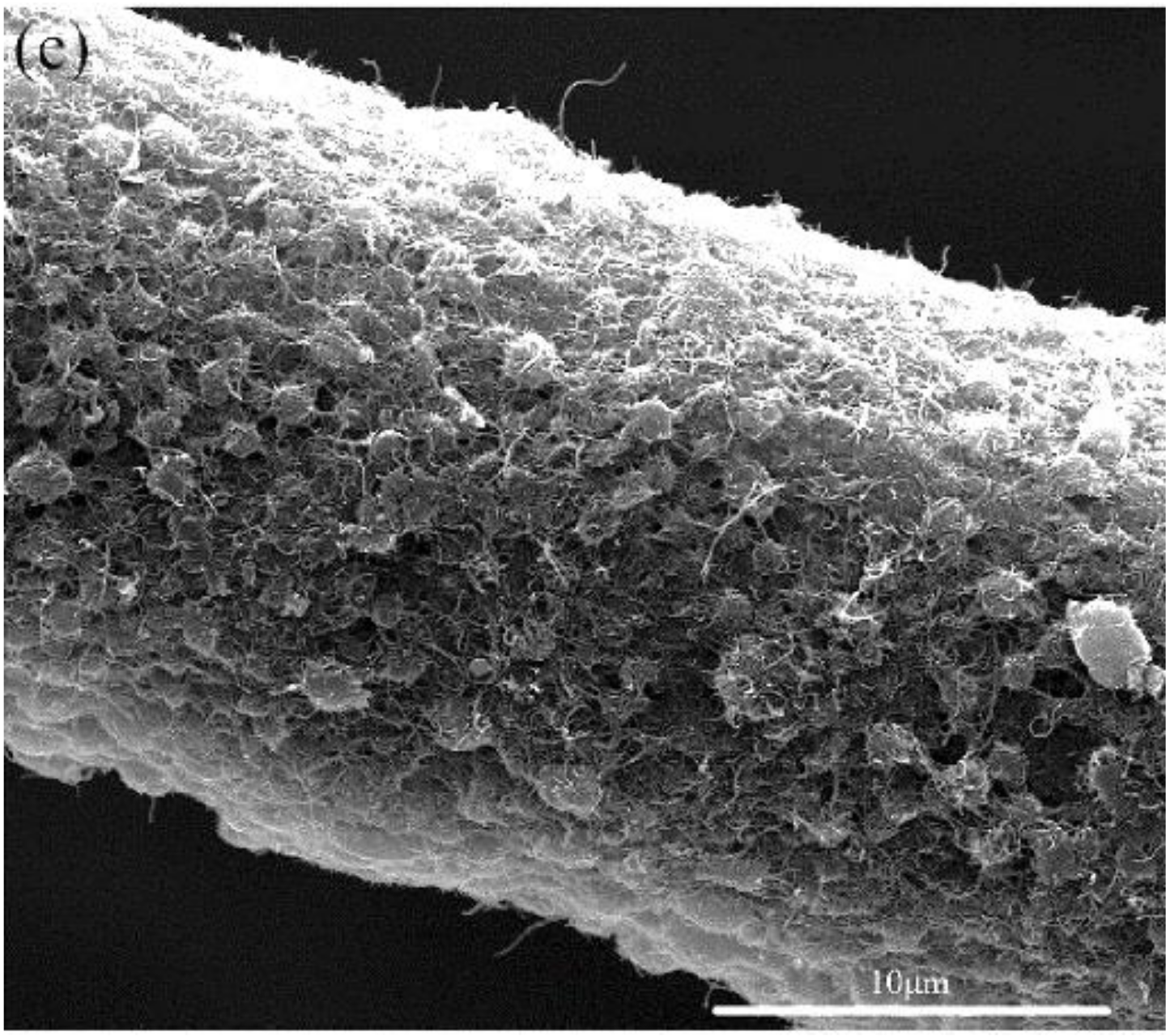
4.1.1. Nanotubes and Nanofibers
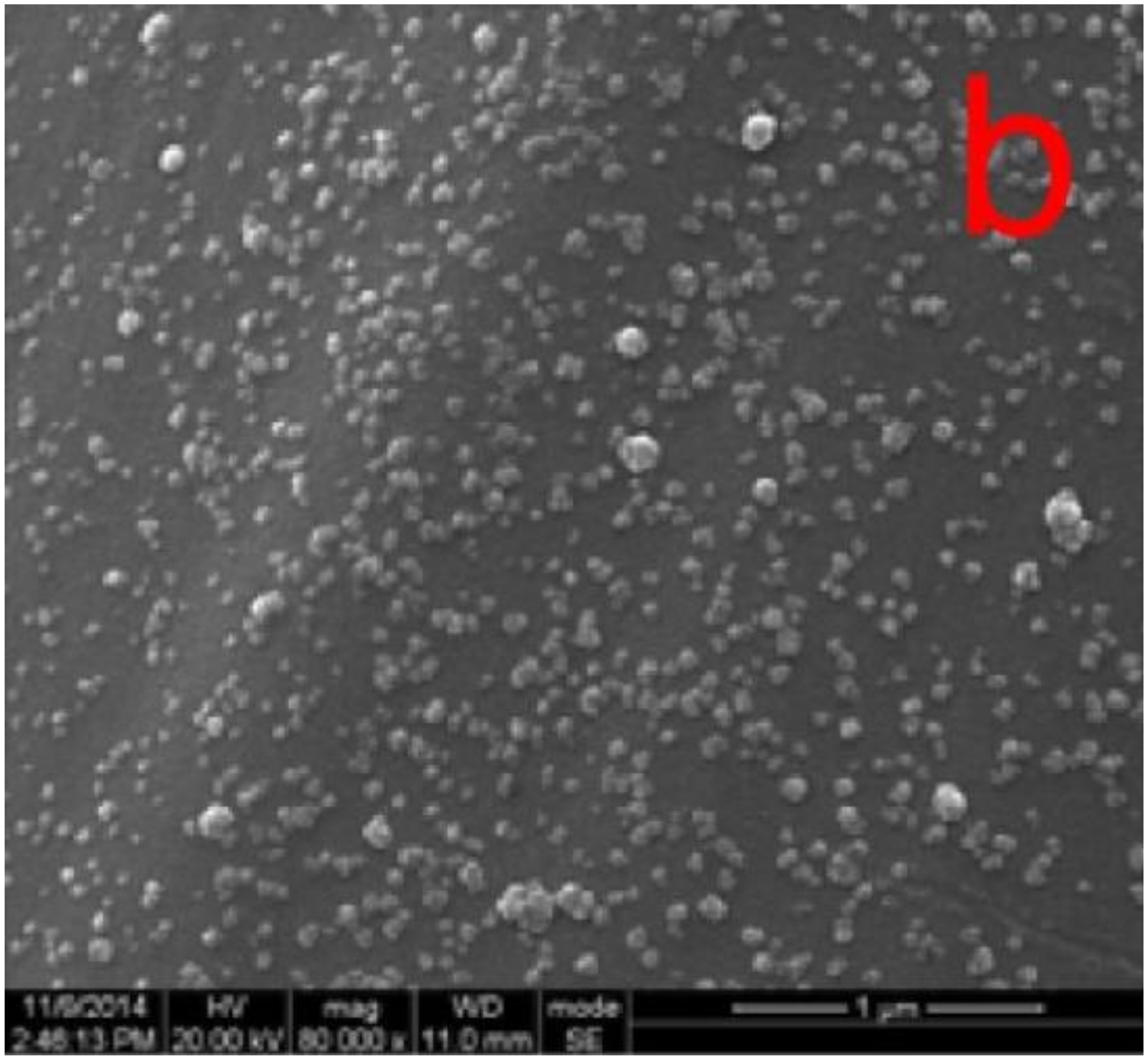
4.1.2. Inorganic Nanoparticles
4.1.3. Polymer Particles
4.2. Growing onto the Fiber Surface
4.2.1. Carbon Nanotubes
4.2.2. Zinc Oxide Nanowires
4.2.3. Nanoparticles
5. Other Methods to Introduce Functionality to the Surface
6. Summary and Future Insights
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Method | Outcome of the Method | Increase of Adhesion [%] | Matrix Used | Adhesion Evaluation | Other Benefits of the Surface Treatment | Effect on Tensile Strength of Fibers [%] | Reference |
|---|---|---|---|---|---|---|---|
| Multiphase coating process | Uniform coating with nano-protrusions | 45 | SMPU | IFSS | +5.7 | [63] | |
| Immersion in graphite oxide suspension | Uniform coverage with graphene sheets | 44 | EP | IFSS | [62] | ||
| Immersion in MWCNT solution and sonication | MWCNTs on the fiber surface | 30 | BMI | ILSS | +12 | [59] | |
| Zinc oxide nanowire growth | ZnO nanowires on the fiber surface | 51 | EP | IFSS | ±0 | [51] | |
| Plasma induced vapor phase graft polymerization of acrylic acid | Poly(acrylic acid) nanoparticle covered fiber surface | 122 | EP | IFSS | ±0 | [102] | |
| Low temperature hydrothermal growth of titanium oxide nanoparticles | Titanium dioxide nanoparticle cover surface | 67 | EP | IFSS | −4 | [118] | |
| High energy gamma ray irradiation | Highly increased surface roughness | 45 | EP | IFSS | N/A | [131] | |
| Irradiation grafting of DEA/ECH to aramid fiber surface | DEA/ECH embellished fiber surface | 31/25 | EP | IFSS/ILSS | −1.3–2.6 | [68] | |
| Deposition of oxidized CNTs onto aramid fibers via sonication | CNT covered hybrid fibers | N/A | N/A | N/A | electrical conductivity | −5.1–27 | [61] |
| Growth of CNTs onto Kevlar fabric via microwave irradiation | Fabric surface covered with iron decorated CNTs | 81 | PES | in-plane shear strength | electrical conductivity, increased impact resistance, strength and modulus of composite | N/A | [60] |
| HPSi grafting onto aramid fibers surface | HPSi covered fiber surface | N/A | N/A | N/A | improved UV-resistance, increased thermal stability and flame retardancy | + | [83] |
| A two-step process to create polydopamine GO coated fibers | two layered structure of polydopamine and GO sheets | N/A | N/A | N/A | improved UV-resistance, thermal stability, surface activity, mechanical properties | +3.8–8.2 | [80] |
| UV assisted PCPA deposition and EDGE grafting | Increased surface roughness with PCPA and EDGE, and epoxy groups | 85.6 | rubber | Pull-out test | - | [79] | |
| A green layer-by-layer self-assembly technique | Layered coating structure or SiO2 and LDH-NS | N/A | N/A | N/A | Improved surface activity, thermal resistance, mechanical properties and UV resistance | +12 | [81] |
| Grafting γ-chloropropyltrimethoxysilane onto Kevlar | Introduction of polar groups and roughened fiber surface | 57 | EP | ILSS | Increased wetting | N/A | [88] |
| “one step” process of silane grafting with PDA | Uniform layer of grated silane on the fiber surface | 62.5 | rubber | IFSS | N/A | [89] | |
| Silane (GPTMS) grafting with PCPA | Uniform coating of GPTMS on the fiber surface | 83.3 | rubber | IFSS | N/A | [90] | |
| ZnO nanoparticle sizing via dip coating | ZnO decorated fiber surface | 18.9 | EP | IFSS | Improved UV resistance | very minimal decrease | [82] |
| Grafting of PEI through Fe3+ coordination | Surface embellished with PEI | 47 | EP | IFSS | ±0 | [85] | |
| EB induced grafting of AA | Roughened fiber surface with nodules of PAA | N/A | N/A | N/A | Improved surface activity, COOH added to the surface | N/A | [104] |
| Cryogenic treatment of Kevlar | Increased surface roughness | 5.7–19 | EP | IFSS | Abrasion resistance increased | +24.9 | [134] |
| Direct fluorination of PBIA fibers | Changes in the surface morphology and polar groups at the surface | 39 | EP | Pull-out strength | N/A | [125] | |
| Direct fluorination and silane grafting of PBIA fibers | Silane grafted onto the fiber surface | 46.7 | EP | IFSS | ±0 | [126] | |
| γ-ray radiation of Armos fiber in N2 | Increased surface roughness, introduction of polar oxygen containing groups | 17.7 | EP | ILSS | Increased wetting | ±0 | [129] |
| γ-ray grafting of Armos fiber in PF/ethanol | Increased surface roughness and polar groups | 25.4 | EP | ILSS | Increased wetting | N/A | [130] |
| γ-ray irradiation and ammonification | Increased surface roughness and –NH2 groups at the surface | 40.55 | EP | IFSS | −0.3 | [132] | |
| Microwave assisted surface treatment | Nanodeposit covered PPTA fiber bundle | 259 | rubber | Modified bundle pull-out | ±0 | [109] | |
| Grafting of CNTs onto PBIA fibers via direct fluorination | CNT and AA+DVB copolymer covered fiber surface | 69.1 | EP | IFSS | Increases electric conductivity and surface energy | ±0 | [98] |
| Grafting ANFs onto PPTA tape | ANF covered fiber surface | 70.27 | EP | IFSS | Increased short beam shear strength by 25.6% | ±0 | [99] |
| Grafting SiO2 and silane coupling agents to PBIA fibers | Increased surface roughness and ability to chemically bond with different type of matrixes | 117/166/ 43 | NR/ BMI/ EP | IFSS | ability to chemically bond with matrixes in a wide polarity range | ±0 | [91] |
References
- Fujun, X.; Yinnan, Z.; Feng, C.; Yiping, Q. Method for Modifying Aramid Fiber Composite by Subzero Treatment. Application No. CN 201410468164, 15 September 2014. [Google Scholar]
- Lee, J.Y.; Kim, S.O.; Yun, J.M.; Lee, K.E. Aramid Fiber Product with Excellent Conductivity and Method of Manufacturing the Same. Patent No. WO2014069853A1, 8 May 2014. [Google Scholar]
- Knijnenberg, A.; Bos, J.; Dingemans, T.J. The synthesis and characterisation of reactive poly (p-phenylene terephthalamide)s: A route towards compression stable aramid fibres. Polymer 2010, 51, 1887–1897. [Google Scholar] [CrossRef]
- Nilakantan, G.; Gillespie, J.W. Yarn pull-out behavior of plain woven Kevlar fabrics: Effect of yarn sizing, pullout rate, and fabric pre-tension. Compos. Struct. 2013, 101, 215–224. [Google Scholar] [CrossRef]
- Hill, H.W.; Kwolek, S.T.; Sweeny, W. Aromatic Polyamides. U.S. Patent Application No. US3380969A, 30 April 1968. [Google Scholar]
- Kwolek, S.L.; Winthrop, M.P.; Sorenson, W.R. Process of Making Wholly Aromatic Polyamides. US Patent Patent No. US3063966A, 13 November 1962. [Google Scholar]
- Kashani, M.R. Aramid-short-fiber reinforced rubber as a tire tread composite. J. Appl. Polym. Sci. 2009, 113, 1355–1363. [Google Scholar] [CrossRef]
- Shirazi, M.; Talma, A.G.; Noordermeer, J.W.M. Adhesion of RFL-coated aramid fibres to sulphur- and peroxide-cured elastomers. J. Adhes. Sci. Technol. 2013, 27, 1048–1057. [Google Scholar] [CrossRef]
- Lange, P.J.D.; Akker, P.G. Adhesion Activation of Twaron Aramid Fibers for Application in Rubber: Plasma Versus Chemical Treatment. J. Adhes. Sci. Technol. 2012, 26, 827–839. [Google Scholar] [CrossRef]
- Sharma, S.C. Adhesion of Rubber to Aramid Cords. Patent No. US4680228A, 14 July 1987. [Google Scholar]
- Song, W.; Gu, A.; Liang, G.; Yuan, L. Effect of the surface roughness on interfacial properties of carbon fibers reinforced epoxy resin composites. Appl. Surf. Sci. 2011, 257, 4069–4074. [Google Scholar] [CrossRef]
- Xie, J.; Xin, D.; Cao, H.; Wang, C.; Zhao, Y.; Yao, L.; Ji, F.; Qiu, Y. Improving carbon fiber adhesion to polyimide with atmospheric pressure plasma treatment. Surf. Coat. Technol. 2011, 206, 191–201. [Google Scholar] [CrossRef]
- Kim, S.Y.; Baek, S.J.; Youn, J.R. New hybrid method for simultaneous improvement of tensile and impact properties of carbon fiber reinforced composites. Carbon 2011, 49, 5329–5338. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, S. The effects of fiber’s surface roughness on the mechanical properties of fiber-reinforced polymer composites. J. Compos. Mater. 2013, 47, 2909–2923. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Suhr, J.; Koratkar, N. Carbon Nanotube/Polycarbonate Composites as Multifunctional Strain Sensors. J. Nanosci. Nanotechnol. 2006, 6, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.V. Advanced Sensors for Multifunctional Applications. Chem. Bus. 2012, 26, 36. [Google Scholar]
- Zhao, X.; Hua, Q.; Yu, R.; Zhang, Y.; Pan, C. Flexible, Stretchable and Wearable Multifunctional Sensor Array as Artifi cial Electronic Skin for Static and Dynamic Strain Mapping. Adv. Electron. Mater. 2015, 1, 1–7. [Google Scholar] [CrossRef]
- Gao, S.-L.; Zhuang, R.-C.; Jie, Z.; Liu, J.-W. Mäder Edith Glass Fibers with Carbon Nanotube Networks as Multifunctional Sensors. Adv. Funct. Mater. 2010, 20, 1885–1893. [Google Scholar] [CrossRef]
- Yokura, M.; Inoue, T. Aramid Paper, Method of Manufacturing the Same and Aramid-Polyester Laminate. Patent No. EP1873307A2, 2 January 2008. [Google Scholar]
- Jung, J.; Sodano, H.A. High strength epoxy nanocomposites reinforced by epoxy functionalized aramid nanofibers. Polymer 2020, 195, 122438. [Google Scholar] [CrossRef]
- Ou, Y.; Lin, M.; Su, L.; Feng, X.; Wang, M.; Li, J.; Liu, D.; Qi, H. Highly mechanical nanostructured aramid-composites with gradient structures. Compos. Part A Appl. Sci. Manuf. 2019, 118, 250–258. [Google Scholar] [CrossRef]
- Xiao, X.F.; Xin, L.; Genyang, C.; Chunhua, Z.; Liangjun, X.; Weilin, X.; Shili, X. Atomic layer deposition TiO2/Al2O3 nanolayer of dyed polyamide/aramid blend fabric for high intensity UV light protection. Polym. Eng. Sci. 2015, 55, 1296–1302. [Google Scholar] [CrossRef]
- Campbell, F.C. Structural Composite Materials; ASM International: Cleveland, OH, USA, 2010; p. 630. [Google Scholar]
- Tam, T.; Bhatnagar, A. High-performance ballistic fibers and tapes. In Lightweight Ballistic Composites, 2nd ed.; Bhatnagar, A., Ed.; Woodhead Publishing: Duxford, UK, 2016; Volume 1, pp. 1–39. [Google Scholar]
- Sharma, M.; Sharma, H.; Shannigrahi, S. 5—Advanced composites with strengthened nanostructured interface. In Hybrid Polymer Composite Materials; Thakur, V.K., Thakur, M.K., Gupta, R.K., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 107–123. [Google Scholar]
- Kim, J.; Hodzic, A. Nanoscale characterisation of thickness and properties of interphase in polymer matrix composites. J. Adhes. 2003, 79, 383–414. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Mahmood, H.; Pegoretti, A. Recent advances in fiber/matrix interphase engineering for polymer composites. Prog. Mater. Sci. 2015, 73, 1–43. [Google Scholar] [CrossRef]
- Jesson, D.A.; Watts, J.F. The Interface and Interphase in Polymer Matrix Composites: Effect on Mechanical Properties and Methods for Identification. Polym. Rev. 2012, 52, 321–354. [Google Scholar] [CrossRef]
- Wardle, M.W. Aramid Fiber Reinforced Plastics—Properties. In Comprehensive Composite Materials; Kelly, A., Zweben, C., Eds.; Pergamon: Oxford, UK, 2000; pp. 199–229. [Google Scholar]
- Carrillo-Baeza, J.G.; Cantwell, W.J.; Gamboa-Castellanos, R.A. Advantages of Low Energy Adhesion PP for Ballistics. In Thermoplastic Elastomers; El-Sonbati, A., Ed.; IntechOpen: London, UK, 2012; pp. 193–212. [Google Scholar]
- Gallini, J. Polyamides, Aromatic. Kirk Othmer Encycl. Chem. Technol. 2001, 3, 558–584. [Google Scholar] [CrossRef]
- Chang, K.K. Aramid Fibers. In ASM Handbooks: Composites; Miracle, D.B., Donaldson, S.L., Eds.; ASM International: Cleveland, OH, USA, 2001; Volume 21, pp. 41–45. [Google Scholar]
- Vara Prasad, V.; Talupula, S. A Review on Reinforcement of Basalt and Aramid (Kevlar 129) fibers. Mater. Today Proc. 2018, 5, 5993–5998. [Google Scholar] [CrossRef]
- Roth, S.; Burghammer, M.; Janotta, A.; Riekel, C. Rotational Disorder in Poly (p-phenylene terephthalamide) Fibers by X-ray Diffraction with a 100 nm Beam. Macromolecules 2003, 36, 1585–1593. [Google Scholar] [CrossRef]
- Qi, G.; Zhang, B.; Du, S.; Yu, Y. Estimation of aramid fiber/epoxy interfacial properties by fiber bundle tests and multiscale modeling considering the fiber skin/core structure. Compos. Struct. 2017, 167, 1–10. [Google Scholar] [CrossRef]
- Luo, L.; Yuan, Y.; Dai, Y.; Cheng, Z.; Wang, X.; Liu, X. The novel high performance aramid fibers containing benzimidazole moieties and chloride substitutions. Mater. Des. 2018, 158, 127–135. [Google Scholar] [CrossRef]
- Dai, Y.; Meng, C.; Cheng, Z.; Luo, L.; Liu, X. Nondestructive modification of aramid fiber based on selective reaction of external cross-linker to improve interfacial shear strength and compressive strength. Compos. Part A Appl. Sci. Manuf. 2019, 119, 217–224. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, Y.; Meng, C.; Dai, Y.; Luo, L.; Liu, X. Constructing a weaving structure for aramid fiber by carbon nanotube-based network to simultaneously improve composites interfacial properties and compressive properties. Compos. Sci. Technol. 2019, 182, 107721. [Google Scholar] [CrossRef]
- de Lange, P.J.; Akker, P.G.; Mäder, E.; Gao, S.; Prasithphol, W.; Young, R.J. Controlled interfacial adhesion of Twaron® aramid fibres in composites by the finish formulation. Compos. Sci. Technol. 2007, 67, 2027–2035. [Google Scholar] [CrossRef]
- de Lange, P.J.; Mäder, E.; Mai, K.; Young, R.J.; Ahmad, I. Characterization and micromechanical testing of the interphase of aramid-reinforced epoxy composites. Compos. Part A Appl. Sci. Manuf. 2001, 32, 331–342. [Google Scholar] [CrossRef]
- Jia, C.; Chen, P.; Li, B.; Wang, Q.; Lu, C.; Yu, Q. Effects of Twaron fiber surface treatment by air dielectric barrier discharge plasma on the interfacial adhesion in fiber reinforced composites. Surf. Coat. Technol. 2010, 204, 3668–3675. [Google Scholar] [CrossRef]
- Sun, J.; Yao, L.; Sun, S.; Qiu, Y. ESR study of atmospheric pressure plasma jet irradiated aramid fibers. Surf. Coat. Technol. 2011, 205, 5312–5317. [Google Scholar] [CrossRef]
- Zhao, J. Effect of surface treatment on the structure and properties of para-aramid fibers by phosphoric acid. Fibers Polym. 2013, 14, 59–64. [Google Scholar] [CrossRef]
- Luo, S.; Ooij, W.J.V. Surface modification of textile fibers for improvement of adhesion to polymeric matrices: A review. J. Adhes. Sci. Technol. 2002, 16, 1715–1735. [Google Scholar] [CrossRef]
- Hirosuke, W.; Masashi, F.; Tadahiko, T.; Masahide, Y. Surface improvements of aramid fibers by physical treatments. Macromol. Symp. 2001, 159, 131–142. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, W.; Yang, D.; Zhou, X.; Zhao, Z.; Zhang, L.; Wang, S.; Feng, J. Hydrophilicity modification of aramid fiber using a linear shape plasma excited by nanosecond pulse. Surf. Coat. Technol. 2018, 344, 614–620. [Google Scholar] [CrossRef]
- On, S.Y.; Kim, M.S.; Kim, S.S. Effects of post-treatment of meta-aramid nanofiber mats on the adhesion strength of epoxy adhesive joints. Compos. Struct. 2017, 159, 636–645. [Google Scholar] [CrossRef]
- Wang, J.; Chen, P.; Xiong, X.; Jia, C.; Yu, Q.; Ma, K. Interface characteristic of aramid fiber reinforced poly (phthalazinone ether sulfone ketone) composite. Surf. Interface Anal. 2017, 49, 788–793. [Google Scholar] [CrossRef]
- Kim, J.G.; Lee, D.; Choi, I.; Seo, I.S. Flame and silane treatments for improving the adhesive bonding characteristics of aramid/epoxy composites. Compos. Struct. 2011, 93, 2696–2705. [Google Scholar] [CrossRef]
- Liu, T.-M.; Zheng, Y.-S.; Jie, H. Surface modification of Aramid fibers with new chemical method for improving interfacial bonding strength with epoxy resin. J. Appl. Polym. Sci. 2010, 118, 2541–2552. [Google Scholar] [CrossRef]
- Ehlert, G.J.; Sodano, H.A. Zinc oxide nanowire interphase for enhanced interfacial strength in lightweight polymer fiber composites. ACS Appl. Mater. Interfaces 2009, 1, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.; de Rooij, M.B.; Talma, A.G.; Noordermeer, J.W.M. Adhesion of RFL-coated aramid fibres to elastomers: The role of elastomer-latex compatibility. J. Adhes. Sci. Technol. 2013, 27, 1886–1898. [Google Scholar] [CrossRef]
- Willemsen, S.; Weening, W.E.; Steenbergen, A. Adhesive-Coated Multifilament Yarn of an Aromatic Polyamide and a Method for the Manufacture Thereof. Patent No. EP0107887A1, 9 May 1984. [Google Scholar]
- de Lange, P.; Akker, P.G.; Maas, A.J.H.; Knoester, A.; Brongersma, H.H. Adhesion activation of Twaron® aramid fibres studied with low-energy ion scattering and x-ray photoelectron spectroscopy. Surf. Interface Anal. 2001, 31, 1079–1084. [Google Scholar] [CrossRef]
- Binetti, R.; Costamagna, F.M.; Marcello, I. Development of carcinogenicity classifications and evaluations: The case of formaldehyde. Ann. Dell’ Ist. Super. Sanità 2006, 42, 132–143. [Google Scholar]
- Info Card European Chemicals Agency, Helsinki, FI. Formaldehyde—Substance Information. 2018. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.000.002 (accessed on 10 June 2020).
- Chawla, K.K. Composite Materials: Science and Engineering, 3rd ed.; Springer: New York, NY, USA, 2012; p. 541. [Google Scholar]
- Mittal, G.; Rhee, K.Y.; Mišković-Stanković, V.; Hui, D. Reinforcements in multi-scale polymer composites: Processing, properties, and applications. Compos. Part B Eng. 2018, 138, 122–139. [Google Scholar] [CrossRef]
- Chen, W.; Qian, X.; He, X.; Liu, Z.; Liu, J. Surface modification of Kevlar by grafting carbon nanotubes. J. Appl. Polym. Sci. 2011, 123, 1983–1990. [Google Scholar] [CrossRef]
- Hazarika, A.; Deka, B.K.; Kim, D.; Park, Y.; Park, H.W. Microwave-induced hierarchical iron-carbon nanotubes nanostructures anchored on polypyrrole/graphene oxide-grafted woven Kevlar® fiber. Compos. Sci. Technol. 2016, 129, 137–145. [Google Scholar] [CrossRef]
- Rodríguez-Uicab, O.; Avilés, F.; Gonzalez-Chi, P.I.; Canché-Escamilla, G.; Duarte-Aranda, S.; Yazdani-Pedram, M.; Toro, P.; Gamboa, F.; Mazo, M.A.; Nistal, A.; et al. Deposition of carbon nanotubes onto aramid fibers using as-received and chemically modified fibers. Appl. Surf. Sci. 2016, 385, 379–390. [Google Scholar] [CrossRef]
- Hussain, S.; Yorucu, C.; Ahmed, I.; Hussain, R.; Chen, B.; Bilal Khan, M.; Siddique, N.A.; Rehman, I.U. Surface modification of aramid fibres by graphene oxide nano-sheets for multiscale polymer composites. Surf. Coat. Technol. 2014, 258, 458–466. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Ni, Q.; Fu, Y.; Fu, X. Surface modification and characterization of aramid fibers with hybrid coating. Appl. Surf. Sci. 2014, 321, 103–108. [Google Scholar] [CrossRef]
- Kim, C.; Randow, C.; Sano, T. (Eds.) Hybrid and Hierarchical Composite Materials; Springer International Publishing: Zurich, Switzerland, 2015; p. 365. [Google Scholar]
- Ortiz, C.; Boyce, M.C. Bioinspired Structural Materials. Science 2008, 319, 1053–1054. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.; Wolff, M.F.H.; Salikov, V.; Heinrich, S.; Schneider, G.A. A novel method for a multi-level hierarchical composite with brick-and-mortar structure. Sci. Rep. 2013, 3, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilatela, J.J.; Khare, R.; Windle, A.H. The hierarchical structure and properties of multifunctional carbon nanotube fibre composites. Carbon 2012, 50, 1227–1234. [Google Scholar] [CrossRef]
- Xing, L.; Liu, L.; Xie, F.; Huang, Y. Mutual irradiation grafting on indigenous aramid fiber-3 in diethanolamine and epichlorohydrin and its effect on interfacially reinforced epoxy composite. Appl. Surf. Sci. 2016, 375, 65–73. [Google Scholar] [CrossRef]
- Warrier, A.; Godara, A.; Rochez, O.; Mezzo, L.; Luizi, F.; Gorbatikh, L.; Lomov, S.V.; VanVuure, A.W.; Verpoest, I. The effect of adding carbon nanotubes to glass/epoxy composites in the fibre sizing and/or the matrix. Compos. Part A Appl. Sci. Manuf. 2010, 41, 532–538. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, R.; Liu, J.; Mäder, E.; Heinrich, G.; Gao, S. Functional interphases with multi-walled carbon nanotubes in glass fibre/epoxy composites. Carbon 2010, 48, 2273–2281. [Google Scholar] [CrossRef]
- Luo, S.; Obitayo, W.; Liu, T. SWCNT-thin-film-enabled fiber sensors for lifelong structural health monitoring of polymeric composites—From manufacturing to utilization to failure. Carbon 2014, 76, 321–329. [Google Scholar] [CrossRef]
- Park, S.; Jin, J. Effect of Silane Coupling Agent on Interphase and Performance of Glass Fibers/Unsaturated Polyester Composites. J. Colloid Interface Sci. 2001, 242, 174–179. [Google Scholar] [CrossRef]
- Wu, H.F.; Dwight, D.W.; Huff, N.T. Effects of silane coupling agents on the interphase and performance of glass-fiber-reinforced polymer composites. Compos. Sci. Technol. 1997, 57, 975–983. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- He, S.; Sun, G.; Cheng, X.; Dai, H.; Chen, X. Nanoporous SiO2 grafted aramid fibers with low thermal conductivity. Compos. Sci. Technol. 2017, 146, 91–98. [Google Scholar] [CrossRef]
- Cheng, Z.; Hong, D.; Dai, Y.; Jiang, C.; Meng, C.; Luo, L.; Liu, X. Highly improved Uv resistance and composite interfacial properties of aramid fiber via iron (III) coordination. Appl. Surf. Sci. 2018, 434, 473–480. [Google Scholar] [CrossRef]
- Kickelbick, G. Introduction to Hybrid Materials. In Hybrid Materials. Synthesis, Characterization and Applications; Kickelbick, G., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 1–48. [Google Scholar]
- Miguel-Ortega, Á.; Trigo-López, M.; García, F.C.; Reglero, J.A.; Martínez-Alonso, M.; Espino, G.; García, J.M. Hybrid aramids, Ir(III)-functionalized aromatic polyamides. Eur. Polym. J. 2017, 95, 119–126. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Y.; Chen, S.; Wang, W.; Tian, M.; Ning, N.; Zhang, L. Highly efficient mussel-like inspired modification of aramid fibers by UV-accelerated catechol/polyamine deposition followed chemical grafting for high-performance polymer composites. Chem. Eng. J. 2017, 314, 583–593. [Google Scholar] [CrossRef]
- Zhu, J.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. A novel strategy of fabricating high performance UV-resistant aramid fibers with simultaneously improved surface activity, thermal and mechanical properties through building polydopamine and graphene oxide bi-layer coatings. Chem. Eng. J. 2017, 310, 134–147. [Google Scholar] [CrossRef]
- Zhou, L.; Yuan, L.; Guan, Q.; Gu, A.; Liang, G. Building unique surface structure on aramid fibers through a green layer-by-layer self-assembly technique to develop new high performance fibers with greatly improved surface activity, thermal resistance, mechanical properties and UV resistance. Appl. Surf. Sci. 2017, 411, 34–45. [Google Scholar] [CrossRef]
- Patterson, B.A.; Sodano, H.A. Enhanced Interfacial Strength and UV Shielding of Aramid Fiber Composites through ZnO Nanoparticle Sizing. ACS Appl. Mater. Interfaces 2016, 8, 33963–33971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yuan, L.; Liang, G.; Gu, A. Effect and origin of the structure of hyperbranched polysiloxane on the surface and integrated performances of grafted Kevlar fibers. Appl. Surf. Sci. 2014, 320, 883–894. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, G.; Gu, A.; Yuan, L. Facile Preparation of Hyperbranched Polysiloxane-Grafted Aramid Fibers with Simultaneously Improved UV Resistance, Surface Activity, and Thermal and Mechanical Properties. Ind. Eng. Chem. Res. 2014, 53, 2684–2696. [Google Scholar] [CrossRef]
- Cheng, Z.; Chen, C.; Huang, J.; Chen, T.; Liu, Y.; Liu, X. Nondestructive grafting of PEI on aramid fiber surface through the coordination of Fe (III) to enhance composite interfacial properties. Appl. Surf. Sci. 2017, 401, 323–332. [Google Scholar] [CrossRef]
- Vleugels, N.; Dierkes, W.K.; Blume, A.; Reuvekamp Louis, A.E.M.; Noordermeer, J.W.M. Understanding the behavior of the coupling agents TESPT and SI 363 in short-cut aramid fibre reinforced elastomers. KGK Kautsch. Gummi Kunstst. 2017, 70, 39–51. [Google Scholar]
- España, J.M.; Samper, M.D.; Fages, E.; Sánchez-Nácher, L.; Balart, R. Investigation of the Effect of Different Silane Coupling Agents on Mechanical Performance of Basalt Fiber Composite Laminates with Biobased Epoxy Matrices. Polym. Compos. 2013, 34, 376–381. [Google Scholar] [CrossRef]
- Ai, T.; Wang, R.; Zhou, W. Effect of grafting alkoxysilane on the surface properties of Kevlar fiber. Polym. Compos. 2007, 28, 412–416. [Google Scholar] [CrossRef]
- Sa, R.; Yan, Y.; Wei, Z.; Zhang, L.; Wang, W.; Tian, M. Surface Modification of Aramid Fibers by Bio-Inspired Poly (dopamine) and Epoxy Functionalized Silane Grafting. ACS Appl. Mater. Interfaces 2014, 6, 21730–21738. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, Y.; Sa, R.; Ning, N.; Wang, W.; Tian, M.; Zhang, L. Surface Modification of Aramid Fibers by Catechol/Polyamine Codeposition Followed by Silane Grafting for Enhanced Interfacial Adhesion to Rubber Matrix. Ind. Eng. Chem. Res. 2016, 55, 12547–12556. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, L.; Jiang, C.; Dai, Y.; Meng, C.; Luo, L.; Liu, X. Aramid fiber with excellent interfacial properties suitable for resin composite in a wide polarity range. Chem. Eng. J. 2018, 347, 483–492. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Li, Y.; Wong, E.; Mai, Z.; Van der Bruggen, B. Fabrication of composite polyamide/Kevlar aramid nanofiber nanofiltration membranes with high permselectivity in water desalination. J. Membr. Sci. 2019, 592, 117396. [Google Scholar] [CrossRef]
- Yang, M.; Cao, K.; Sui, L.; Qi, Y.; Zhu, J.; Waas, A.; Arruda, E.M.; Kieffer, J.; Thouless, M.D.; Kotov, N.A. Dispersions of Aramid Nanofibers: A New Nanoscale Building Block. ACS Nano 2011, 5, 6945–6954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Li, Y.; Zhu, J.; Lejarazu-Larrañaga, A.; Yuan, S.; Ortega, E.; Shen, J.; Gao, C.; Bruggen, B.V.D. Thin and robust organic solvent cation exchange membranes for ion separation. J. Mater. Chem. A 2019, 7, 13903–13909. [Google Scholar] [CrossRef]
- O’Connor, I.; Hugh, H.; Coleman, J.N.; Gun’ko, Y.K. High-Strength, High-Toughness Composite Fibers by Swelling Kevlar in Nanotube Suspensions. Small 2009, 5, 466–469. [Google Scholar] [CrossRef]
- Fan, W.; Wang, Y.; Wang, C.; Chen, J.; Wang, Q.; Yuan, Y.; Niu, F. High efficient preparation of carbon nanotube-grafted carbon fibers with the improved tensile strength. Appl. Surf. Sci. 2016, 364, 539–551. [Google Scholar] [CrossRef]
- Lv, J.; Cheng, Z.; Wu, H.; He, T.; Qin, J.; Liu, X. In-situ polymerization and covalent modification on aramid fiber surface via direct fluorination for interfacial enhancement. Compos. Part B Eng. 2020, 182, 107608. [Google Scholar] [CrossRef]
- Nasser, J.; Lin, J.; Steinke, K.; Sodano, H.A. Enhanced interfacial strength of aramid fiber reinforced composites through adsorbed aramid nanofiber coatings. Compos. Sci. Technol. 2019, 174, 125–133. [Google Scholar] [CrossRef]
- Hwang, H.; Malakooti, M.H.; Patterson, B.A.; Sodano, H.A. Increased interyarn friction through ZnO nanowire arrays grown on aramid fabric. Compos. Sci. Technol. 2015, 107, 75–81. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Wang, X. Nanoparticle Coatings for UV Protective Textiles. Res. J. Text. Appar. 2010, 14, 9–20. [Google Scholar] [CrossRef]
- Wang, C.X.; Du, M.; Lv, J.C.; Zhou, Q.Q.; Ren, Y.; Liu, G.L.; Gao, D.W.; Jin, L.M. Surface modification of aramid fiber by plasma induced vapor phase graft polymerization of acrylic acid. I. Influence of plasma conditions. Appl. Surf. Sci. 2015, 349, 333–342. [Google Scholar] [CrossRef]
- Graham, J.F.; McCague, C.; Warren, O.L.; Norton, P.R. Spatially resolved nanomechanical properties of Kevlar® fibers. Polymer 2000, 41, 4761–4764. [Google Scholar] [CrossRef]
- Xu, L.; Hu, J.; Ma, H.; Wu, G. Electron-beam-induced post-grafting polymerization of acrylic acid onto the surface of Kevlar fibers. Radiat. Phys. Chem. 2018, 145, 74–79. [Google Scholar] [CrossRef]
- Mathur, R.B.; Chatterjee, S.; Singh, B.P. Growth of carbon nanotubes on carbon fibre substrates to produce hybrid/phenolic composites with improved mechanical properties. Compos. Sci. Technol. 2008, 68, 1608–1615. [Google Scholar] [CrossRef]
- De Greef, N.; Zhang, L.; Magrez, A.; Forró, L.; Locquet, J.; Verpoest, I.; Seo, J.W. Direct growth of carbon nanotubes on carbon fibers: Effect of the CVD parameters on the degradation of mechanical properties of carbon fibers. Diam. Relat. Mater. 2015, 51, 39–48. [Google Scholar] [CrossRef]
- Boehle, M.; Jiang, Q.; Li, L.; Lagounov, A.; Lafdi, K. Carbon nanotubes grown on glass fiber as a strain sensor for real time structural health monitoring. Int. J. Smart Nano Mater. 2012, 3, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, R.; Wagner, H.D. Fast growth of carbon nanotubes using a microwave oven. Carbon 2015, 82, 327–336. [Google Scholar] [CrossRef]
- Palola, S.; Sarlin, E.; Kolahgar Azari, S.; Koutsos, V.; Vuorinen, J. Microwave induced hierarchical nanostructures on aramid fibers and their influence on adhesion properties in a rubber matrix. Appl. Surf. Sci. 2017, 410, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Cui, J. Zinc oxide nanowires. Mater. Charact. 2012, 64, 43–52. [Google Scholar] [CrossRef]
- Hazarika, A.; Deka, B.K.; Kim, D.; Kong, K.; Park, Y.; Park, H.W. Growth of aligned ZnO nanorods on woven Kevlar® fiber and its performance in woven Kevlar® fiber/polyester composites. Compos. Part A Appl. Sci. Manuf. 2015, 78, 284–293. [Google Scholar] [CrossRef]
- Hwang, H.; Malakooti, M.H.; Sodano, H.A. Tailored interyarn friction in aramid fabrics through morphology control of surface grown ZnO nanowires. Compos. Part A Appl. Sci. Manuf. 2015, 76, 326–333. [Google Scholar] [CrossRef]
- Zeng, X.S.; Tan, V.B.C.; Shim, V.P.W. Modelling inter-yarn friction in woven fabric armour. Int. J. Numer. Methods Eng. 2005, 66, 1309–1330. [Google Scholar] [CrossRef]
- Nilakantan, G.; Gillespie, J.W. Ballistic impact modeling of woven fabrics considering yarn strength, friction, projectile impact location, and fabric boundary condition effects. Compos. Struct. 2012, 94, 3624–3634. [Google Scholar] [CrossRef]
- Gawandi, A.; Thostenson, E.; Gilllespie, J., Jr. Tow pullout behavior of polymer-coated Kevlar fabric. J. Mater. Sci. 2011, 46, 77–89. [Google Scholar] [CrossRef]
- Malakooti, M.H.; Hwang, H.; Goulbourne, N.C.; Sodano, H.A. Role of ZnO nanowire arrays on the impact response of aramid fabrics. Compos. Part B Eng. 2017, 127, 222–231. [Google Scholar] [CrossRef]
- Chen, X.; Sun, D.; Wells, G.M. Effect of Inter-yarn Friction on Ballistic Performance of Woven Fabrics. In Polymeric Protective Technical Textiles; McCarthy, B.J., Ed.; Smithers Rapra: Akron, OH, USA, 2013; pp. 41–60. [Google Scholar]
- Wang, B.; Duan, Y.; Zhang, J. Titanium dioxide nanoparticles-coated aramid fiber showing enhanced interfacial strength and UV resistance properties. Mater. Des. 2016, 103, 330–338. [Google Scholar] [CrossRef]
- Yanjun, X.; Xin, D. UV photo-stabilization of tetrabutyl titanate for aramid fibers via sol-gel surface modification. J. Appl. Polym. Sci. 2007, 103, 3113–3119. [Google Scholar] [CrossRef]
- Hui, D.; Hongda, Z. In situ synthesis and hydrothermal crystallization of nanoanatase TiO2–SiO2 coating on aramid fabric (HTiSiAF) for UV protection. Microsc. Res. Tech. 2015, 78, 918–925. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Taege, R.; Ferrier, G.; Teplyakov, V.V.; Syrtsova, D.A.; Koops, G. Direct fluorination—Useful tool to enhance commercial properties of polymer articles. J. Fluor. Chem. 2005, 126, 251–263. [Google Scholar] [CrossRef]
- Jeong, E.; Lee, B.H.; Doh, S.J.; Park, I.J.; Lee, Y. Multifunctional surface modification of an aramid fabric via direct fluorination. J. Fluor. Chem. 2012, 141, 69–75. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, P.; Li, B.; Chen, T.; Liu, Y.; Ren, M.; Wang, Z.; Lai, W.; Wang, X.; Liu, X. Surface chain cleavage behavior of PBIA fiber induced by direct fluorination. Appl. Surf. Sci. 2016, 384, 480–486. [Google Scholar] [CrossRef]
- Luo, L.; Wu, P.; Cheng, Z.; Hong, D.; Li, B.; Wang, X.; Liu, X. Direct fluorination of para-aramid fibers 1: Fluorination reaction process of PPTA fiber. J. Fluor. Chem. 2016, 186, 12–18. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, P.; Gao, J.; Wang, X.; Ren, M.; Li, B.; Luo, L.; Liu, X. Structural evolution of fluorinated aramid fibers with fluorination degree and dominant factor for its adhesion property. J. Fluor. Chem. 2016, 188, 139–146. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, B.; Huang, J.; Chen, T.; Liu, Y.; Wang, X.; Liu, X. Covalent modification of Aramid fibers’ surface via direct fluorination to enhance composite interfacial properties. Mater. Des. 2016, 106, 216–225. [Google Scholar] [CrossRef]
- Saunders, C.B.; Singh, A.; Lopata, V.J.; Seier, S.; Boyer, G.D.; Kremers, W.; Mason, V.A. Electron-Beam Curing of Aramid-Fiber-Reinforced Composites. In Radiation Effects on Polymers; Clough, R.L., Shalaby, S.W., Eds.; American Chemical Society: Washington, DC, USA, 1991; Volume 475, pp. 251–261. [Google Scholar]
- Clough, R.L. High-energy radiation and polymers: A review of commercial processes and emerging applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2001, 185, 8–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Liu, L.; Wu, L. Surface modification of aramid fibers with γ-ray radiation for improving interfacial bonding strength with epoxy resin. J. Appl. Polym. Sci. 2007, 106, 2251–2262. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Huang, Y.D.; Liu, L.; Cai, K.L. Effects of γ-ray radiation grafting on aramid fibers and its composites. Appl. Surf. Sci. 2008, 254, 3153–3161. [Google Scholar] [CrossRef]
- Xing, L.; Liu, L.; Huang, Y.; Jiang, D.; Jiang, B.; He, J. Enhanced interfacial properties of domestic aramid fiber-12 via high energy gamma ray irradiation. Compos. Part B Eng. 2015, 69, 50–57. [Google Scholar] [CrossRef]
- Xie, F.; Xing, L.; Liu, L.; Liu, Y.; Zhong, Z.; Jia, C.; Wang, W.; Wang, C.; Zhao, M.; Huang, Y. Surface ammonification of the mutual-irradiated aramid fibers in 1,4-dichlorobutane for improving interfacial properties with epoxy resin. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Nasser, J.; Groo, L.; Zhang, L.; Sodano, H. Laser induced graphene fibers for multifunctional aramid fiber reinforced composite. Carbon 2020, 158, 146–156. [Google Scholar] [CrossRef]
- Xu, F.; Fan, W.; Zhang, Y.; Gao, Y.; Jia, Z.; Qiu, Y.; Hui, D. Modification of tensile, wear and interfacial properties of Kevlar fibers under cryogenic treatment. Compos. Part B Eng. 2017, 116, 398–405. [Google Scholar] [CrossRef]
- Fan, J.; Shi, Z.; Zhang, L.; Wang, J.; Yin, J. Aramid nanofiber-functionalized graphene nanosheets for polymer reinforcement. Nanoscale 2012, 4, 7046–7055. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Bang, S.H.; Malakooti, M.H.; Sodano, H.A. Isolation of Aramid Nanofibers for High Strength and Toughness Polymer Nanocomposites. ACS Appl. Mater. Interfaces 2017, 9, 11167–11175. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Al-Sagheer, F.; Shiju, J. Aramid-multiwalled carbon nanotube nanocomposites: Effect of compatibilization through oligomer wrapping of the nanotubes. Polym. Int. 2016, 65, 1204–1213. [Google Scholar] [CrossRef]
- Shiju, J.; Al-Sagheer, F.; Bumajdad, A.; Ahmad, Z. In-Situ Preparation of Aramid-Multiwalled CNT Nano-Composites: Morphology, Thermal Mechanical and Electric Properties. Nanomaterials 2018, 8, 309. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Cao, W.; Yue, M.; Hou, Y.; Han, J.; Yang, M. Strong and stiff aramid nanofiber/carbon nanotube nanocomposites. ACS Nano 2015, 9, 2489–2501. [Google Scholar] [CrossRef] [PubMed]







| Type of Method | Increase in IFSS [%] | Change in Mechanical Strength [%] | Reference | |
|---|---|---|---|---|
| Coating | Hybrid coating | 19…47 | 0…6 | [62,63,82,85] |
| Silane coupling | 63…166 | 0 | [90,91,98] | |
| Grafting | Nanotubes/fibers | 70 | 0 | [98,99] |
| Nanoparticles | 31…122 | −3…−1 | [68,102] | |
| Growing | Nanowires | 51 | 0 | [51] |
| Nanoparticles | 67 | −4 | [118] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palola, S.; Vuorinen, J.; Noordermeer, J.W.M.; Sarlin, E. Development in Additive Methods in Aramid Fiber Surface Modification to Increase Fiber-Matrix Adhesion: A Review. Coatings 2020, 10, 556. https://doi.org/10.3390/coatings10060556
Palola S, Vuorinen J, Noordermeer JWM, Sarlin E. Development in Additive Methods in Aramid Fiber Surface Modification to Increase Fiber-Matrix Adhesion: A Review. Coatings. 2020; 10(6):556. https://doi.org/10.3390/coatings10060556
Chicago/Turabian StylePalola, Sarianna, Jyrki Vuorinen, Jacques W. M. Noordermeer, and Essi Sarlin. 2020. "Development in Additive Methods in Aramid Fiber Surface Modification to Increase Fiber-Matrix Adhesion: A Review" Coatings 10, no. 6: 556. https://doi.org/10.3390/coatings10060556
APA StylePalola, S., Vuorinen, J., Noordermeer, J. W. M., & Sarlin, E. (2020). Development in Additive Methods in Aramid Fiber Surface Modification to Increase Fiber-Matrix Adhesion: A Review. Coatings, 10(6), 556. https://doi.org/10.3390/coatings10060556





