Gas Technique of Simultaneous Borocarburizing of Armco Iron Using Trimethyl Borate
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Simultaneous Gas Borocarburizing
2.3. Microstructure Analysis
2.4. Microhardness Test
2.5. Wear Resistance Test
3. Results and Discussion
3.1. Thermodynamic Fundamentals of the Gas Borocarburizing Process
3.2. Microstructure Characterization
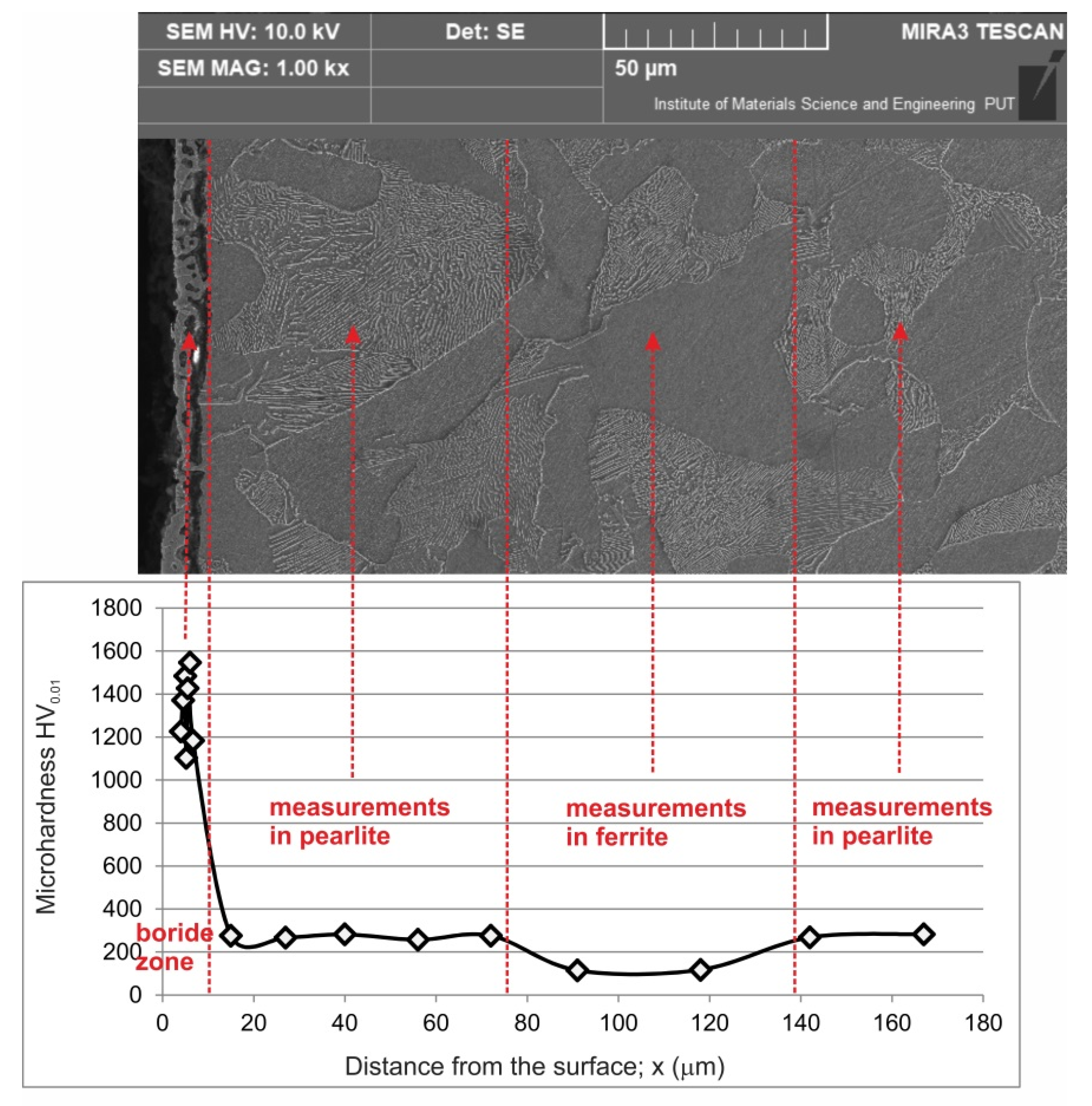
3.3. Microhardness and Wear Resistance
4. Conclusions
- -
- The microstructure of the produced diffusion layer consisted of two zones: an outer zone of Fe2B iron borides and an inner zone of carbon diffusion (carburized zone),
- -
- The important reaction, proceeding during simultaneous gas borocarburizing with the use of trimethyl borate, was the reduction of trimethyl borate with hydrogen. As a consequence, atomic carbon was produced. Therefore, the appropriate conditions for a carburizing process were realised. The next reaction in this atmosphere provided atomic boron, which could diffuse into the substrate material producing the boride layer,
- -
- The compact boride zone was characterized by low thickness (average value of 7.8 µm) which was caused by the hindered diffusion of boron atoms into the carburized zone,
- -
- Simultaneously formed, due to the intensive carburizing—the zone of carbon diffusion was characterized by a considerable thickness of 396.7 µm,
- -
- The presence of the outer Fe2B zone caused an increase in hardness up to 1546 HV0.01,
- -
- Gas borocarburizing in a N2–H2–B(CH3O)3 atmosphere caused an increase in the wear resistance in comparison with untreated Armco iron.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kulka, M. Current Trends in Boriding: Techniques; Engineerings Materials series; Springer International Publishing, Springer Nature Switzerland AG: Basel, Switzerland, 2019; pp. 1–282. ISBN 978-3-030-06781-6. [Google Scholar]
- Asthana, P.; Liang, H.; Usta, M.; Ucisik, A.H. Wear and surface characterization of boronized pure Iron. J. Tribol. 2007, 129, 1–10. [Google Scholar] [CrossRef]
- Elias-Espinosa, M.; Ortiz-Dominguez, M.; Keddam, M.; Flores-Renteria, M.A.; Damian-Mejia, O.; Zuno-Silva, J.; Hernandez-Avila, J.; Cardoso-Legorreta, E.; Arenas-Flores, A. Growth kinetics of the Fe2B layers and adhesion on Armco iron substrate. J. Mater. Eng. Perform. 2014, 23, 2014–2943. [Google Scholar] [CrossRef]
- Venkataraman, B.; Sundararajan, G. The high speed sliding wear behaviour of boronized medium carbon steel. Surf. Coat. Technol. 1995, 73, 177–184. [Google Scholar] [CrossRef]
- Xu, C.H.; Xi, J.K.; Gao, W. Improving the mechanical properties of boronized layers by superplastic boronizing. J. Mater. Process. Technol. 1997, 65, 94–98. [Google Scholar] [CrossRef]
- Gutierrez-Noda, L.; Cuao-Moreu, C.A.; Perez-Acosta, O.; Lorenzo-Bonet, E.; Zambrano-Robledo, P.; Hernandez-Rodriguez, M.A.L. The effect of a boride diffusion layer on the tribological properties of AISI M2 steel. Wear 2019, 426–427, 1667–1671. [Google Scholar] [CrossRef]
- Hernandez-Sanchez, E.; Rodriguez-Castro, G.; Meneses-Amador, A.; Bravo-Barcenas, D.; Arzate-Vazquez, I.; Martinez-Gutierrez, H.; Romero-Romo, M.; Campos-Silva, I. Effect of the anisotropic growth on the fracture toughness measurements obtained in the Fe2B layer. Surf. Coat. Technol. 2013, 237, 292–298. [Google Scholar] [CrossRef]
- Spence, T.W.; Makhlouf, M.M. Characterization of the operative mechanism in potassium fluoborate activated pack boriding of steels. J. Mater. Process. Technol. 2005, 168, 127–136. [Google Scholar] [CrossRef]
- Meneses-Amador, A.; Blancas-Perez, D.; Corpus-Mejia, R.; Rodriguez-Castro, G.A.; Martinez-Trinidad, J.; Jimenez-Tinoco, L.F. Adhesive and cohesive strength in FeB/Fe2B systems. J. Mater. Eng. Perform. 2018, 27, 2018–2089. [Google Scholar] [CrossRef]
- Türkmen, I.; Yalamac, E.; Keddam, M. Investigation of tribological behaviour and diffusion model of Fe2B layer formed by pack-boriding on SAE 1020 steel. Surf. Coat. Technol. 2019, 377, 124888. [Google Scholar] [CrossRef]
- Türkmen, I.; Yalamac, E. Growth of the Fe2B layer on SAE 1020 steel employed a boron source of H3BO3 during the powder-pack boriding method. J. Alloys Compd. 2018, 744, 658–666. [Google Scholar] [CrossRef]
- Kartal, G.; Eryilmaz, O.L.; Krumdick, G.; Erdemir, A.; Timur, S. Kinetics of electrochemical boriding of low carbon steel. Appl. Surf. Sci. 2011, 257, 6928–6934. [Google Scholar] [CrossRef]
- Allaoui, O.; Bouaouadja, N.; Saindernan, G. Characterization of boronized layers on a XC38 steel. Surf. Coat. Technol. 2006, 201, 3475–3482. [Google Scholar] [CrossRef]
- Sen, S.; Sen, U.; Bindal, C. The growth kinetics of borides formed on boronized AISI 4140 steel. Vacuum 2005, 77, 195–202. [Google Scholar] [CrossRef]
- Sen, S.; Sen, U.; Bindal, C. An approach to kinetic study of borided steels. Surf. Coat. Technol. 2005, 191, 274–285. [Google Scholar] [CrossRef]
- Wierzchon, T.; Bielinski, P.; Sikorski, K. Formation and properties of multicomponent and composite borided layers on steel. Surf. Coat. Technol. 1995, 73, 121–124. [Google Scholar] [CrossRef]
- Hunger, H.J.; Löbig, G. Generation of boride layers on steel and nickel alloys by plasma activation of boron trifluoride. Thin Solid Films 1997, 310, 244–250. [Google Scholar] [CrossRef]
- Miyashita, F.; Yokota, K. Plasma-assisted low temperature boridation of pure iron and steels. Surf. Coat. Technol. 1996, 84, 334–337. [Google Scholar] [CrossRef]
- Rodriguez Cabeo, E.; Laudien, G.; Biemer, S.; Rie, K.T.; Hoppe, S. Plasma-assisted boriding of industrial components in a pulsed d.c. glow discharge. Surf. Coat. Technol. 1999, 116–119, 229–233. [Google Scholar] [CrossRef]
- Kashaev, N.; Stock, H.R.; Mayr, P. Plasmaborieren mit Triethylboran. Haerterei Tech. Mit. 2003, 58, 1–9. [Google Scholar]
- Qiao, X.; Stock, H.R.; Küper, A.; Jarms, C. Effects of B(CH3O)3 content on a PACVD plasma-boriding process. Surf. Coat. Technol. 2000, 131, 291–293. [Google Scholar] [CrossRef]
- Küper, A. Plasma-assisted boronizing. Adv. Mater. Process. 2003, 161, 20–22. [Google Scholar]
- Kulka, M.; Makuch, N.; Pertek, A. Microstructure and properties of laser-borided 41Cr4 steel. Opt. Laser Technol. 2013, 45, 308–318. [Google Scholar] [CrossRef]
- Kulka, M.; Mikolajczak, D.; Makuch, N.; Dziarski, P.; Miklaszewski, A. Wear resistance improvement of austenitic 316L steel by laser alloying with boron. Surf. Coat. Technol. 2016, 291, 292–313. [Google Scholar] [CrossRef]
- Sashank, S.; Dinesh Babu, P.; Marimuthu, P. Experimental studies of laser borided low alloy steel and optimization of parameters using response surface methodology. Surf. Coat. Technol. 2019, 363, 255–264. [Google Scholar] [CrossRef]
- Kulka, M.; Makuch, N.; Piasecki, A. Nanomechanical characterization and fracture toughness of FeB and Fe2B iron borides produced by gas boriding of Armco iron. Surf. Coat. Technol. 2017, 325, 515–532. [Google Scholar] [CrossRef]
- Kulka, M.; Makuch, N.; Pertek, A.; Małdziński, L. Simulation of the growth kinetics of boride layers formed on Fe during gas boriding in H2– BCl3 atmosphere. J. Solid State Chem. 2013, 199, 196–203. [Google Scholar] [CrossRef]
- Keddam, M.; Kulka, M.; Makuch, N.; Pertek, A.; Małdziński, L. A kinetic model for estimating the boron activation energies in the FeB and Fe2B layers during the gas-boriding of Armco iron: Effect of boride incubation times. Appl. Surf. Sci. 2014, 298, 155–163. [Google Scholar] [CrossRef]
- Pertek, A. The Structure Formation and the Properties of Boronized Layers Obtained in Gaseous Boriding Process; Dissertation No. 365; Publishing House of Poznan University of Technology: Poznan, Poland, 2001; pp. 1–194. [Google Scholar]
- Pertek, A.; Kulka, M. Two-step treatment carburizing followed by boriding on medium-carbon steel. Surf. Coat. Technol. 2003, 173, 309–314. [Google Scholar] [CrossRef]
- Kulka, M.; Pertek, A. Gradient formation of boride layers by borocarburizing. Appl. Surf. Sci. 2008, 254, 5281–5290. [Google Scholar] [CrossRef]
- Kulka, M.; Pertek, A.; Makuch, N. The importance of carbon concentration–depth profile beneath iron borides for low-cycle fatigue strength. Mater. Sci. Eng. A 2011, 528, 8641–8650. [Google Scholar] [CrossRef]
- Kulka, M.; Makuch, N.; Pertek, A.; Piasecki, A. An alternative method of gas boriding applied to the formation of borocarburized layer. Mater. Charact. 2012, 72, 59–67. [Google Scholar] [CrossRef]
- Kulka, M.; Makuch, N.; Dziarski, P.; Mikołajczak, D.; Przestacki, D. Gradient boride layers formed by diffusion carburizing and laser boriding. Opt. Lasers Eng. 2015, 67, 163–175. [Google Scholar] [CrossRef]
- Wang, B.; Xue, W.; Wu, J.; Jin, X.; Hua, M.; Wu, Z. Characterization of surface hardened layers on Q235 low-carbon steel treated by plasma electrolytic borocarburizing. J. Alloys Compd. 2013, 578, 162–169. [Google Scholar] [CrossRef]
- Wang, B.; Jin, X.; Xue, W.; Wu, Z.; Du, J.; Wu, J. High temperature tribological behaviors of plasma electrolytic borocarburized Q235 low-carbon steel. Surf. Coat. Technol. 2013, 232, 142–149. [Google Scholar] [CrossRef]
- Wang, B.; Xue, W.; Wu, Z.; Jin, X.; Wu, J.; Du, J. Influence of discharge time on properties of plasma electrolytic borocarburized layers on Q235 low-carbon steel. Mater. Chem. Phys. 2015, 168, 10–17. [Google Scholar] [CrossRef]
- Makuch, N.; Kulka, M. Microstructural characterization and some mechanical properties ofgas-borided Inconel 600-alloy. Appl. Surf. Sci. 2014, 314, 1007–1018. [Google Scholar] [CrossRef]
- Makuch, N.; Kulka, M.; Piasecki, A. The effects of chemical composition of Nimonic 80A-alloy on the microstructure and properties of gas-borided layer. Surf. Coat. Technol. 2015, 276, 440–455. [Google Scholar] [CrossRef]
- Balci, S.; Sezgi, N.; Eren, E. Boron oxide production kinetics using boric acid as raw material. Ind. Eng. Chem. Res. 2012, 51, 11091–11096. [Google Scholar] [CrossRef]
- Przybyłowicz, K. Teoria i Praktyka Borowania Stali (Theory and Practice of Steel Boronizing); PL ISSN 0239-4979; Publishing House of Kielce University of Technology: Kielce, Poland, 2000. (In Polish) [Google Scholar]
- Hirsch, T.; Hoffmann, F.; Mayr, P. Röntgenographische untersuchungen mikrostruktureller kenngrößen von verbindungsschichten gasnitrierter stähle. Haerterei Tech. Mit. 1996, 51, 390–398. (In German) [Google Scholar]
- Hirsch, T.; Hoffmann, F.; Mayr, P. Effect of different compound layer and base material microstructures on microstrain and domain size of nitrided steel. Surf. Eng. 1998, 14, 481–488. [Google Scholar] [CrossRef]
- Kraus, I.; Ganev, N.; Gosmanova, G.; Tietz, H.D.; Pfeiffer, L.; Böhm, S. Residual stress measurement in alumina coatings. Mater. Sci. Eng. A 1995, 199, L15–L17. [Google Scholar] [CrossRef]









| Element | C | Mn | Si | Cr | P | S | Ni | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|
| (wt %) | 0.035 | 0.20 | 0.22 | 0.10 | 0.025 | 0.025 | 0.12 | 0.10 | balance |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makuch, N.; Dziarski, P.; Kulka, M. Gas Technique of Simultaneous Borocarburizing of Armco Iron Using Trimethyl Borate. Coatings 2020, 10, 564. https://doi.org/10.3390/coatings10060564
Makuch N, Dziarski P, Kulka M. Gas Technique of Simultaneous Borocarburizing of Armco Iron Using Trimethyl Borate. Coatings. 2020; 10(6):564. https://doi.org/10.3390/coatings10060564
Chicago/Turabian StyleMakuch, Natalia, Piotr Dziarski, and Michał Kulka. 2020. "Gas Technique of Simultaneous Borocarburizing of Armco Iron Using Trimethyl Borate" Coatings 10, no. 6: 564. https://doi.org/10.3390/coatings10060564





