Oxygen Plasma Improved Shear Strength of Bonding between Zirconia and Composite Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zirconia Preparation
2.2. Plasma Treatment
2.3. Resin Binding to Zirconia
2.4. Morphology and Phase Analyses
2.5. Roughness and Contact Angle
2.6. Shear Bond Strength
2.7. Statistical Analysis
3. Results
3.1. Surface Morphology
3.2. Phase Composition
3.3. Chemical Composition
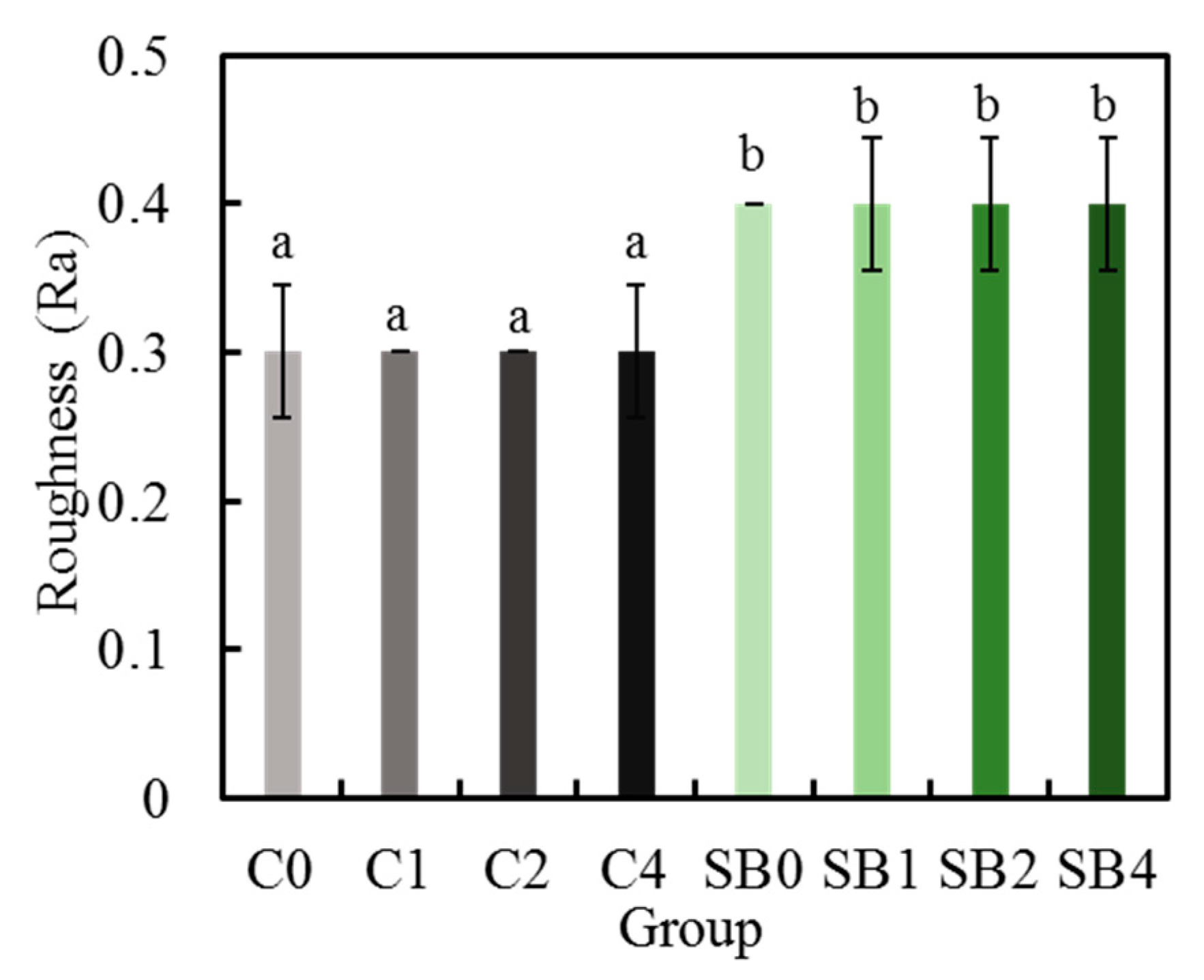
3.4. Surface Roughness
3.5. Contract Angle
3.6. Cross-Sectional Structure
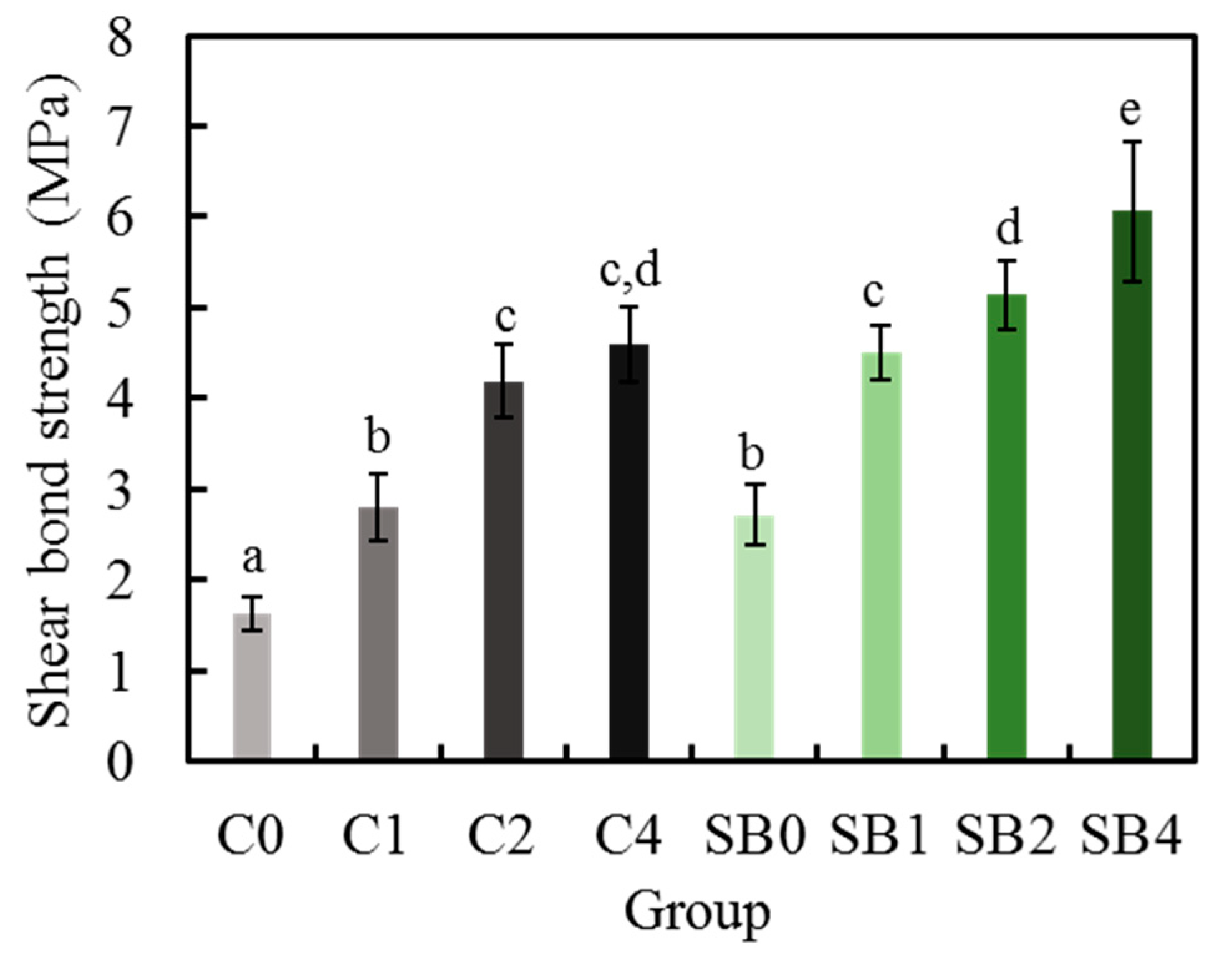
3.7. Shear Bond Strength
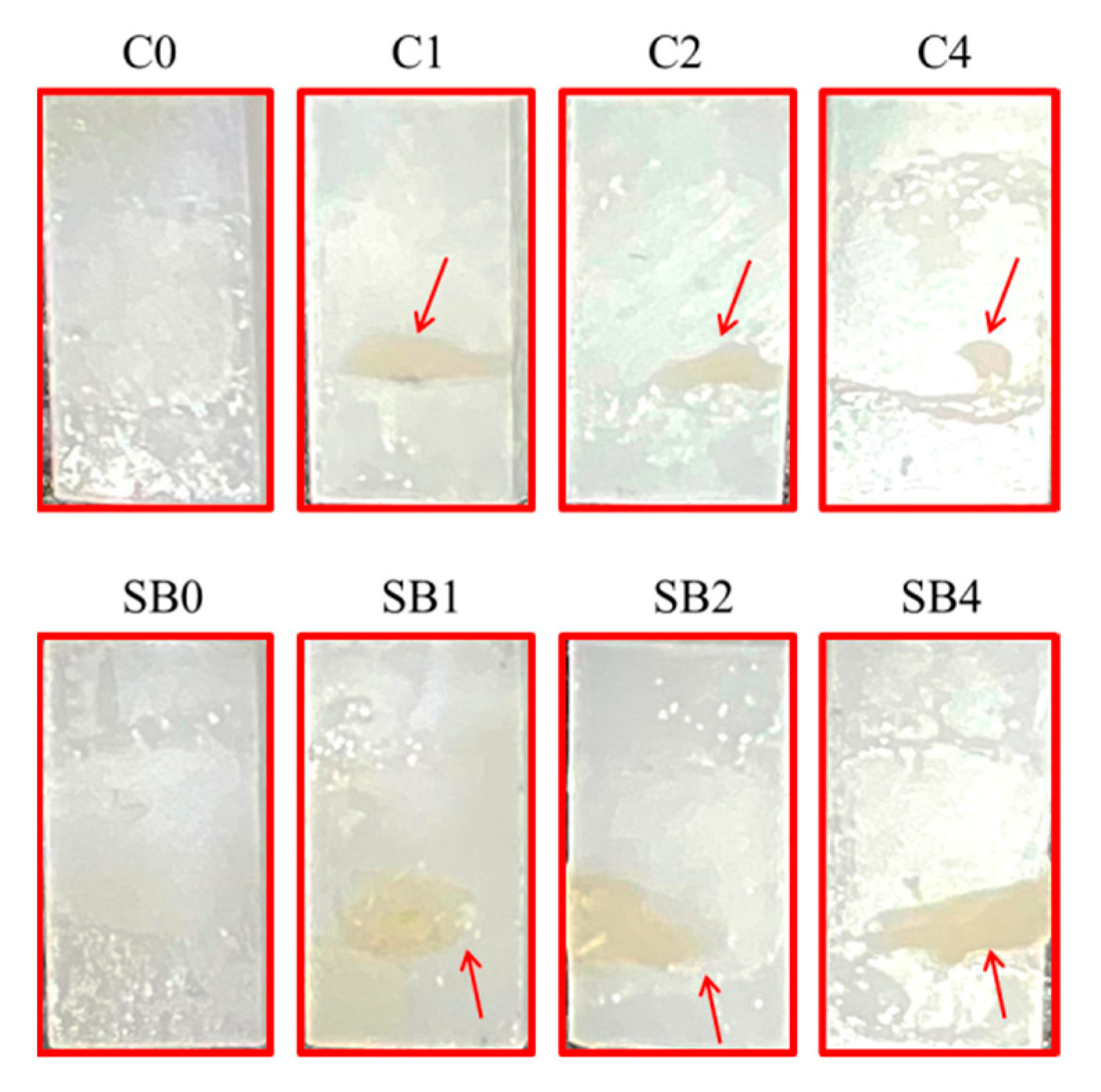
3.8. Fracture Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anusavice, K.; Shen, C.; Ralph Rawls, H. Phillips’ Science of Dental Materials, 12th ed.; Saunders: Philadelphia, PA, USA, 2013; pp. 30–68. [Google Scholar]
- Takeda, T.; Shigami, K.; Shimada, A.; Ohki, K. A study of discoloration of the gingiva by artificial crowns. Int. J. Prosthodont. 1996, 9, 197–202. [Google Scholar] [PubMed]
- Yan, M.; Kao, C.-T.; Ye, J.-S.; Huang, T.-H.; Ding, S.-J. Effect of preoxidation of titanium on the titanium–ceramic bonding. Surf. Coat. Technol. 2007, 202, 288–293. [Google Scholar] [CrossRef]
- Buga, C.; Hunyadi, M.; Gácsi, Z.; Hegedűs, C.; Hakl, J.; Schmidt, U.; Ding, S.J.; Csík, A. Calcium silicate layer on titanium fabricated by electrospray deposition. Mater. Sci. Eng. C 2019, 98, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Höland, W.; Rheinberger, V.; Apel, E.; Ritzberger, C.; Rothbrust, F.; Kappert, H.; Krumeich, F.; Nesper, R. Future perspectives of biomaterials for dental restoration. J. Eur. Ceram. Soc. 2009, 29, 1291–1297. [Google Scholar] [CrossRef]
- Yan, M.; Wei, C.-K.; Lin, Y.-Y.; Hu, S.-W.; Ding, S.-J. Impact behavior of three notched all-ceramic restorations after soaking in artificial saliva. Materials 2015, 8, 4479–4490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komine, F.; Strub, J.R.; Matsumura, H. Bonding between layering materials and zirconia frameworks. Jpn. Dent. Sci. Rev. 2012, 48, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Gale, M.; Darvell, B. Thermal cycling procedures for laboratory testing of dental restorations. J. Dent. 1999, 27, 89–99. [Google Scholar] [CrossRef]
- Vaidyanathan, J.; Vaidyanathan, T.K. Flexural creep deformation and recovery in dental composites. J. Dent. 2001, 29, 545–551. [Google Scholar] [CrossRef]
- Kobayashi, K.; Komine, F.; Blatz, M.B.; Saito, A.; Koizumi, H.; Matsumura, H.J.Q.I. Influence of priming agents on the short-term bond strength of an indirect composite veneering material to zirconium dioxide ceramic. Quintessence Int. 2009, 40, 545–551. [Google Scholar]
- Komine, F.; Fushiki, R.; Koizuka, M.; Taguchi, K.; Kamio, S.; Matsumura, H. Effect of surface treatment on bond strength between an indirect composite material and a zirconia framework. J. Oral Sci. 2012, 54, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Han, I.H.; Kang, D.W.; Chung, C.H.; Choe, H.C.; Son, M.K. Effect of various intraoral repair systems on the shear bond strength of composite resin to zirconia. J. Adv. Prosthodont. 2013, 5, 248–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, T.; Komine, F.; Fushiki, R.; Kubochi, K.; Shinohara, M.; Matsumura, H. Shear bond strengths of an indirect composite layering material to a tribochemically silica-coated zirconia framework material. Dent. Mater. J. 2016, 35, 461–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sari, F.; Secilmis, A.; Simsek, I.; Ozsevik, S. Shear bond strength of indirect composite material to monolithic zirconia. J. Adv. Prosthodont. 2016, 8, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Jansen van Vuuren, W.A.; Jansen van Vuuren, L.; Torr, B.; Waddell, J.N. Adhesion between zirconia and indirect composite resin. Int. J. Adhes. Adhes. 2016, 69, 72–78. [Google Scholar] [CrossRef]
- Lin, T.H.; Chang, H.J.; Chung, K.H. Interfacial strengths of various alloy surface treatment for resin-bonded fixed partial dentures. J. Prosthet. Dent. 1990, 64, 158–162. [Google Scholar] [CrossRef]
- Mukai, M.; Fukui, H.; Hasegawa, J. Relationship between sandblasting and composite resin-alloy bond strength by a silica coating. J. Prosthet. Dent. 1995, 74, 151–155. [Google Scholar] [CrossRef]
- Wolfart, M.; Lehmann, F.; Wolfart, S.; Kern, M. Durability of the resin bond strength to zirconia ceramic after using different surface conditioning methods. Dent. Mater. 2007, 23, 45–50. [Google Scholar] [CrossRef]
- Shimoe, S.; Tanoue, N.; Kusano, K.; Okazaki, M.; Satoda, T. Influence of air-abrasion and subsequent heat treatment on bonding between zirconia framework material and indirect composites. Dent. Mater. J. 2012, 31, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lawn, B.R.; Rekow, E.D.; Thompson, V.P. Effect of sandblasting on the long-term performance of dental ceramics. J. Biomed. Mater. Res. B 2004, 71, 381–386. [Google Scholar] [CrossRef]
- Guazzato, M.; Quach, L.; Albakry, M.; Swain, M.V. Influence of surface and heat treatments on the flexural strength of Y-TZP dental ceramic. J. Dent. 2005, 33, 9–18. [Google Scholar] [CrossRef]
- Denry, I.L.; Holloway, J.A. Microstructural and crystallographic surface changes after grinding zirconia-based dental ceramics. J. Biomed. Mater. Res. B 2006, 76, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Guess, P.C.; Zhang, Y.; Kim, J.W.; Rekow, E.D.; Thompson, V.P. Damage and reliability of Y-TZP after cementation surface treatment. J. Dent. Res. 2010, 89, 592–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, M.; Taketa, H.; Torii, Y.; Irie, M.; Matsumoto, T. Optimal sandblasting conditions for conventional-type yttria-stabilized tetragonal zirconia polycrystals. Dent. Mater. 2019, 35, 169–175. [Google Scholar] [CrossRef]
- Lung, C.Y.K.; Matinlinna, J.P. Aspects of silane coupling agents and surface conditioning in dentistry: An overview. Dent. Mater. 2012, 28, 467–477. [Google Scholar] [CrossRef]
- Lung, C.Y.K.; Liu, D.; Matinlinna, J.P. Silica coating of zirconia by silicon nitride hydrolysis on adhesion promotion of resin to zirconia. Mater. Sci. Eng. C 2015, 46, 103–110. [Google Scholar] [CrossRef]
- Scaminaci Russo, D.; Cinelli, F.; Sarti, C.; Giachetti, L.; Scaminaci Russo, D.; Cinelli, F.; Sarti, C.; Giachetti, L. Adhesion to zirconia: A systematic review of current conditioning methods and bonding materials. Dent. J. 2019, 7, 74. [Google Scholar] [CrossRef] [Green Version]
- El-Shrkawy, Z.R.; El-Hosary, M.M.; Saleh, O.; Mandour, M.H. Effect of different surface treatments on bond strength, surface and microscopic structure of zirconia ceramic. Future Dent. J. 2016, 2, 41–53. [Google Scholar] [CrossRef]
- Wolter, S.D.; Piascik, J.R.; Stoner, B.R. Characterization of plasma fluorinated zirconia for dental applications by X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2011, 257, 10177–10182. [Google Scholar] [CrossRef]
- Silva, N.R.; Coelho, P.G.; Valverde, G.B.; Becker, K.; Ihrke, R.; Quade, A.; Thompson, V.P. Surface characterization of Ti and Y-TZP following non-thermal plasma exposure. J. Biomed. Mater. Res. B 2011, 99, 199–206. [Google Scholar] [CrossRef]
- Derand, T.; Molin, M.; Kvam, K. Bond strength of composite luting cement to zirconia ceramic surfaces. Dent. Mater. 2005, 21, 1158–1162. [Google Scholar] [CrossRef]
- Wu, C.C.; Wei, C.K.; Ho, C.C.; Ding, S.J. Enhanced hydrophilicity and biocompatibility of dental zirconia ceramics by oxygen plasma treatment. Materials 2015, 8, 684–699. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Okawa, T.; Fukumoto, T.; Tsurumi, A.; Tatsuta, M.; Fujii, T.; Tanaka, J.; Tanaka, M. Influence of atmospheric pressure low-temperature plasma treatment on the shear bond strength between zirconia and resin cement. J. Prosthodont. Res. 2016, 60, 289–293. [Google Scholar] [CrossRef]
- Liu, T.; Hong, L.; Hottel, T.; Dong, X.; Yu, Q.; Chen, M. Non-thermal plasma enhanced bonding of resin cement to zirconia ceramic. Clin. Plasma Med. 2016, 4, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Park, S.W.; Yun, K.D.; Ji, M.K.; Kim, S.; Yang, Y.P.; Lim, H.P. Effect of plasma treatment and its post process duration on shear bonding strength and antibacterial effect of dental zirconia. Materials 2018, 11, 2233. [Google Scholar] [CrossRef] [Green Version]
- Elias, A.B.; Simao, R.A.; Prado, M.; Cesar, P.F.; Botelho Dos Santos, G.; Moreira da Silva, E. Effect of different times of nonthermal argon plasma treatment on the microtensile bond strength of self-adhesive resin cement to yttria-stabilized tetragonal zirconia polycrystal ceramic. J. Prosthet. Dent. 2019, 121, 485–491. [Google Scholar] [CrossRef]
- Kim, D.S.; Ahn, J.J.; Bae, E.B.; Kim, G.C.; Jeong, C.M.; Huh, J.B.; Lee, S.H. Influence of non-thermal atmospheric pressure plasma treatment on shear bond strength between Y-TZP and self-adhesive resin cement. Materials 2019, 12, 3321. [Google Scholar] [CrossRef] [Green Version]
- ISO/TS 11405. Dental Materials—Testing of Adhesion to Tooth Structure; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Cavalcanti, A.N.; Pilecki, P.; Foxton, R.M.; Watson, T.F.; Oliveira, M.T.; Gianinni, M.; Marchi, G.M.; Surgery, L. Evaluation of the surface roughness and morphologic features of Y-TZP ceramics after different surface treatments. Photomed. Laser Surg. 2009, 27, 473–479. [Google Scholar] [CrossRef]
- Lee, P.R.; Ho, C.C.; Hwang, C.S.; Ding, S.J. Improved physicochemical properties and biocompatibility of stainless steel implants by PVA/ZrO2-based composite coatings. Surf. Coat. Technol. 2014, 258, 374–380. [Google Scholar] [CrossRef]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. A 2005, 74, 49–58. [Google Scholar] [CrossRef]
- Valverde, G.B.; Coelho, P.G.; Janal, M.N.; Lorenzoni, F.C.; Carvalho, R.M.; Thompson, V.P.; Weltemann, K.-D.; Silva, N.R.F.A. Surface characterisation and bonding of Y-TZP following non-thermal plasma treatment. J. Dent. 2013, 41, 51–59. [Google Scholar] [CrossRef]






| Material | Composition (%) | Lot. No. | Manufacturer |
|---|---|---|---|
| Vita YZ-55 In-Ceram | ZrO2: 91–94%; Y2O3: 4–6%; HfO2: 2–4%; Al2O3: < 0.1 | 29730 | VITA, Germany |
| Ceraresin Bond I and II | Bond I: Ethanol, silane coupling agent, etc. Bond II: Acetone, 4-AET, UDMA, polymerization initiator, etc. | 121926 101925 | Shofu, Japan |
| Ceramage (body A3B) | UDMA, Urethane diacrylate, Zirconium silicate, colouring materials, etc. | 051918 | Shofu, Japan |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, M.; Yang, C.-C.; Chen, Y.-H.; Ding, S.-J. Oxygen Plasma Improved Shear Strength of Bonding between Zirconia and Composite Resin. Coatings 2020, 10, 635. https://doi.org/10.3390/coatings10070635
Yan M, Yang C-C, Chen Y-H, Ding S-J. Oxygen Plasma Improved Shear Strength of Bonding between Zirconia and Composite Resin. Coatings. 2020; 10(7):635. https://doi.org/10.3390/coatings10070635
Chicago/Turabian StyleYan, Min, Chun-Chuan Yang, Yi-Hsuan Chen, and Shinn-Jyh Ding. 2020. "Oxygen Plasma Improved Shear Strength of Bonding between Zirconia and Composite Resin" Coatings 10, no. 7: 635. https://doi.org/10.3390/coatings10070635






