Study on the Wear and Seawater Corrosion Resistance of Ni–Co–P Alloy Coatings with Jet Electrodeposition in Different Jet Voltages and Temperatures of Plating Solution
Abstract
:1. Introduction
2. Experimental Device and Procedure
2.1. Experimental Device
2.2. Materials and Pretreatments
2.3. Preparation of Ni–Co–P Alloy Coatings
2.4. Characterization and Tests
3. Results and Discussion
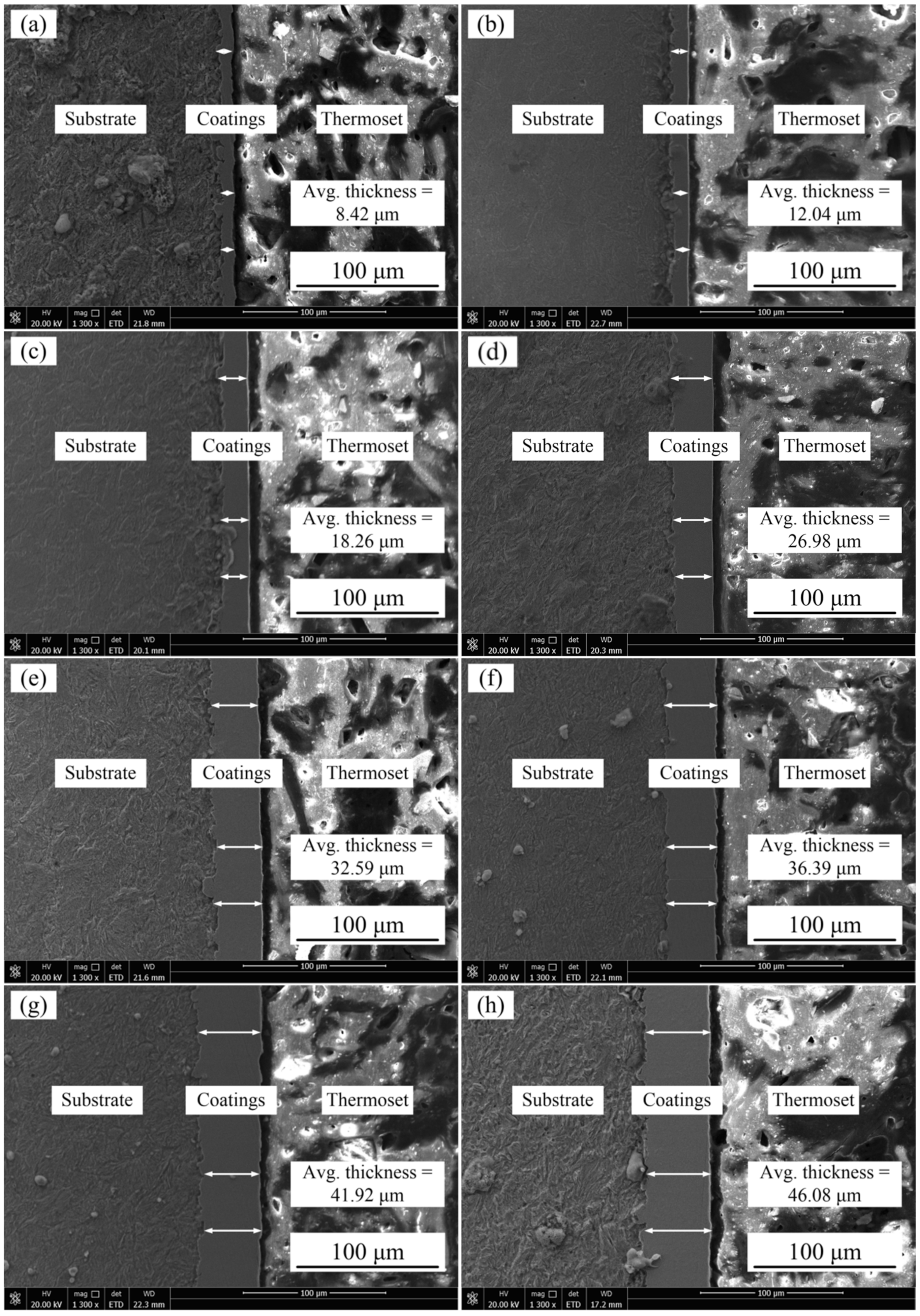
3.1. Effects of Jet Voltages and Temperature of Plating Solution on the Cross-Section Morphologies of Ni–Co–P Alloy Coatings
3.2. Effect of Jet Voltages and Temperature of Plating Solution on the EDS Patterns of Ni–Co–P Alloy Coatings
3.3. Effect of Jet Voltages and Temperature of Plating Solution on the XRD Patterns of Ni–Co–P Alloy Coatings
3.4. Effect of Jet Voltages and Temperature of Plating Solution on the Microhardness of Ni–Co–P Alloy Coatings
3.5. Effect of Jet Voltages and the Temperature of the Plating Solution on the Wear Resistance of Ni–Co–P Alloy Coatings
3.6. Effect of Jet Voltage and Plating Solution Temperature on the Seawater Corrosion Resistance of Ni–Co–P Alloy Coatings
3.6.1. Effect of Jet Voltages on the Seawater Corrosion Resistance of Ni–Co–P Alloy Coatings
3.6.2. Effect of Plating Solution Temperature on the Seawater Corrosion Resistance of Ni–Co–P Alloy Coatings
4. Conclusions
- (1)
- The variation of jet voltage and temperature of plating solution affected the cross-section morphologies, thickness, chemical composition, and crystalline structure of Ni–Co–P alloy coatings. The content of Co in Ni–Co–P alloy coatings reached a maximum value of 47.46 wt·% when the jet voltage was 12 V and the temperature of plating solution was 60 °C. The XRD patterns of Ni–Co–P alloy coatings showed that there was an obvious preferred orientation in the (111) plane.
- (2)
- When the jet voltage and temperature of the plating solution increased, the microhardness of the Ni–Co–P alloy coatings first increased and then decreased, with the maximum value obtained being 634.9 HV0.1. When the jet voltage was 12 V and the temperature of the plating solution was 60 °C, the wear scar width of Ni–Co–P alloy coatings reached a minimum value of 463.4 µm.
- (3)
- Jet voltage and temperature of the plating solution significantly influenced seawater corrosion resistance. When the jet voltage was 12 V and the temperature of the plating solution was 60 °C, the polarization curve in the electrochemical test showed that the samples exhibited a lower corrosion current density (Icorr) of 1.72 µA/cm2 and a higher polarization resistance (Rp) of 19.61 kΩ·cm−2, which indicated that the coatings had better seawater corrosion resistance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, F.; Li, J.; Zeng, Z.; Gao, Y. Tribocorrosion behavior in artificial seawater and anti-microbiologically influenced corrosion properties of TiSiN-Cu coating on F690 steel. J. Mater. Sci. Technol. 2019, 35, 448–459. [Google Scholar] [CrossRef]
- Totolin, V.; Pejakovic, V.; Csanyi, T.; Hekele, O.; Huber, M.; Ripoll, M.R. Surface engineering of Ti6Al4V surfaces for enhanced tribocorrosion performance in artificial seawater. Mater. Des. 2016, 104, 10–18. [Google Scholar] [CrossRef]
- Momen, G.; Farzaneh, M. Facile approach in the development of icephobic hierarchically textured coatings as corrosion barrier. Appl. Surf. Sci. 2014, 299, 41–46. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Lu, Z. Advantage of super-hydrophobic surface as a barrier against atmospheric corrosion induced by salt deliquescence. Corros. Sci. 2015, 90, 23–32. [Google Scholar] [CrossRef]
- Dong, T.; Liu, L.; Li, G.; Wang, R.; Yuan, J.; Feng, Y. Effect of induction remelting on microstructure and wear resistance of plasma sprayed NiCrBSiNb coatings. Surf. Coat. Technol. 2019, 364, 347–357. [Google Scholar] [CrossRef]
- Do, Q.; An, H.; Wang, G.; Meng, G.; Wang, Y.; Liu, B.; Wang, J.; Wang, F. Effect of cupric sulfate on the microstructure and corrosion behavior of nickel-copper nanostructure coatings synthesized by pulsed electrodeposition technique. Corros. Sci. 2019, 147, 246–259. [Google Scholar] [CrossRef]
- Pang, J.; Li, Q.; Wang, W.; Xu, X.; Zhai, J. Preparation and characterization of electroless Ni-Co-P ternary alloy on fly ash cenospheres. Surf. Coat. Technol. 2011, 205, 4237–4242. [Google Scholar] [CrossRef]
- Fetohi, A.E.; Hameed, R.M.A.; El-Khatib, K.M.; Souaya, E.R. Ni-P and Ni-Co-P coated aluminum alloy 5251 substrates as metallic bipolar plates for PEM fuel cell applications. Int. J. Hydrog. Energy 2012, 37, 7677–7688. [Google Scholar] [CrossRef]
- Khan, M.R.; Nicholson, E.L. Magnetic properties and microstructure of electroless plated Co-Ni-P and Co-P thin films for longitudinal recording. J. Magn. Magn. Mater. 1986, 54, 1654–1656. [Google Scholar] [CrossRef]
- Lew, K.S.; Raja, M.; Thanikaikarasan, S.; Kim, T.; Kim, Y.D.; Mahalingam, T. Effect of pH and current density in electrodeposited Co-Ni-P alloy thin films. Mater. Chem. Phys. 2008, 112, 249–253. [Google Scholar] [CrossRef]
- Ji, L.; Chen, F.; Huang, H.; Sun, X.; Yan, Y.; Tang, X. Preparation of nickel–graphene composites by jet electrodeposition and the influence of graphene oxide concentration on the morphologies and properties. Surf. Coat. Technol. 2018, 351, 212–219. [Google Scholar] [CrossRef]
- Xia, F.F.; Jia, W.C.; Ma, C.Y.; Yang, R.; Wang, Y.; Potts, M. Synthesis and characterization of Ni-doped TiN thin films deposited by jet electrodeposition. Appl. Surf. Sci. 2018, 434, 228–233. [Google Scholar] [CrossRef]
- Jiang, W.; Shen, L.; Qiu, M.; Wang, X.; Fan, M.; Tian, Z. Preparation of Ni-SiC composite coatings by magnetic field-enhanced jet electrodeposition. J. Alloys Compd. 2018, 762, 115–124. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, L.; Jiang, W.; Wang, X.; Fan, M.; Tian, Z.; Han, X. Laser processing as an alternative electrodeposition pretreatment. Surf. Coat. Technol. 2019, 357, 957–964. [Google Scholar] [CrossRef]
- Rajput, M.S.; Pandey, P.M.; Jha, S. Modelling of high speed selective jet electrodeposition process. J. Manuf. Process. 2015, 17, 98–107. [Google Scholar] [CrossRef]
- Ning, D.; Zhang, A.; Murtaza, M.; Wu, H. Effect of surfactants on the electrodeposition of Cu-TiO2 composite coatings prepared by jet electrodeposition. J. Alloys Compd. 2019, 777, 1245–1250. [Google Scholar] [CrossRef]
- Qiao, G.; Jing, T.; Wang, N.; Gao, Y.; Zhao, X.; Zhou, J.; Wang, W. High-speed jet electrodeposition and microstructure of nanocrystalline Ni-Co alloys. Electrochim. Acta 2005, 51, 85–92. [Google Scholar] [CrossRef]
- Liu, X.; Shen, L.D.; Qiu, M.B.; Tian, Z.J.; Wang, Y.H.; Zhao, K.L. Jet electrodeposition of nanocrystalline nickel assisted by controllable friction. Surf. Coat. Technol. 2016, 305, 231–240. [Google Scholar] [CrossRef]
- Xia, F.; Jia, W.; Jiang, M.; Cui, W.; Wang, J. Microstructure and corrosion properties of Ni-TiN nanocoatings prepared by jet pulse electrodeposition. Ceram. Int. 2017, 43, 14623–14628. [Google Scholar] [CrossRef]
- Tóth-Kádár, E.; Bakonyi, I.; Pogány, L.; Cziráki, Á. Microstructure and electrical transport properties of pulse-plated nanocrystalline nickel electrodeposits. Surf. Coat. Technol. 1997, 88, 57–65. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, D.; Wang, G.; Shen, L.; Liu, Z.; Huang, Y. Microstructure and properties of nanocrystalline nickel coatings prepared by pulse jet electrodeposition. Trans. Nonferr. Met. Soc. China 2010, 20, 1037–1042. [Google Scholar] [CrossRef]
- Qiao, G.; Jing, T.; Mang, N.; Gao, Y.; Zhao, X.; Zhou, J.; Wang, W. Effect of current density on microstructure and properties of bulk nanocrystalline Ni-Co alloys prepared by JED. J. Electrochem. Soc. 2006, 153, C305–C308. [Google Scholar] [CrossRef]
- Parente, M.V.; Mattos, O.R.; Diaz, S.L.; De Lima-Neto, P.; Miranda, F.F. Electrochemical characterization of Ni-P and Ni-Co-P amorphous alloy deposits obtained by electrodeposition. J. Appl. Electrochem. 2001, 31, 677–683. [Google Scholar] [CrossRef]
- Yao, S.; Liu, B.; Guo, H.; Takao, S. Study of Ni-Co-P amorphous alloy electrodeposition and its properties. J. Chem. Ind. Eng. 1996, 1, 48–52. [Google Scholar]
- Wang, C.; Shen, L.; Qiu, M.; Tian, Z.; Jiang, W. Characterizations of Ni-CeO2 nanocomposite coating by interlaced jet electrodeposition. J. Alloys Compd. 2017, 727, 269–277. [Google Scholar] [CrossRef]
- Li, H.; Kang, M.; Zhang, Y.; Liu, Y.; Jin, M.; Mbugua, N.S.; Zhu, G.; Liu, C. Fabrication of Ni-Co-BN (h) nanocomposite coatings with jet electrodeposition in different pulse parameters. Coatings 2019, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Du, X.; Gao, S.; Zhang, X. Effect of artificial seawater temperature on electrochemical behavior of Ni-P and Ni-Cu-P alloy coatings. Trans. Nonferr. Met. Soc. China 2018, 6, 1176–1181. (In Chinese) [Google Scholar]
- Zhang, X.; Kang, M.; Shao, Y.; Zhang, Y.; Cai, G. Process parameters optimization of electric plating Ni-P alloy coating. Electromach. Mould. 2015, 4, 42–47. (In Chinese) [Google Scholar]
- Li, Z.; Deng, Y.; Shen, B.; Liu, L.; Hu, W. Synthesis, characterization and microwave properties of Ni-Co-P hollow spheres. J. Alloys Compd. 2010, 491, 406–410. [Google Scholar] [CrossRef]
- Apachitei, I.; Duszczyk, J. Autocatalytic nickel coatings on aluminium with improved abrasive wear resistance. Surf. Coat. Technol. 2000, 132, 89–98. [Google Scholar] [CrossRef]
- Apachitei, I.; Tichelaar, F.D.; Duszczyk, J.; Katgerman, L. The effect of heat treatment on the structure and abrasive wear resistance of autocatalytic Ni-P and Ni-P-SiC coatings. Surf. Coat. Technol. 2002, 149, 263–278. [Google Scholar] [CrossRef]
- Zimmerman, A.F.; Palumbo, G.; Aust, K.T.; Erb, U. Mechanical properties of nickel silicon carbide nanocomposites. Mater. Sci. Eng. A 2002, 328, 137–146. [Google Scholar] [CrossRef]
- Gilewicz, A.; Chmielewska, P.; Murzynski, D.; Dobruchowska, E.; Warcholinski, B. Corrosion resistance of CrN and CrCN/CrN coatings deposited using cathodic arc evaporation in Ringer’s and Hank’s solutions. Surf. Coat. Technol. 2016, 299, 7–14. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical polarization: I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Kang, M.; Zhang, Y.; Li, H. Study on the performances of Ni-Co-P/BN(h) nanocomposite coatings made by jet electrodeposition. Procedia CIRP 2018, 68, 221–226. [Google Scholar] [CrossRef]
- Liu, C.; Su, F.; Liang, J. Fabrication of Co-Ni-P film with excellent wear and corrosion resistance by electroplating with supercritical CO2 emulsion. Trans. Nonferr. Met. Soc. China 2018, 28, 2489–2498. [Google Scholar] [CrossRef]
- Meng, G.Z.; Li, Y.; Shao, Y.W.; Zhang, T.; Wang, Y.Q.; Wang, F.H.; Cheng, X.Q.; Dong, C.F.; Li, X.G. Effect of microstructures on corrosion behavior of nickel coatings: (II) competitive effect of grain size and twins density on corrosion behavior. J. Mater. Sci. Technol. 2016, 32, 465–469. [Google Scholar] [CrossRef]
- Dai, N.W.; Zhang, L.C.; Zhang, J.X.; Chen, Q.M.; Wu, M.L. Corrosion behavior of selective laser melted Ti-6Al-4V alloy in NaCl solution. Corros. Sci. 2016, 102, 484–489. [Google Scholar] [CrossRef]
- Mbugua, N.S.; Kang, M.; Li, H.; Liu, Y.; Joseph, N.; Zhang, Y. The influence of Co concentration on the properties of conventionally electrodeposited Ni-Co-Al2O3-SiC nanocomposite coatings. Prot. Met. Phys. Chem. Surf. 2020, 56, 94–102. [Google Scholar] [CrossRef]
- Bakhit, B.; Akbari, A.; Nasirpouri, F.; Hosseini, M.G. Corrosion resistance of Ni-Co alloy and Ni-Co/SiC nanocomposite coatings electrodeposited by sediment codeposition technique. Appl. Surf. Sci. 2014, 307, 351–359. [Google Scholar] [CrossRef]














| Solution | Composition | Concentration (g·L−1) |
|---|---|---|
| Electric Cleaning Solution | NaOH | 25.0 |
| Na2CO3 | 21.7 | |
| Na3PO4 | 50.0 | |
| NaCl | 2.4 | |
| Strong Activation Solution | Hydrochloric acid | 25.0 |
| NaCl | 140.1 | |
| Weak Activation Solution | H3C6H5O7·H2O | 94.3 |
| NiCl2·6H2O | 3.0 | |
| Na3C6H5O7·2H2O | 141.2 |
| Solution | Composition | Concentration (g·L−1) |
|---|---|---|
| Plating Solution | NiSO4·6 H2O | 200.0 |
| NiCl2·6 H2O | 30.0 | |
| CoSO4·7 H2O | 20.0 | |
| H3BO3 | 30.0 | |
| H3PO3 | 20.0 | |
| C6H8O7 | 60.0 | |
| Sodium dodecyl sulfate | 0.08 | |
| Sulfourea | 0.02 |
| Constituent | NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl | NaH CO3 | KBr | H3BO3 | SrCl2 | NaF |
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (g·L−1) | 24.530 | 5.200 | 4.090 | 1.160 | 0.695 | 0.201 | 0.101 | 0.027 | 0.025 | 0.003 |
| Jet Voltages (V) | Ni (wt·%) | Co (wt·%) | P (wt·%) |
|---|---|---|---|
| 6 | 68.26 | 30.76 | 0.98 |
| 8 | 67.20 | 31.52 | 1.28 |
| 10 | 58.69 | 40.22 | 1.09 |
| 12 | 49.29 | 47.46 | 3.25 |
| 14 | 51.97 | 45.12 | 2.91 |
| 16 | 55.43 | 42.48 | 2.09 |
| 18 | 56.87 | 40.99 | 2.14 |
| 20 | 57.54 | 40.54 | 1.92 |
| Plating Solution Temperature (°C) | Ni (wt·%) | Co (wt·%) | P (wt·%) |
|---|---|---|---|
| 40 | 55.29 | 42.98 | 1.73 |
| 45 | 54.46 | 43.55 | 1.99 |
| 50 | 53.67 | 43.98 | 2.35 |
| 55 | 50.19 | 47.35 | 2.46 |
| 60 | 49.29 | 47.46 | 3.25 |
| 65 | 53.05 | 43.88 | 3.07 |
| 70 | 58.05 | 39.24 | 2.71 |
| 75 | 63.59 | 33.55 | 2.86 |
| Jet Voltage | Ecorr (mV) | Icorr (µA·cm−2) | Corrosion Rate (µm·year−1) | βa (mV/dec) | βc (mV/dec) | Rp (kΩ·cm−2) |
|---|---|---|---|---|---|---|
| 6 | −610 | 41.60 | 503.9 | 426 | 366 | 2.05 |
| 8 | −509 | 10.02 | 121.2 | 223 | 537 | 6.83 |
| 10 | −433 | 2.45 | 29.6 | 78 | 789 | 12.59 |
| 12 | −220 | 1.72 | 20.8 | 94 | 444 | 19.61 |
| 14 | −325 | 2.35 | 24.8 | 241 | 175 | 18.76 |
| 16 | −240 | 3.14 | 36.8 | 81 | 470 | 9.56 |
| 18 | −304 | 9.32 | 112.8 | 168 | 716 | 6.34 |
| 20 | −514 | 11.41 | 138.2 | 238 | 292 | 4.99 |
| Jet Voltage | Rs (Ω·cm−2) | CPE-T (10−5 μF·cm−2) | CPE-P | Rct (104 Ω·cm−2) |
|---|---|---|---|---|
| 6 | 6.91 | 15.65 | 0.48 | 0.48 |
| 8 | 6.40 | 2.89 | 0.93 | 1.34 |
| 10 | 6.79 | 2.41 | 0.91 | 3.73 |
| 12 | 7.01 | 3.39 | 0.92 | 5.63 |
| 14 | 5.49 | 2.84 | 0.93 | 4.14 |
| 16 | 6.64 | 3.89 | 0.92 | 3.31 |
| 18 | 7.16 | 1.80 | 0.87 | 2.96 |
| 20 | 7.25 | 3.49 | 0.92 | 2.55 |
| Temperature of Plating Solution (°C) | Ecorr (mV) | Icorr (µA·cm−2) | Corrosion Rate (µm·year−1) | βa (mV/dec) | βc (mV/dec) | Rp (kΩ·cm−2) |
|---|---|---|---|---|---|---|
| 40 | −438 | 7.99 | 116.6 | 104 | 981 | 5.11 |
| 45 | −525 | 5.94 | 71.8 | 165 | 251 | 7.28 |
| 50 | −499 | 5.37 | 66.3 | 315 | 504 | 15.69 |
| 55 | −315 | 2.12 | 24.4 | 146 | 220 | 17.99 |
| 60 | −220 | 1.72 | 20.9 | 94 | 444 | 19.61 |
| 65 | −331 | 1.83 | 22.8 | 104 | 194 | 16.08 |
| 70 | −395 | 2.08 | 26.7 | 118 | 210 | 15.79 |
| 75 | −368 | 3.47 | 42.0 | 193 | 352 | 15.61 |
| Temperature of Plating Solution (°C) | Rs (Ω·cm−2) | CPE-T (10−5 μF·cm−2) | CPE-P | Rct (104 Ω·cm−2) |
|---|---|---|---|---|
| 40 | 6.95 | 3.17 | 0.90 | 0.88 |
| 45 | 6.42 | 5.39 | 0.84 | 1.75 |
| 50 | 6.33 | 3.45 | 0.93 | 2.42 |
| 55 | 6.32 | 2.92 | 0.94 | 3.71 |
| 60 | 7.01 | 3.39 | 0.92 | 5.63 |
| 65 | 6.06 | 3.41 | 0.86 | 5.07 |
| 70 | 6.25 | 3.46 | 0.93 | 4.01 |
| 75 | 6.25 | 2.66 | 0.90 | 3.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Kang, M.; Yao, L.; Mbugua, N.S.; Jin, M.; Zhu, J. Study on the Wear and Seawater Corrosion Resistance of Ni–Co–P Alloy Coatings with Jet Electrodeposition in Different Jet Voltages and Temperatures of Plating Solution. Coatings 2020, 10, 639. https://doi.org/10.3390/coatings10070639
Zhang Y, Kang M, Yao L, Mbugua NS, Jin M, Zhu J. Study on the Wear and Seawater Corrosion Resistance of Ni–Co–P Alloy Coatings with Jet Electrodeposition in Different Jet Voltages and Temperatures of Plating Solution. Coatings. 2020; 10(7):639. https://doi.org/10.3390/coatings10070639
Chicago/Turabian StyleZhang, Yin, Min Kang, Liang Yao, Nyambura Samuel Mbugua, Meifu Jin, and Jiping Zhu. 2020. "Study on the Wear and Seawater Corrosion Resistance of Ni–Co–P Alloy Coatings with Jet Electrodeposition in Different Jet Voltages and Temperatures of Plating Solution" Coatings 10, no. 7: 639. https://doi.org/10.3390/coatings10070639





