Study on the Influencing Factors in the Process of Surface Strippable Decontaminant
Abstract
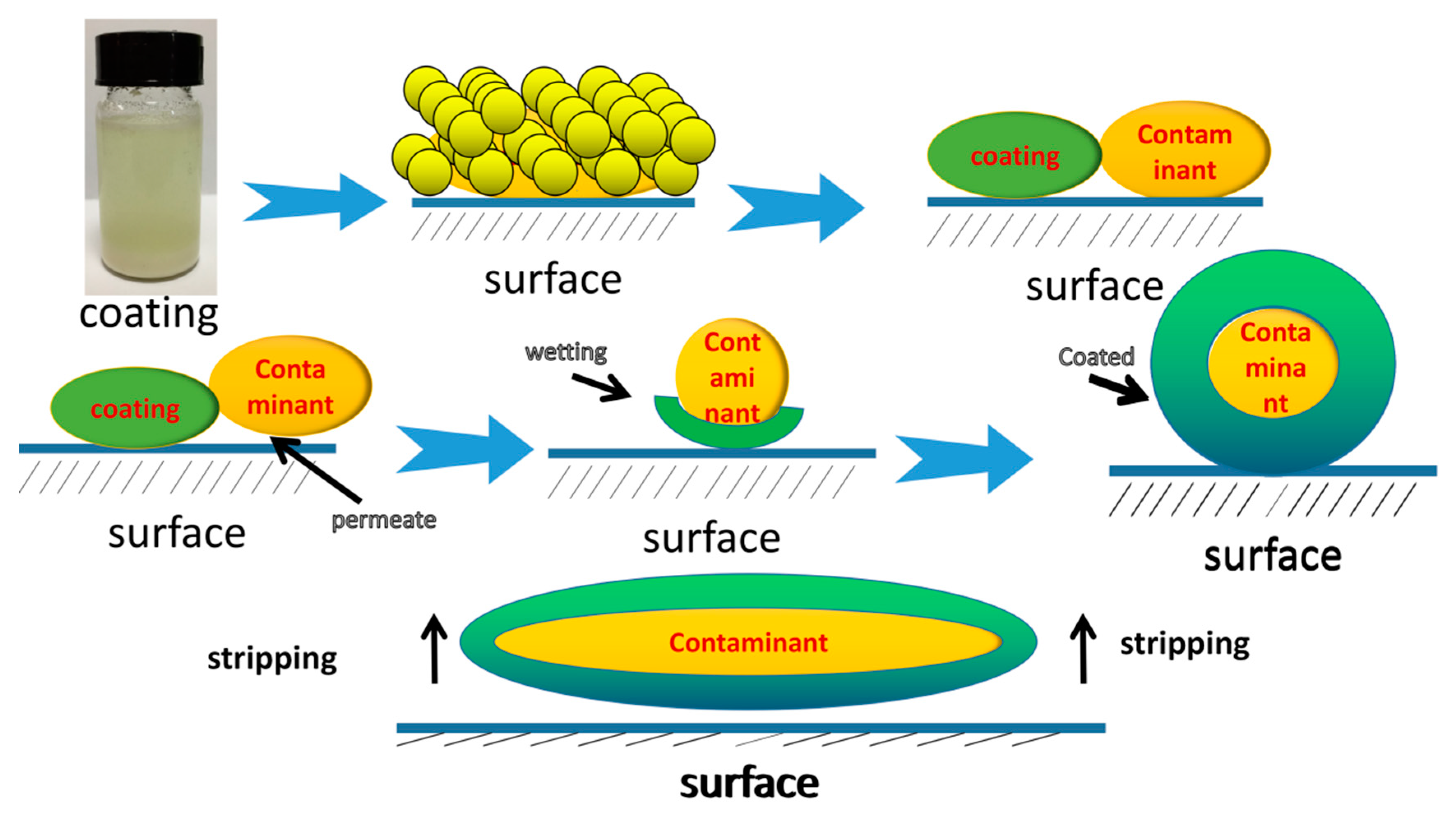
1. Introduction
- Short curing time, good physical properties, no corrosion of the equipment surface, and friendly to the environment.
- The film body formed by the coating has excellent sealing and mechanical properties such as better elongation at break and tensile strength.
- The painting process is simple, and the adhesion is moderate. It can adapt to different object interfaces and can be quickly and completely peeled off.
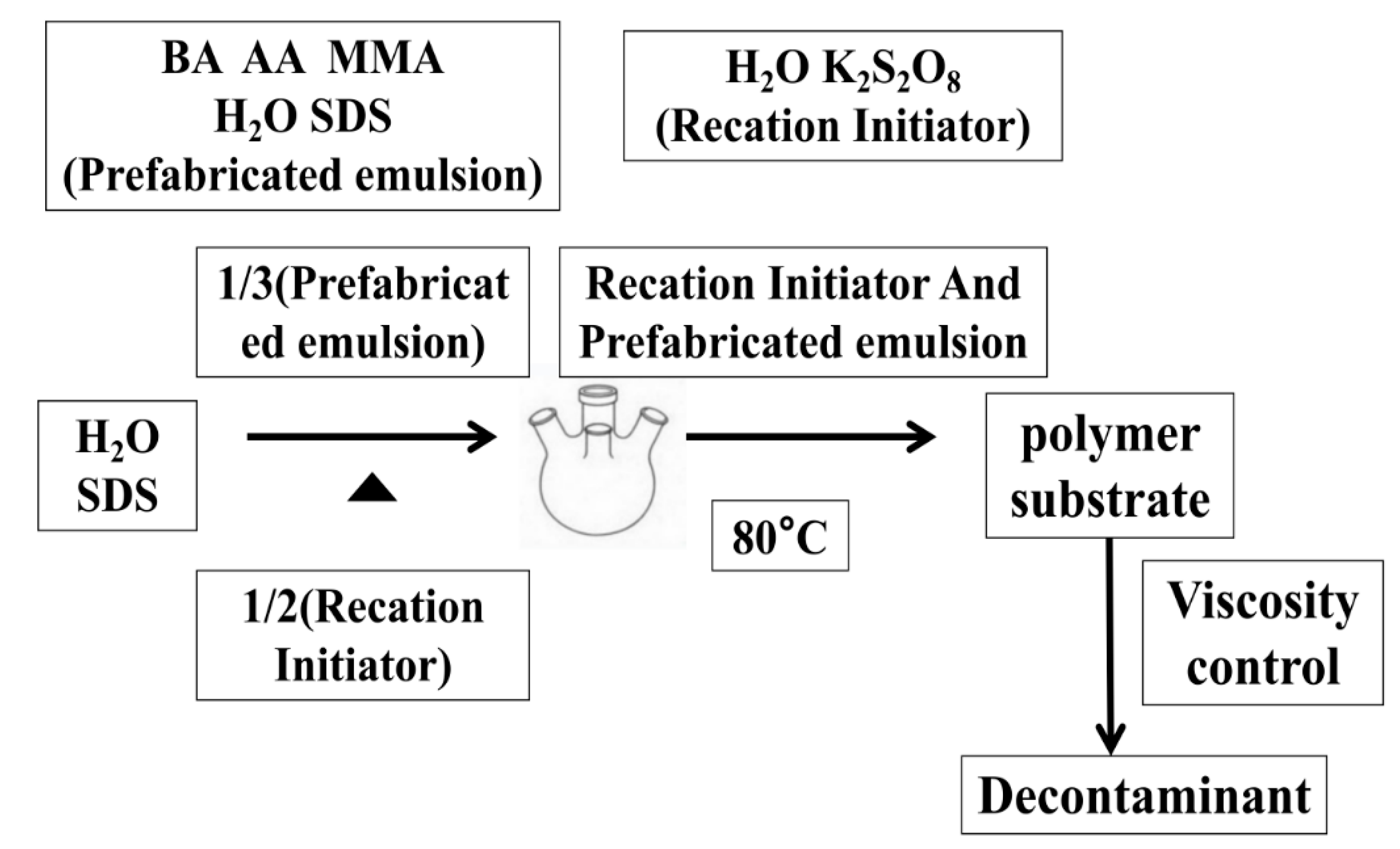
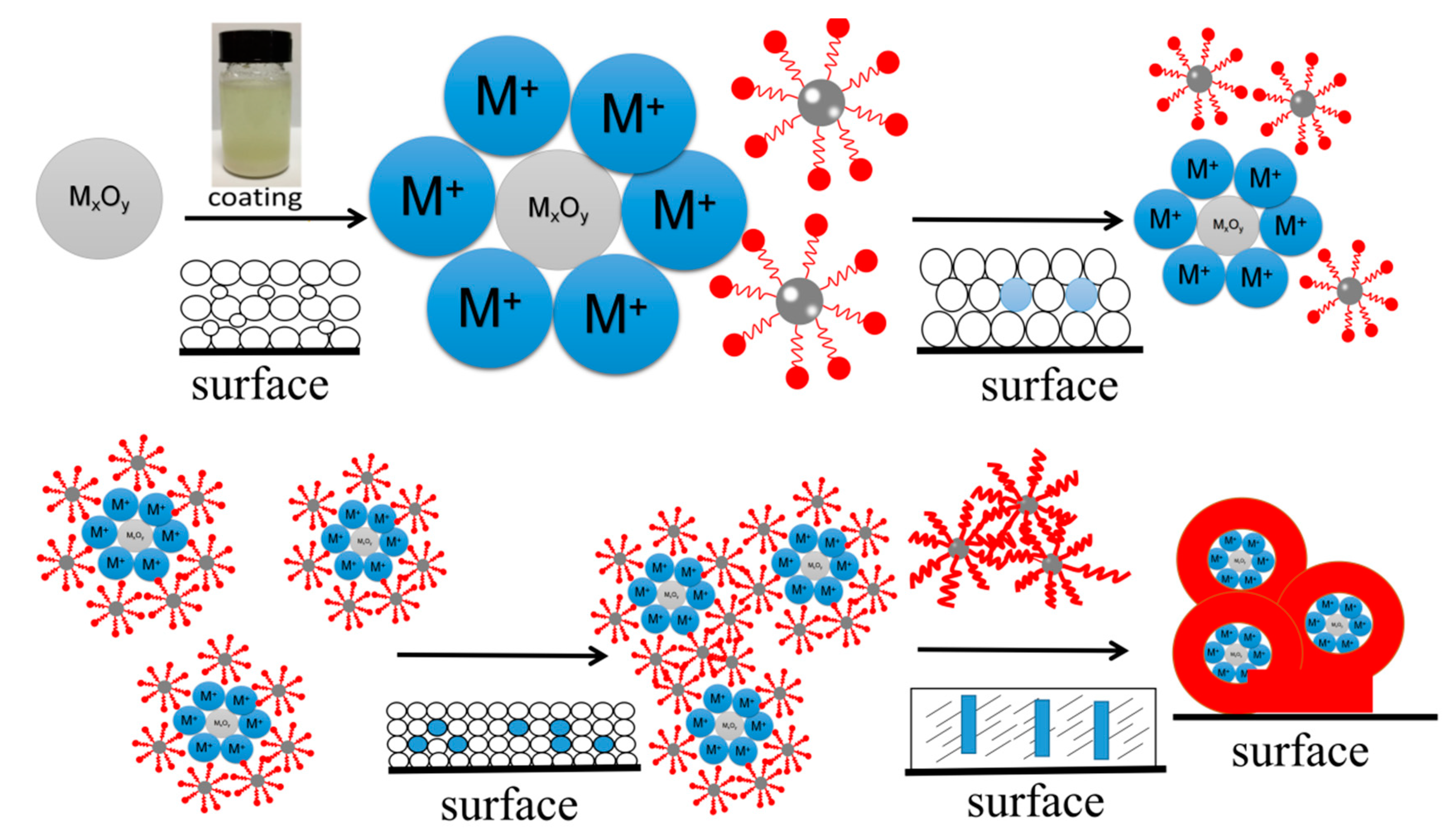
2. Experimental Methods
2.1. Chemicals
2.2. Synthesis
- (1)
- First, 5 g of OP-10 emulsifier was dissolved in 450 mL of distilled water and was stirred and dissolved in a 30 °C water bath. Then, 5 g of sodium dodecyl sulfate was dissolved in a clear solution containing an emulsifier and was stirred until clear. Then, 500 mL of water was added, stirred well, and set aside. Afterward, 6 g of potassium persulfate was added to the water and dissolved.
- (2)
- We added 100 g of monomer, 100 mL of emulsifier, and 100 mL of distilled water into a beaker at room temperature, and an emulsifying machine was used for the pre-emulsification of the fluid.
- (3)
- We added the distilled water and emulsifier into four-mouth flasks, which were each equipped with a reflux condensing tube thermometer with an agitator and a constant pressure drip funnel, and then the water was heated to 75 °C. Next, we added 100 mL of pre-emulsion and 50 mL of potassium persulfate solution. The remaining pre-emulsion was preserved in a constant pressure funnel for later use.
- (4)
- When the emulsion was blue, the remaining pre-emulsion and potassium persulfate solution were added synchronously and slowly, which lasted 2–3 h, and then the temperature was raised to 80 °C. After 2 h of insulation, the reaction ended. Next, cooling and discharging were completed.
- (5)
- Then, the pH value was adjusted to the desired viscosity in order to obtain the polymer substrate.
2.3. Structural Characterization
3. Results and Discussion
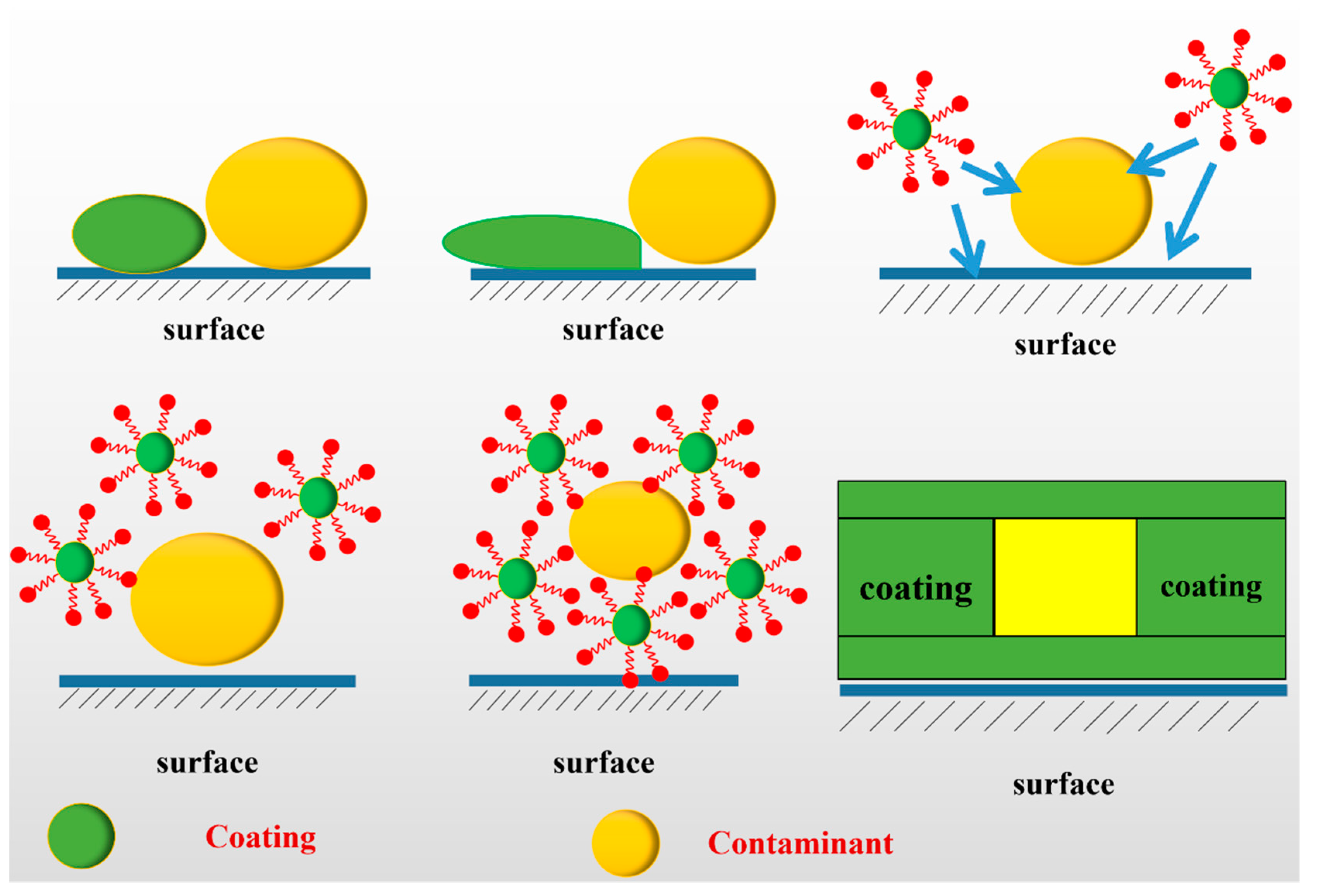
3.1. Study on the Influencing Factors of the Decontamination Process
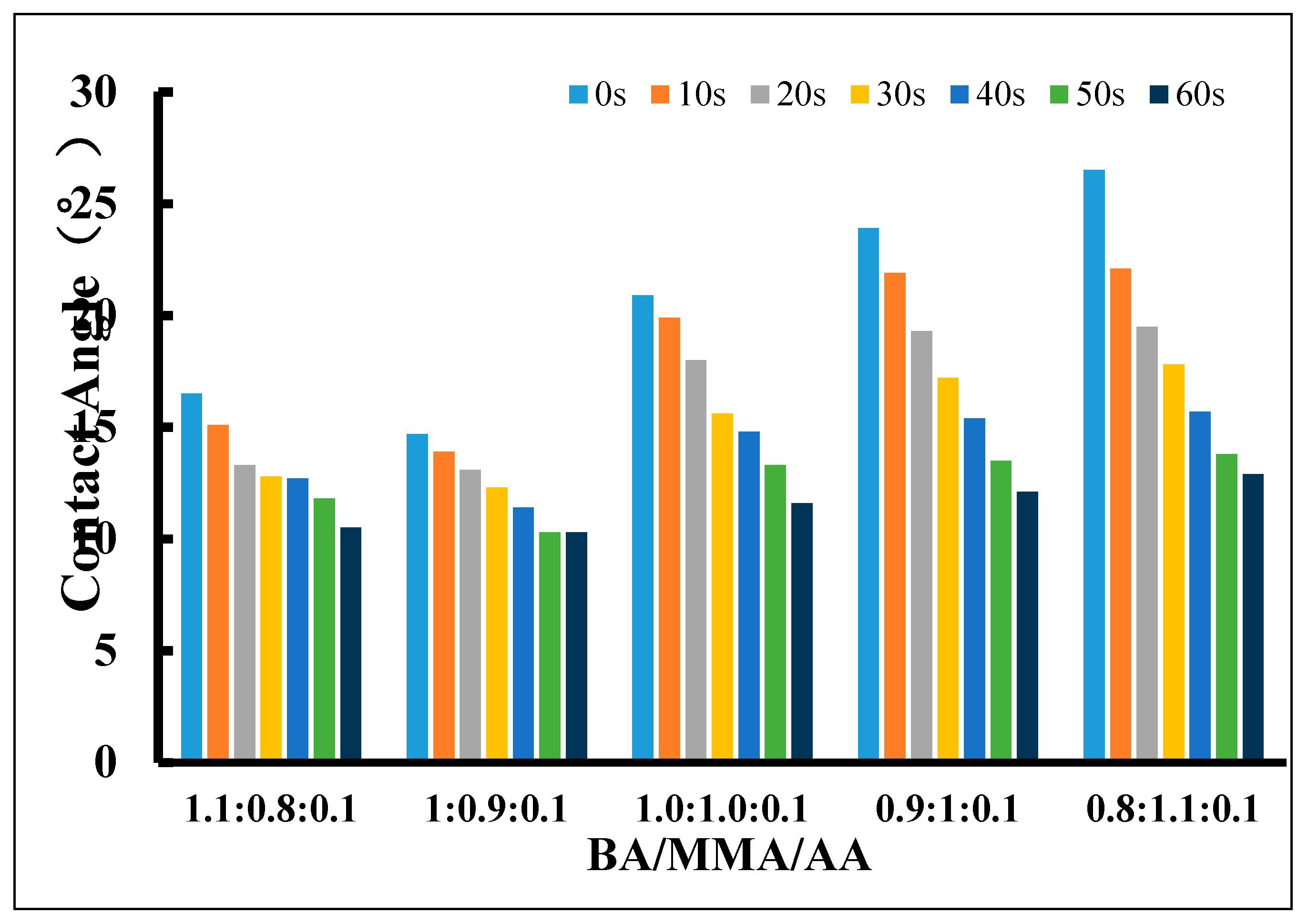
3.1.1. The Wettability of the Decontaminant
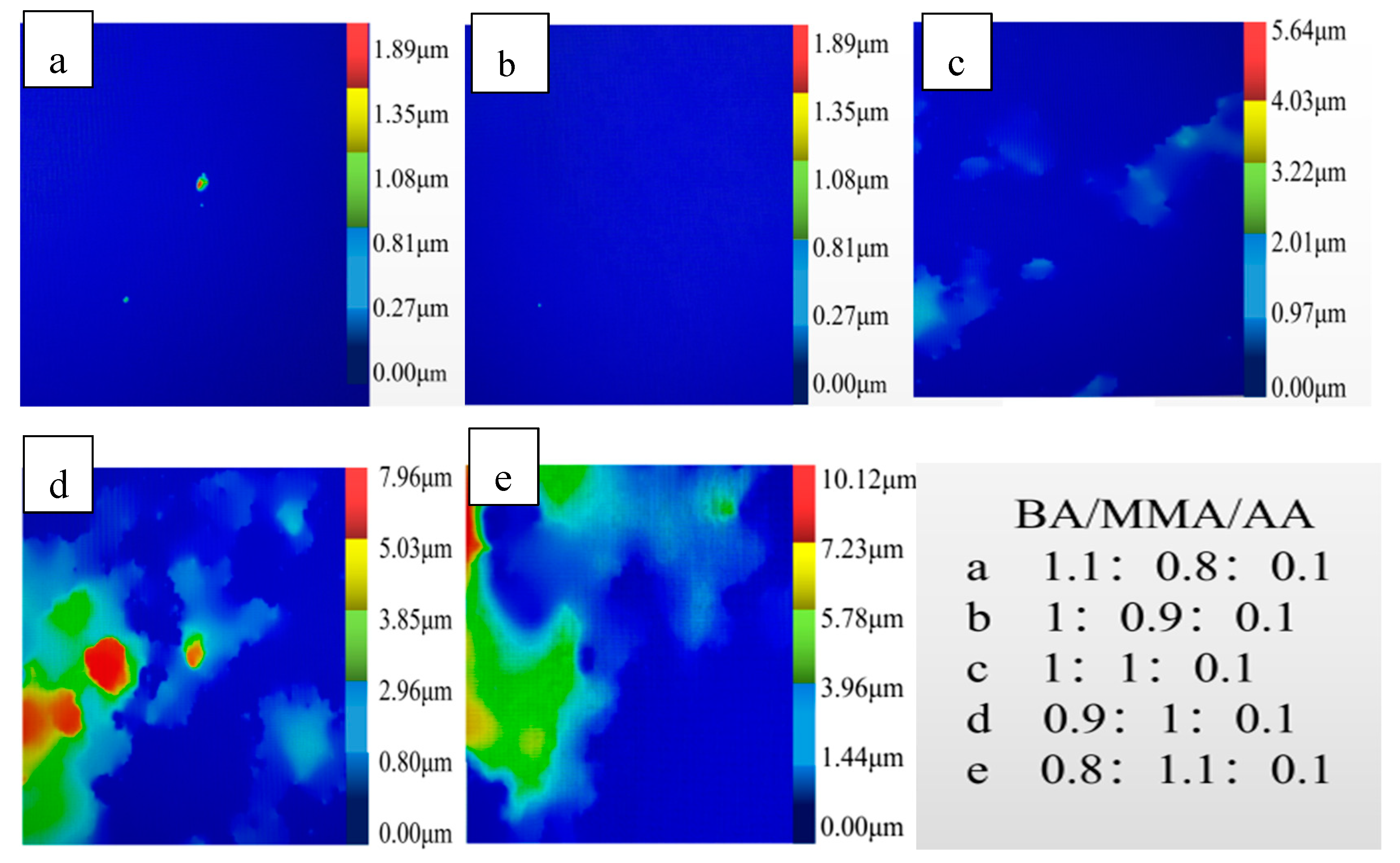
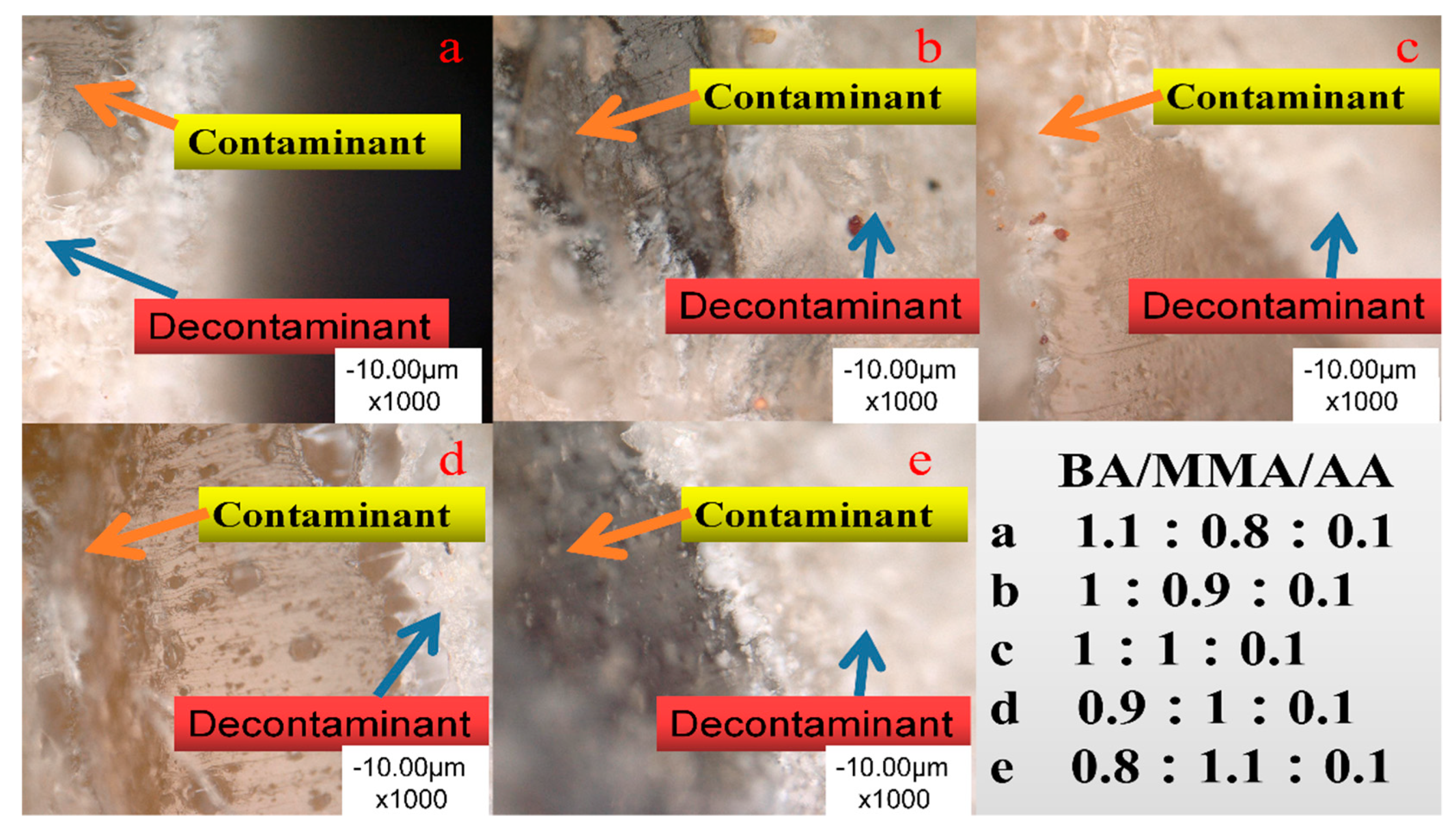
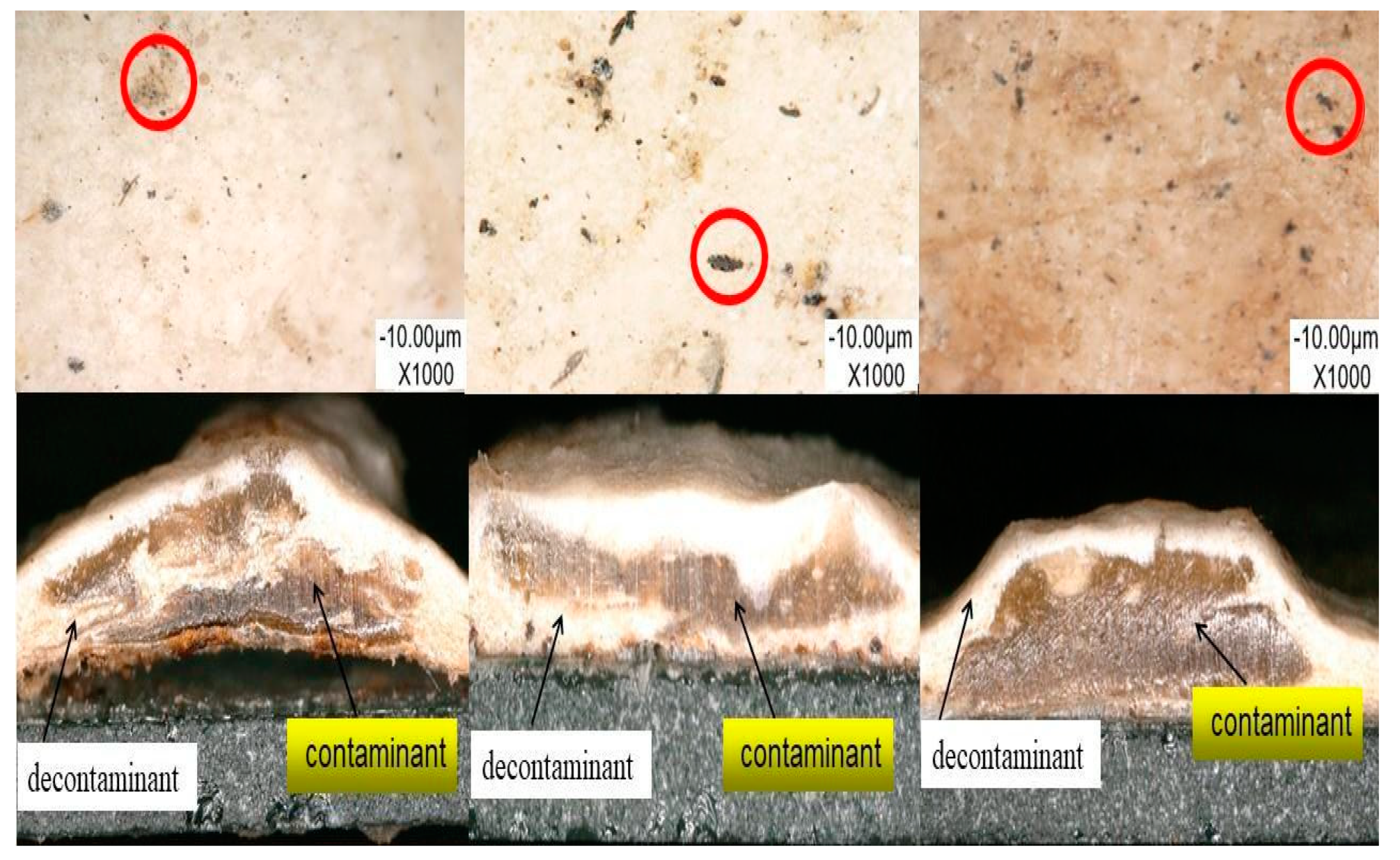
3.1.2. Surface Morphology
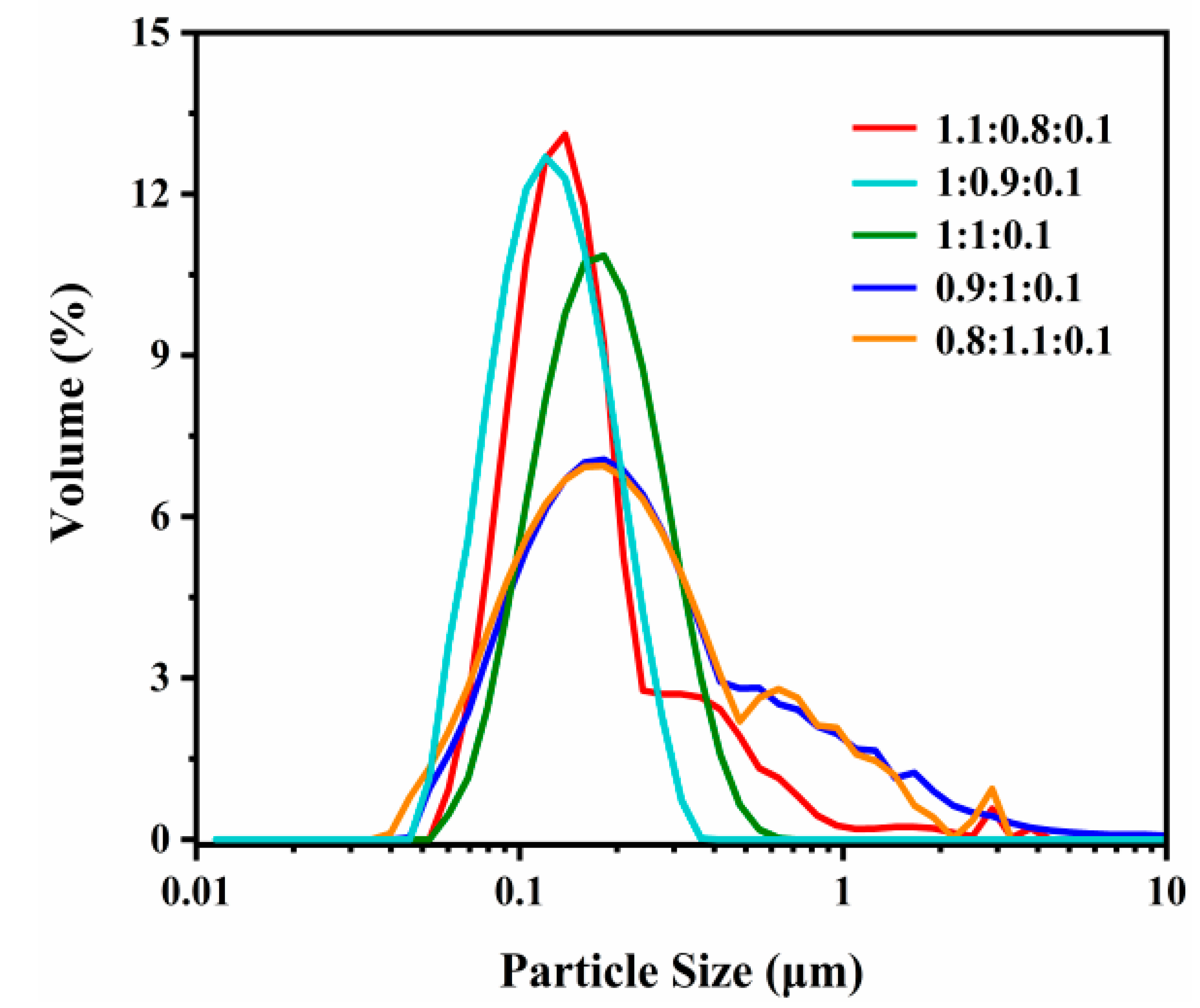
3.1.3. Effect of Particle Size on the Decontamination Process
3.2. The Decontamination Efficiency of the Decontaminant BA/MMA/AA (1:0.9:0.1)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Q. Current status and recommendations of mechanical decontamination technology. In Proceedings of the National Symposium on Radiation Protection for Young and Middle Aged, Hangzhou, China, 17–21 October 1999. [Google Scholar]
- Myodo, M. Application of laser to decontamination and decommissioning of nuclear facilities at JAERI. Proc. SPIE Int. Soc. Opt. Eng. 2000, 3887, 94–103. [Google Scholar]
- Aniruddh, K.; Bhatt, R.B.; Behere, P.G.; Afzal, M. Ultrasonic decontamination of prototype fast breeder reactor fuel pins. Ultrasonics 2014, 54, 1052–1056. [Google Scholar]
- Lu, C.H.; Sun, Y. Development of chemical decontamination technology and its application in decommissioning of nuclear facilities. Environ. Eng. 2002, 20, 25–32. [Google Scholar]
- Ishida, K.; Nagase, M.; Uetake, N.; Anazawa, K.; Nakamura, F.; Yoshikawa, H.; Tamagawa, T.; Furukawa, K. Low corrosive chemical decontamination method using pH control, (II) decomposition of reducing agent by using catalyst with hydrogen peroxide. J. Nucl. Sci. Tech. 2002, 39, 941–949. [Google Scholar] [CrossRef]
- Bargues, S.; Favier, F.; Pascal, J.L.; Lecourt, J.P.; Damerval, F. Organomineral Decontamination Gel and Use Thereof for Surface Decontamination. U.S. Patent 6,203,624, 20 March 2001. [Google Scholar]
- Zhou, Y.L.; Li, Y.T.; Xie, C.Q.; Li, Y.J. Preparation of a Self-Brittle Radioactive Decontamination Coating. CN Patent 102,690,579B, 2014. [Google Scholar]
- International Atomic Energy Agency. Report of the International Mission on Remediation of Large Contaminated Areas Off-Site the Fukushima Dai-Ichi NPP; International Atomic Energy Agency: Vienna, Austria, 2001. [Google Scholar]
- Real, J.; Persin, F.; Camarasa-Claret, C. Mechanisms of desorption of 134Cs and 85Sr aerosols deposited on urban surfaces. J. Environ. Radioact. 2002, 62, 1–15. [Google Scholar] [CrossRef]
- Samuleev, P.V.; Andrews, W.S.; Creber, K.; Azmi, P.; Velicogna, D.; Juang, W.; Volchek, K. Decontamination of radionuclides on construction materials. J. Radioanal. Nucl. Chem. 2013, 296, 811–815. [Google Scholar] [CrossRef]
- Kaminski, M.D.; Lee, S.D.; Magnuson, M. Wide-area decontamination in an urban environment after radiological dispersion: A review and perspectives. J. Hazard. Mater. 2016, 305, 67–86. [Google Scholar] [CrossRef]
- Chen, E.; Li, Y.; Guo, Y.; Liu, X.; Zhou, Y. Preparation and Performance Study of a Self-embrittling Strippable Decontaminant with Controllable Morphology. J. Southwest Univ. Sci. Tech. 2017, 32, 71–76. [Google Scholar]
- Fujii, K.; Ochi, K.; Ohbuchi, A.; Koike, Y. Evaluation of physicochemical properties of radioactive cesium in municipal solid waste incineration fly ash by particle size classification and leaching tests. J. Environ. Manag. 2018, 217, 157–463. [Google Scholar] [CrossRef]
- DirtyBomb. NOVA Public Broadcasting System Program Transcript. Available online: http://www.pbs.org/wgbh/nova/transcripts/3007_dirtybom.html (accessed on 15 July 2004).
- Lumia, M.; Gentile, C.A.; Efthimion, P.; Robson, M.G. The use of the removable thin film coating technique as an alternative to traditional decontamination methods to mitigate and abate hazardous particulates. Remediat. J. 2008, 18, 115–130. [Google Scholar] [CrossRef]
- Yang, H.M.; Park, C.W.; Lee, K.W. Polymeric coatings for surface-contamination and ecofriendly volume reduction of radinactive waste after use. Prog. Nucl. Energy 2018, 104, 67. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Li, J.; Zheng, L.; Li, J.; Lv, L. Experimental study on hot-melt pressure sensitive coil material for decontaminating radioactive dust. Radiat. Prot. 2018, 38, 326–330. [Google Scholar]
- Zu Qun, D.; Nian Qing, F.U.; Yun-Ying, W. Synthesis of Waterborne Polyurethane and the Properties of Strippable Coating. Surf. Tech. 2017, 46, 60–65. [Google Scholar]
- Zhang, H.; Zhou, Y.; Li, Y.; Liu, R.; Wu, H.; Shanqiang, W. Preparation and Performance of One Kind Resin for Capturing and Encapsulating Radioactive Aerosols. Polymer Mater. Sci. Eng. 2016, 32, 131–136. [Google Scholar]
- Wu, H.; Li, Y.; Zhang, H.; Zhou, Y. Preparation and Characterization of P(AM-co-AA) for Capturing and Encapsulating Radioactive Aerosols. Fine Chem. 2017, 34, 116–120. [Google Scholar]
- Liu, R.; Li, Y.; Zhou, Y.; Zhang, H.Y.; Zhang, Q.P.; Zheng, J.; Wang, S.Q. Fabrication of poly (methyl methacrylate)-block-poly (methacrylic acid) diblock copolymer as self-embrittling strippable coating for radioactive decontamination. Chem. Lett. 2016, 45, 793–794. [Google Scholar] [CrossRef]
- Gertzmann, R.; Petzoldt, J.; Miller, H. Emulsion Polymer for Stripping Coatings. CN Patent 1,585,805A, 23 February 2005. [Google Scholar]
- William, M.M.; Trachan, R.J. Water Stripping Coating Composition for Pressure-Sensitive Adhesives. CN Patent 1,089,284A, 13 July 1994. [Google Scholar]
- Yamashita, X.Y.; Matsumoto, M.; Honggui, S.W. Water dispersion of peel coatings. CN Patent 1,215,073A, 28 April 1999. [Google Scholar]
- Yin, H.L.; Tan, Z.Y.; Liao, Y.T.; Feng, Y.J. Application of SO42_/TiO2 solid superacid in decontaminating radioactive pollutants. J. Environ. Radioact. 2006, 87, 227–235. [Google Scholar] [CrossRef]
- Hu, K.H.; Hu, X.G.; Xu, Y.F.; Sun, D.J. Synthesis of nano MoS2/TiO2 composite and its catalytic degradation effect on methylorange. J. Mater. Sci. 2010, 4, 2640–2648. [Google Scholar] [CrossRef]
- Zhang, W.P.; Xiao, X.Y.; Zheng, L.L.; Wan, C. Fabrication of TiO2 /MoS2 @ zeolite photocatalyst and its photocatalytic activity for degradation of methyl orange under visible light. Appl. Surf. Sci. 2015, 358, 468–478. [Google Scholar] [CrossRef]
- Sun, F.C.; Shen, B. Synthesis and properties of novel UV curable strippable metallic coatings. Chem. Ind. Times 2010, 24, 12–16. [Google Scholar]
- Wang, X.F.; Liang, F.H.; Huang, N.B. Formulation and properties of PVC resin solventable plastics. Corros. Prot. 2009, 30, 184–185. [Google Scholar]
- Li, Y.; Li, J.; Hao, Y. Sol-gel method synthesis of high temperature stable macroporous TiO2. Funct. Mater. 2011, 42, 1738–1741. [Google Scholar]
- Yuan, X.; Huo, D.; Wang, H. Research Status of Film Formation Theory of Polymer Emulsion. Polym. Bull. 2006, 8, 66–71. [Google Scholar]
- He, S.Z. Study on Preparation and Characterization of Waterborne Cellulose Acetate Emulsion; Shaanxi University of Science and Technology: Xi’an, China, 2019. [Google Scholar]





| Zeta Potential (mv) | The Different Monomers’ Ratios (BA/MMA/AA) |
|---|---|
| −43.52 | 1.1:0.8:0.1 |
| −49.25 | 1:0.9:0.1 |
| −39.91 | 1:1:0.1 |
| −30.25 | 0.9:0.1:0.1 |
| −29.12 | 0.8:1.1:0.1 |
| Sample | Peel Strength (N/M) | Stripping Degree |
|---|---|---|
| Glass | 0.562 | 100% |
| Iron sheets | 0.855 | 100% |
| Plastic piece | 0.663 | 100% |
| Pollutant | Polluted Area (%) | Decontamination Efficiency (%) | Film-Forming Property | Stripping Degree (%) | |
|---|---|---|---|---|---|
| Prior to the Decontamination | After Decontamination | ||||
| Refined SiO2 | 100% | 3% | 97% | Continuous film | 100% |
| Soil dust | 100% | 6% | 94% | Continuous film | 100% |
| Fly ash | 100% | 4% | 96% | Continuous film | 100% |
| Starch | 100% | 5% | 95% | Continuous film | 100% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.-y.; Li, Y.-T.; Zhang, Q.-p.; Li, Y.-j.; Liu, D.-l.; Xiao, Z.-q.; Zhang, S.-f.; Zhou, Y.-L.; Luo, D.l. Study on the Influencing Factors in the Process of Surface Strippable Decontaminant. Coatings 2020, 10, 649. https://doi.org/10.3390/coatings10070649
He Z-y, Li Y-T, Zhang Q-p, Li Y-j, Liu D-l, Xiao Z-q, Zhang S-f, Zhou Y-L, Luo Dl. Study on the Influencing Factors in the Process of Surface Strippable Decontaminant. Coatings. 2020; 10(7):649. https://doi.org/10.3390/coatings10070649
Chicago/Turabian StyleHe, Zhi-yu, Yin-Tao Li, Quan-ping Zhang, Ying-jun Li, Dong-liang Liu, Zhi-qiang Xiao, Shi-feng Zhang, Yuan-Lin Zhou, and De li Luo. 2020. "Study on the Influencing Factors in the Process of Surface Strippable Decontaminant" Coatings 10, no. 7: 649. https://doi.org/10.3390/coatings10070649
APA StyleHe, Z.-y., Li, Y.-T., Zhang, Q.-p., Li, Y.-j., Liu, D.-l., Xiao, Z.-q., Zhang, S.-f., Zhou, Y.-L., & Luo, D. l. (2020). Study on the Influencing Factors in the Process of Surface Strippable Decontaminant. Coatings, 10(7), 649. https://doi.org/10.3390/coatings10070649





