Effect of Oxygen Source on the Various Properties of SnO2 Thin Films Deposited by Plasma-Enhanced Atomic Layer Deposition
Abstract
:1. Introduction
2. Experiment
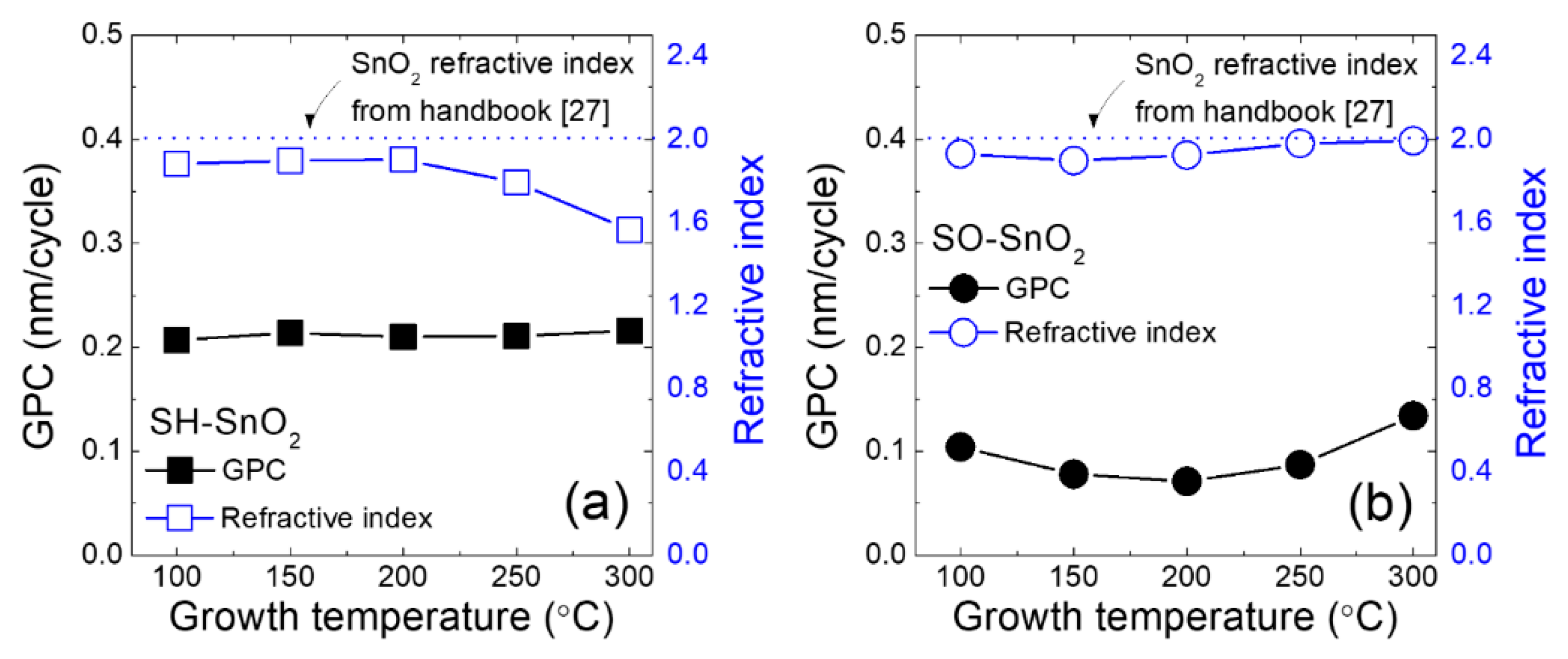
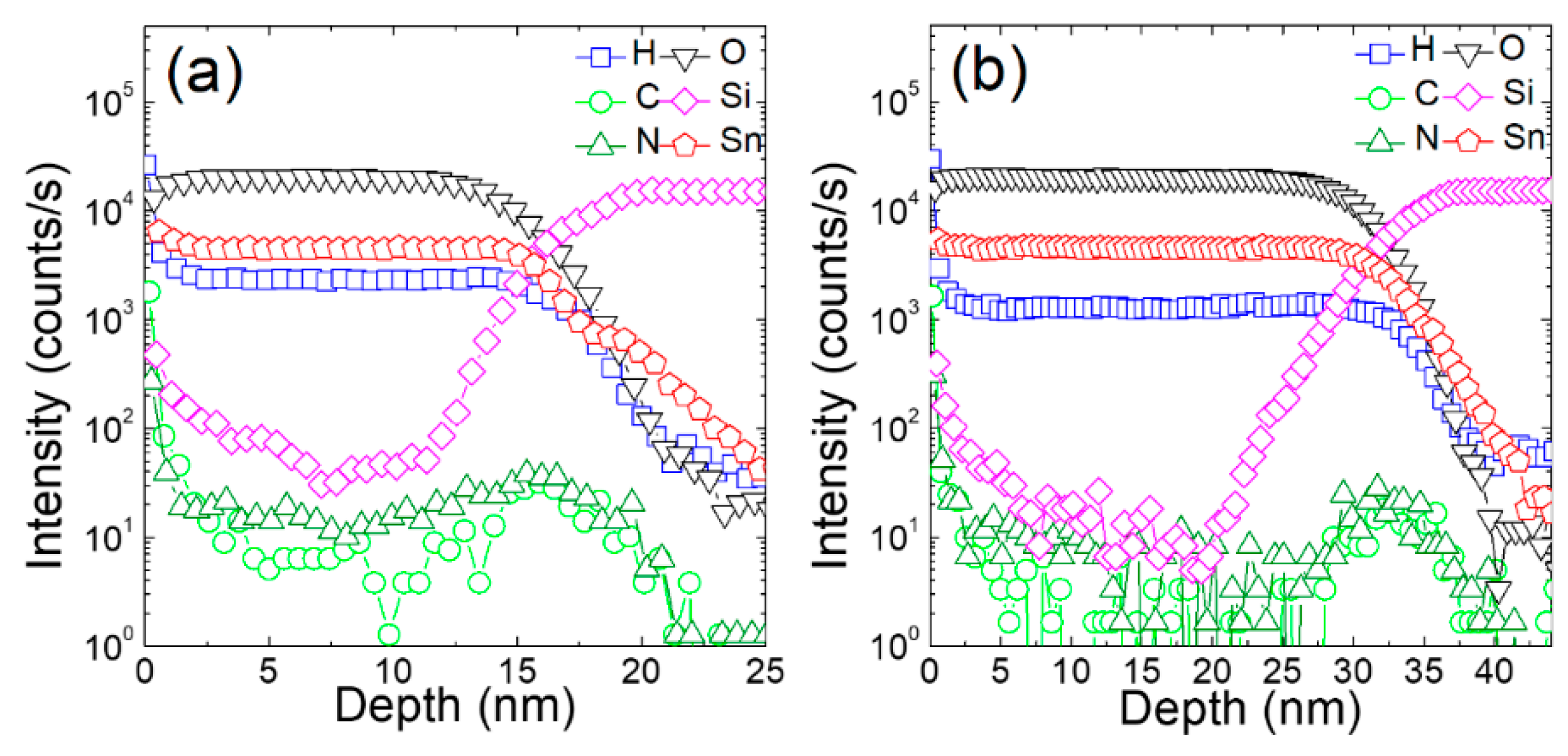
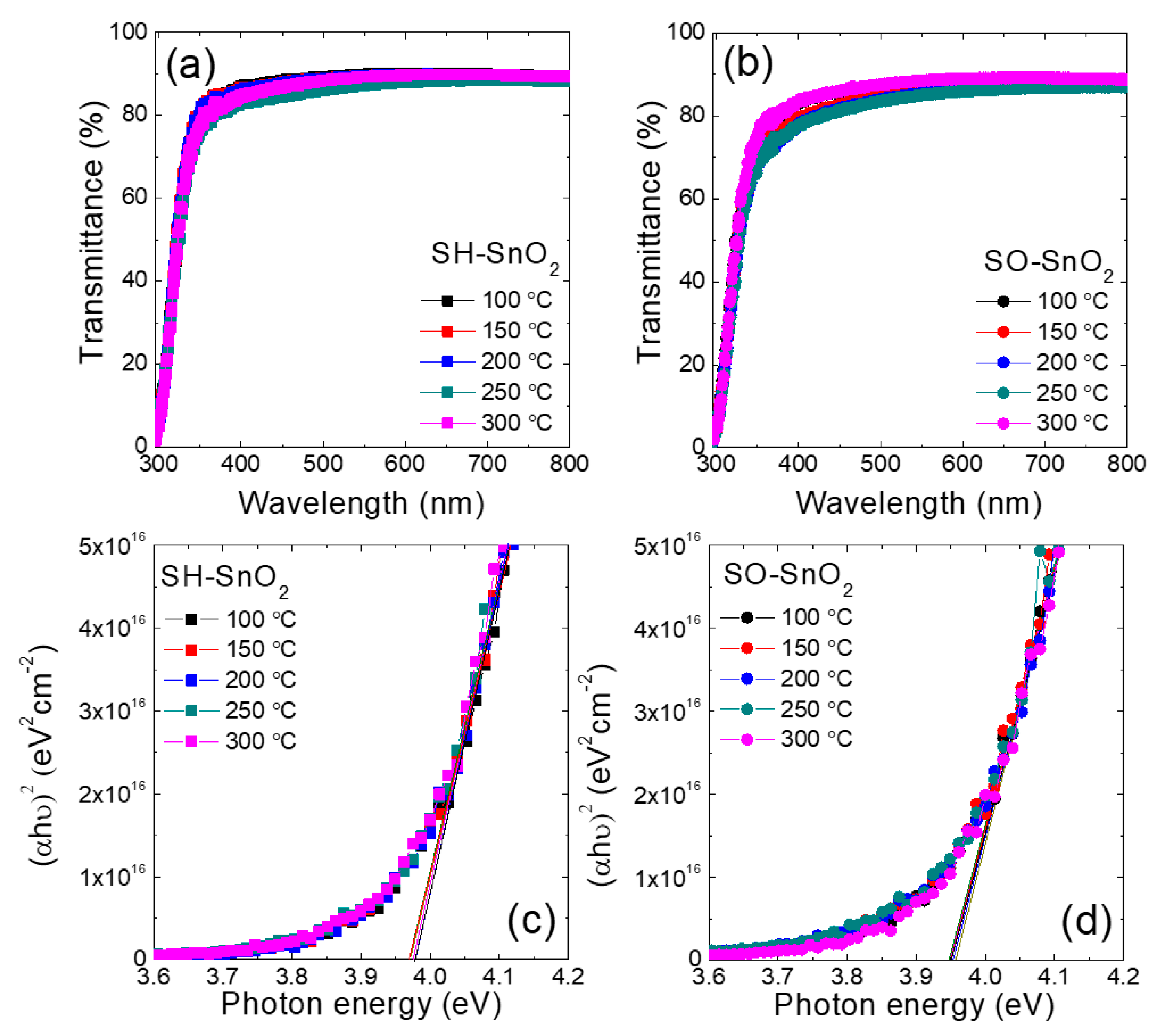
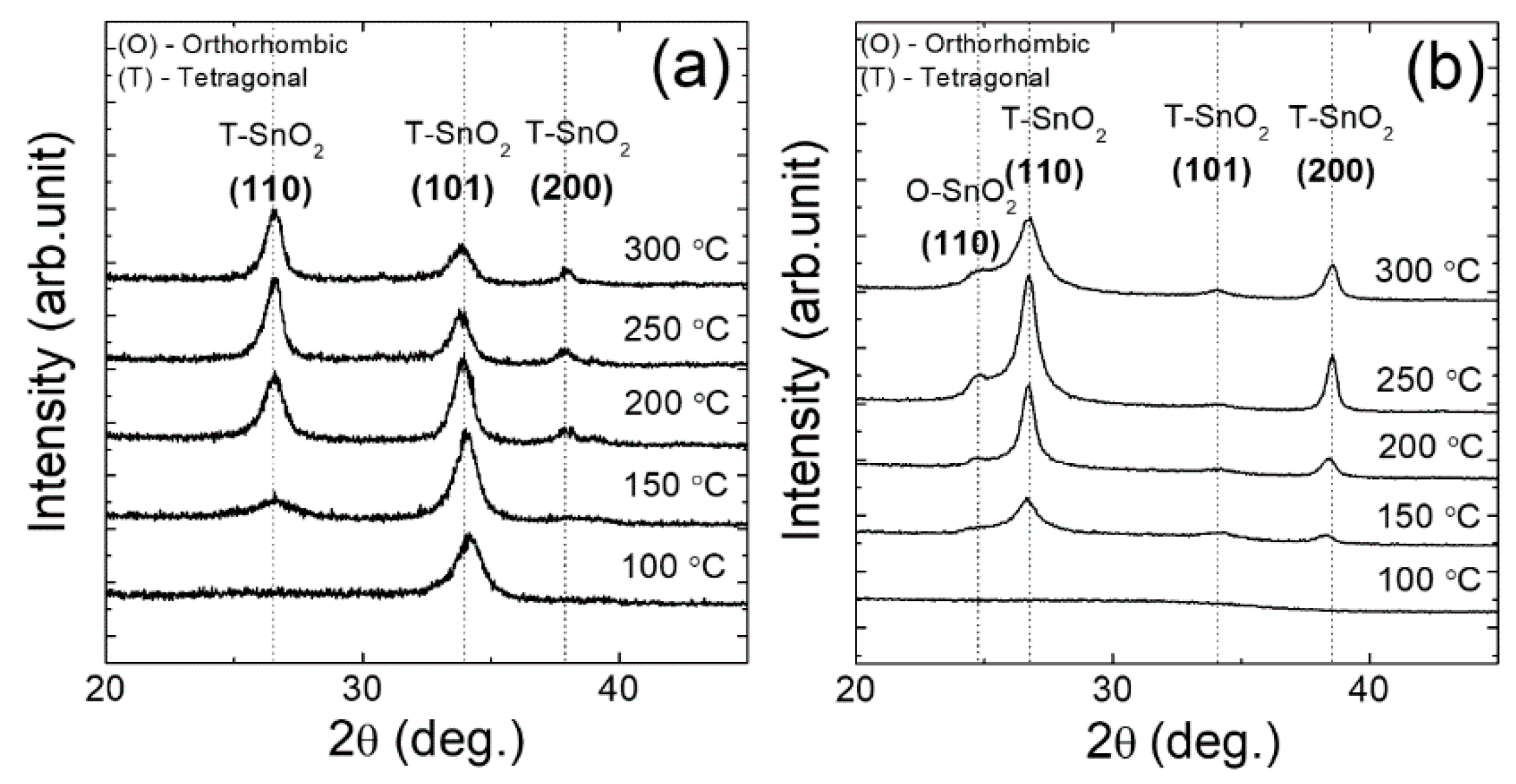
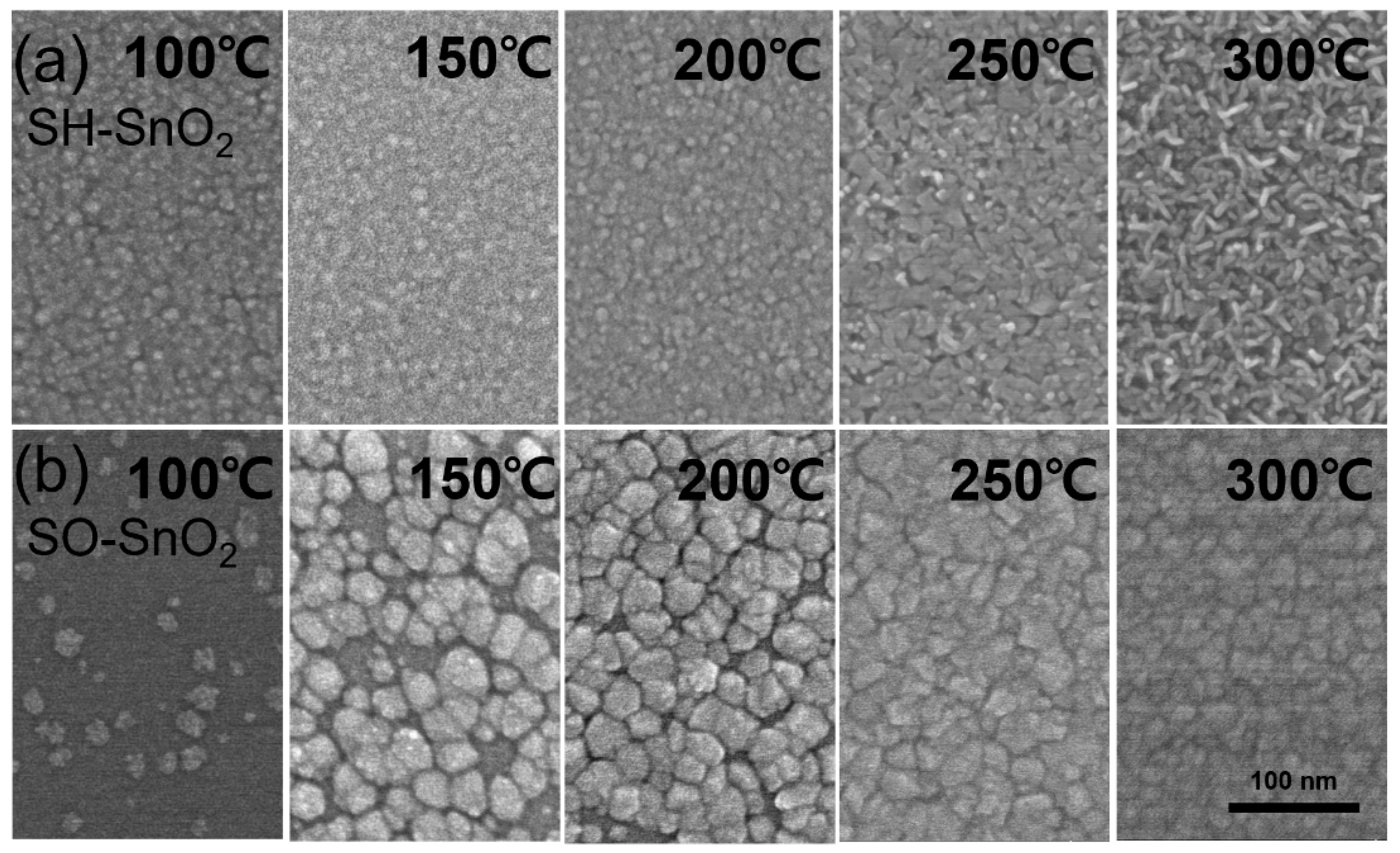
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paulowicz, I.; Hrkac, V.; Kaps, S.; Cretu, V.; Lupan, O.; Braniste, T.; Duppel, V.; Tiginyanu, I.; Kienle, L.; Adelung, R. Three-dimensional SnO2 nanowire networks for multifunctional applications: From high-temperature stretchable ceramics to ultra responsive sensors. Am. Ceram. Soc. Bull. 2015, 94, 21. [Google Scholar]
- Guan, C.; Wang, X.; Zhang, Q.; Fan, Z.; Zhang, H.; Fan, H.J. Highly stable and reversible lithium storage in SnO2 nanowires surface coated with a uniform hollow shell by atomic layer deposition. Nano Lett. 2014, 14, 4852–4858. [Google Scholar] [CrossRef]
- Kim, D.H.; Shin, D.Y.; Lee, Y.G.; An, G.H.; Han, J.H.; Ahn, H.J.; Choi, B.J. Effects of SnO2 layer coated on carbon nanofiber for the methanol oxidation reaction. Ceram. Int. 2018, 44, 19554–19559. [Google Scholar] [CrossRef]
- Park, M.; Kim, J.Y.; Son, H.J.; Lee, C.H.; Jang, S.S.; Ko, M.J. Low-temperature solution-processed Li-doped SnO2 as an effective electron transporting layer for high-performance flexible and wearable perovskite solar cells. Nano Energy 2016, 26, 208–215. [Google Scholar] [CrossRef]
- Chu, H.C.; Shen, Y.S.; Hsieh, C.H.; Huang, J.H.; Wu, Y.H. Low-voltage operation of ZrO2-gated n-type thin-film transistors based on a channel formed by hybrid phases of SnO and SnO2. ACS Appl. Mater. Interfaces 2015, 7, 15129–15137. [Google Scholar] [CrossRef]
- Jarzebski, Z.M.; Marton, J.P. Physical properties of SnO2 materials. J. Electrochem. Soc. 1976, 123, 299–310. [Google Scholar] [CrossRef]
- Manifacier, J.C.; De Murcia, M.; Fillard, J.P.; Vicario, E. Optical and electrical properties of SnO2 thin films in relation to their stoichiometric deviation and their crystalline structure. Thin Solid Films 1977, 41, 127–135. [Google Scholar] [CrossRef]
- Minami, T.; Nanto, H.; Takata, S. Highly conducting and transparent SnO2 thin films prepared by RF magnetron sputtering on low-temperature substrates. Jpn. J. Appl. Phys. 1988, 27, L287–L289. [Google Scholar] [CrossRef]
- Liu, Y.; Koep, E.; Liu, M. A highly sensitive and fast-responding SnO2 sensor fabricated by combustion chemical vapor deposition. Chem. Mater. 2005, 17, 3997–4000. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Schwank, J.; Dibattista, M.; Asiliev, A. Peculiarities of SnO2 thin film deposition by spray pyrolysis for gas sensor application. Sens. Actuators B 2001, 77, 244–252. [Google Scholar] [CrossRef]
- Kim, H.; Piqué, A. Transparent conducting Sb-doped SnO2 thin films grown by pulsed-laser deposition Transparent conducting Sb-doped SnO2 thin films grown by pulsed-laser deposition. Appl. Phys. Lett. 2004, 218, 10–13. [Google Scholar]
- Lu, J.; Sundqvist, J.; Ottosson, M.; Tarre, A.; Rosental, A.; Aarik, J.; Hårsta, A. Microstructure characterisation of ALD-grown epitaxial SnO2 thin films. J. Cryst. Growth. 2004, 260, 191–200. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today. 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, D.; Grice, C.R.; Liao, W.; Yu, Y.; Cimaroli, A.; Shrestha, N.; Roland, P.J.; Chen, J.; Yu, Z.; et al. Low-temperature plasma-enhanced atomic layer deposition of tin oxide electron selective layers for highly efficient planar perovskite solar cells. J. Mater. Chem. A. 2016, 4, 12080–12087. [Google Scholar] [CrossRef]
- Lee, D.K.; Wan, Z.; Bae, J.S.; Lee, H.B.R.; Ahn, J.H.S.; Kim, D.; Kim, J.; Kwon, S.H. Plasma-enhanced atomic layer deposition of SnO2 thin films using SnCl4 and O2 plasma. Mater. Lett. 2016, 166, 163–166. [Google Scholar] [CrossRef]
- Choi, M.J.; Cho, C.J.; Kim, K.C.; Pyeon, J.J.; Park, H.H.; Kim, H.S.; Han, J.H.; Kim, C.G.; Chung, T.M.; Park, T.J.; et al. SnO2 thin films grown by atomic layer deposition using a novel Sn precursor. Appl. Surf. Sci. 2014, 320, 188–194. [Google Scholar] [CrossRef]
- Lim, S.S.; Baek, I.H.; Kim, K.C.; Baek, S.H.; Park, H.H.; Kim, J.S.; Kim, S.K. Atomic layer deposition of SnO2 thin films using tetraethyltin and H2O2. Ceram. Int. 2019, 45, 20600–20605. [Google Scholar] [CrossRef]
- Kim, H.Y.; Nam, J.H.; George, S.M.; Park, J.S.; Park, B.K.; Kim, G.H.; Jeon, D.J.; Chung, T.M.; Han, J.H. Phase-controlled SnO2 and SnO growth by atomic layer deposition using Bis(N-ethoxy-2,2-dimethyl propanamido)tin precursor. Ceram. Int. 2019, 45, 5124–5132. [Google Scholar] [CrossRef]
- won Choi, D.; Park, J.S. Highly conductive SnO2 thin films deposited by atomic layer deposition using tetrakis-dimethyl-amine-tin precursor and ozone reactant. Surf. Coatings Technol. 2014, 259, 238–243. [Google Scholar] [CrossRef]
- Chang, S.; Selvaraj, S.K.; Choi, Y.-Y.; Hong, S.; Nakhmanson, S.M.; Takoudis, C.G. Atomic layer deposition of environmentally benign SnTiOx as a potential ferroelectric material. J. Vac. Sci. Technol. A 2016, 34, 01A119. [Google Scholar] [CrossRef] [Green Version]
- Tarlov, M.J.; Evans, J.F.; Newman, J.G. Static SIMS and XPS study of water plasma exposed tin oxide films. Appl. Surf. Sci. 1993, 64, 115–125. [Google Scholar] [CrossRef]
- Tarlov, M.J.; Evans, J.F. Surface characterization of radio frequency water plasma treated and annealed polycrystalline tin oxide thin films. Chem. Mater. 1990, 2, 49–60. [Google Scholar] [CrossRef]
- Kawamura, Y.; Hattori, N.; Miyatake, N.; Uraoka, Y. Comparison between ZnO films grown by plasma-assisted atomic layer deposition using H2O plasma and O2 plasma as oxidant. J. Vac. Sci. Technol. A. 2013, 31, 01A142. [Google Scholar] [CrossRef]
- Heo, J.; Kim, S.B.; Gordon, R.G. Atomic layer deposition of tin oxide with nitric oxide as an oxidant gas. J. Mater. Chem. 2012, 22, 4599–4602. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.; Hock, A.S.; Gordon, R.G. Low temperature atomic layer deposition of tin oxide. Chem. Mater. 2010, 22, 4964–4973. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill Companies, Inc.: New York, NY, USA, 2003; p. 940. ISBN 0-07-049439-8. [Google Scholar]
- Stuckert, E.P.; Miller, C.J.; Fisher, E.R. Gas-phase diagnostics during H2 and H2O plasma treatment of SnO2 nanomaterials: Implications for surface modification. J. Vac. Sci. Technol. B 2017, 35, 021802. [Google Scholar] [CrossRef]
- Stuckert, E.P.; Fisher, E.R. Ar/O2 and H2O plasma surface modification of SnO2 nanomaterials to increase surface oxidation. Sens. Actuators B Chem. 2015, 208, 379–388. [Google Scholar] [CrossRef]
- Choi, M.S.; Lee, Y.J.; Kwon, J.D.; Jeong, Y.; Park, J.Y.; Kang, Y.C.; Song, P.K.; Kim, D.H. Effects of hydrogen plasma treatment on SnO2:F substrates for amorphous Si thin film solar cells. Curr. Appl. Phys. 2013, 13, 1589–1593. [Google Scholar] [CrossRef]
- Xie, Q.; Musschoot, J.; Deduytsche, D.; Van Meirhaeghe, R.L.; Detavernier, C.; Van Den Berghe, S.; Jiang, Y.L.; Ru, G.P.; Li, B.Z.; Qu, X.P. Growth kinetics and crystallization behavior of TiO2 films prepared by plasma enhanced atomic layer deposition. J. Electrochem. Soc. 2008, 155, 688–692. [Google Scholar] [CrossRef]
- Dymond, E.G. Scattering of electrons in helium. Nature 1926, 118, 336–337. [Google Scholar] [CrossRef]
- Husein, S.; Stuckelberger, M.; West, B.; Ding, L.; Dauzou, F.; Morales-Masis, M.; Duchamp, M.; Holman, Z.; Bertoni, M.I. Carrier scattering mechanisms limiting mobility in hydrogen-doped indium oxide. J. Appl. Phys. 2018, 123, 245102. [Google Scholar] [CrossRef]
- Villamagua, L.; Stashans, A.; Carini, M.; Maldonado, F. Doping of SnO2 with H atoms: An alternative way to attain n-type conductivity. AIP Adv. 2016, 6, 115217. [Google Scholar] [CrossRef] [Green Version]
- Baco, S.; Chik, A.; Yassin, F.M. Study on optical properties of tin oxide thin film at different annealing temperature. J. Sci. Technol. 2012, 4, 61–72. [Google Scholar]
- Tauc, J.C. Optical Properties of Solid; Abeles, F., Ed.; North-Holland Publishing: Amsterdam, The Netherlands, 1972; p. 279. [Google Scholar]
- Kim, S.; Kim, D.-H.; Hong, S.-H. Epitaxial growth of orthorhombic SnO2 films on various YSZ substrates by plasma enhanced atomic layer deposition. J. Cryst. Growth 2012, 348, 15–19. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, J.H.; Han, S.H.; Park, B.K.; Chung, T.-M.; Han, J.H. Effect of Oxygen Source on the Various Properties of SnO2 Thin Films Deposited by Plasma-Enhanced Atomic Layer Deposition. Coatings 2020, 10, 692. https://doi.org/10.3390/coatings10070692
Won JH, Han SH, Park BK, Chung T-M, Han JH. Effect of Oxygen Source on the Various Properties of SnO2 Thin Films Deposited by Plasma-Enhanced Atomic Layer Deposition. Coatings. 2020; 10(7):692. https://doi.org/10.3390/coatings10070692
Chicago/Turabian StyleWon, Jong Hyeon, Seong Ho Han, Bo Keun Park, Taek-Mo Chung, and Jeong Hwan Han. 2020. "Effect of Oxygen Source on the Various Properties of SnO2 Thin Films Deposited by Plasma-Enhanced Atomic Layer Deposition" Coatings 10, no. 7: 692. https://doi.org/10.3390/coatings10070692



