Diamond-Like Carbon Films with Low Internal Stress by a Simple Bilayer Approach
Abstract
1. Introduction
2. Materials and Methods
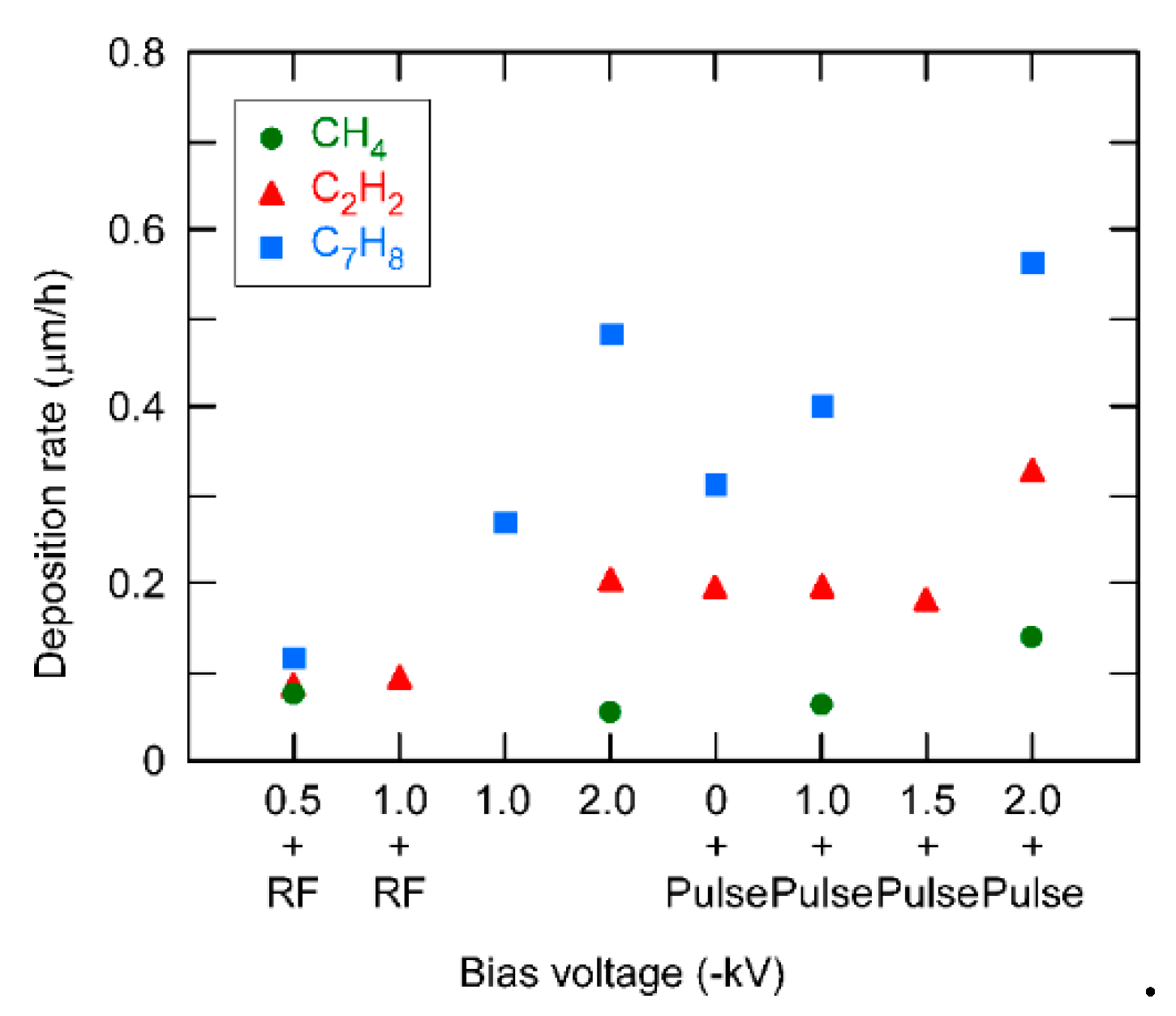
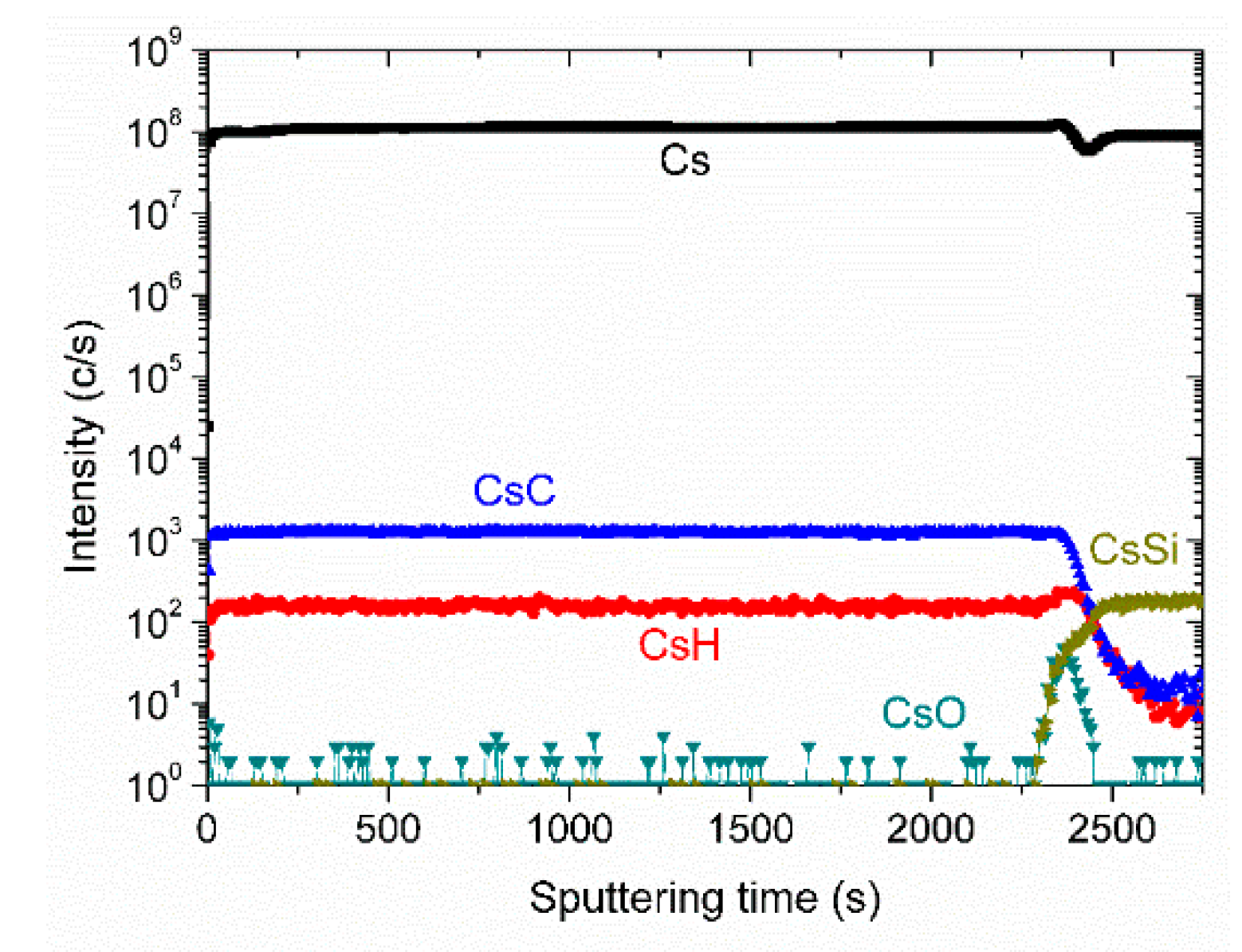
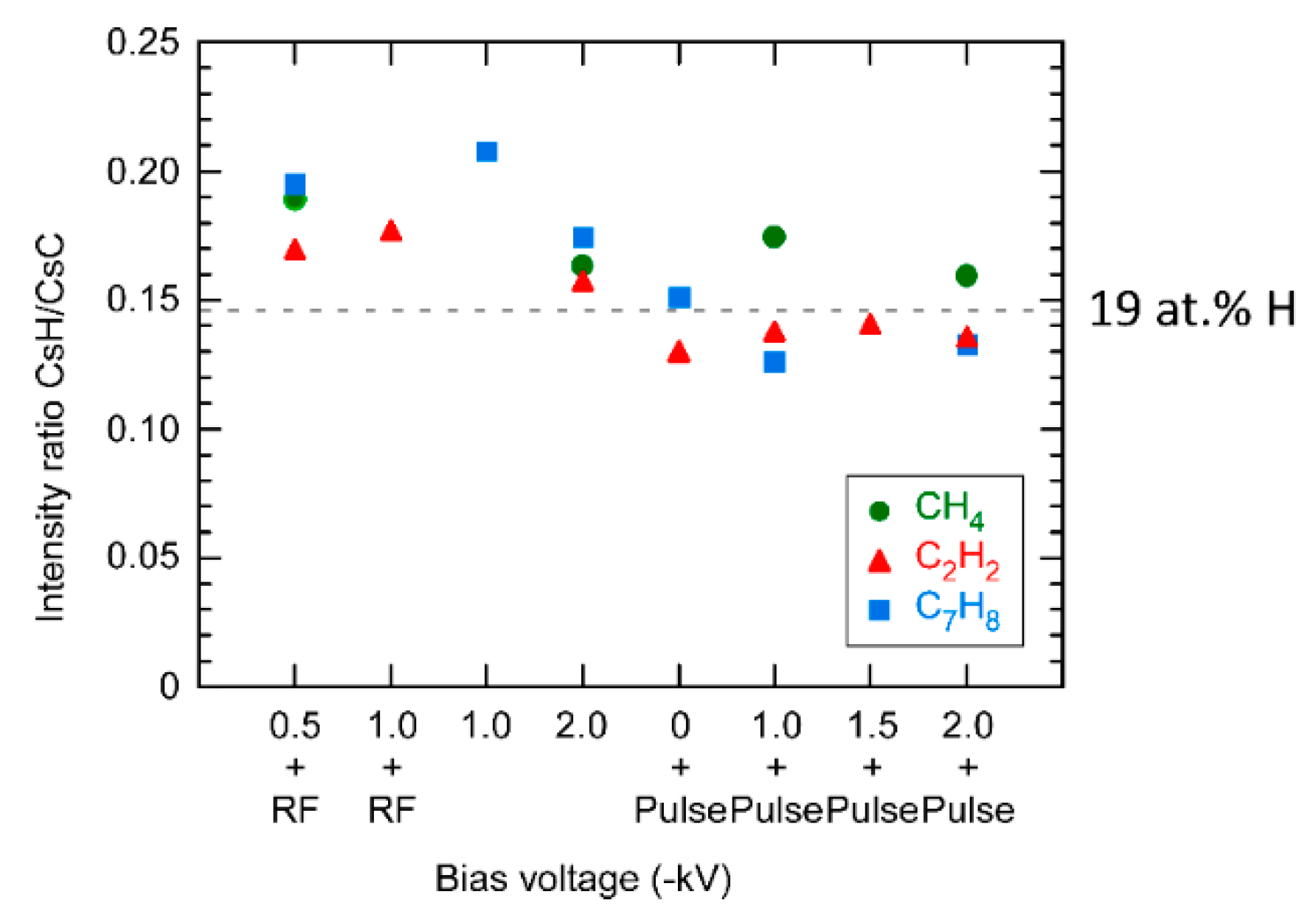
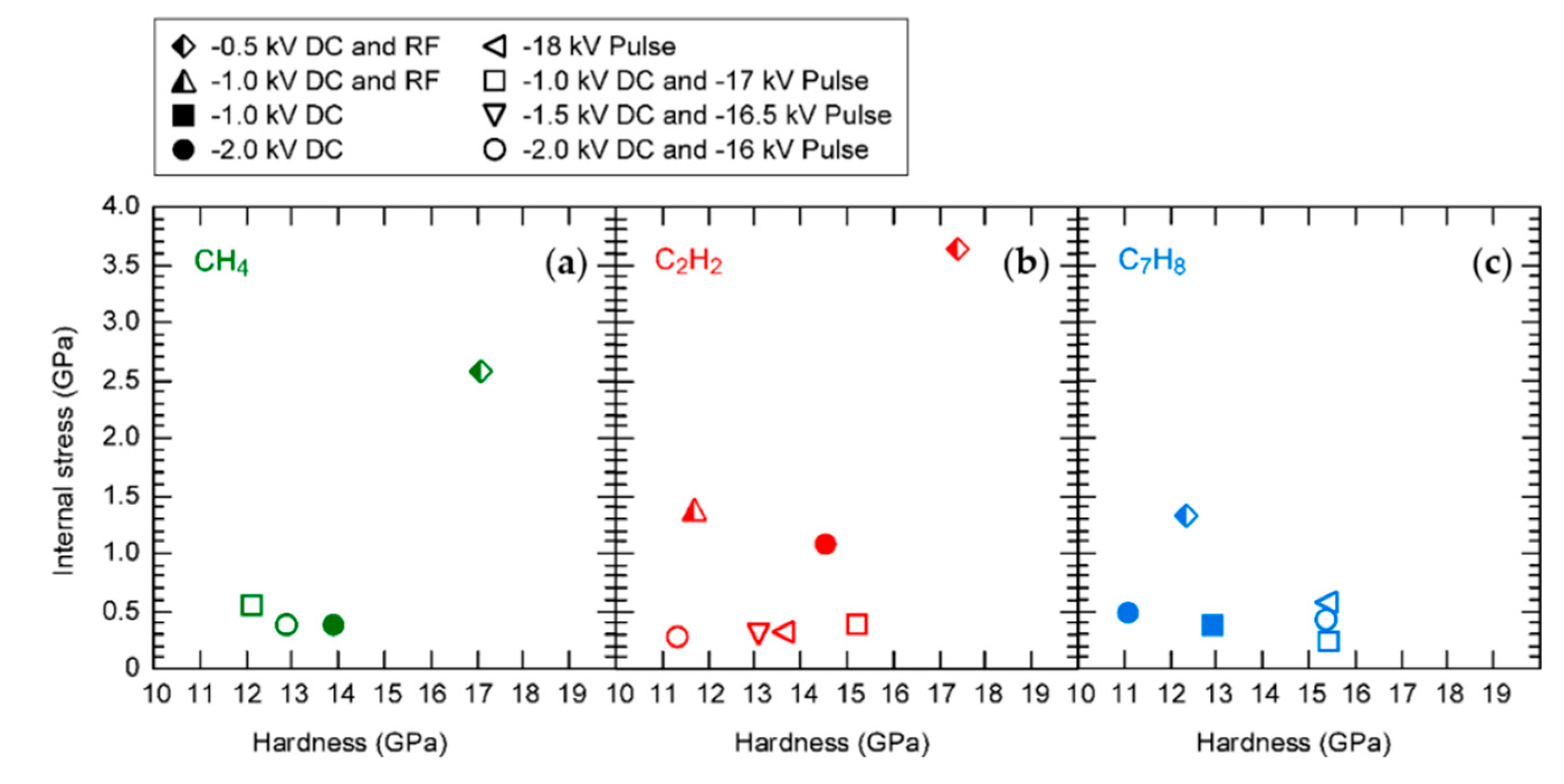
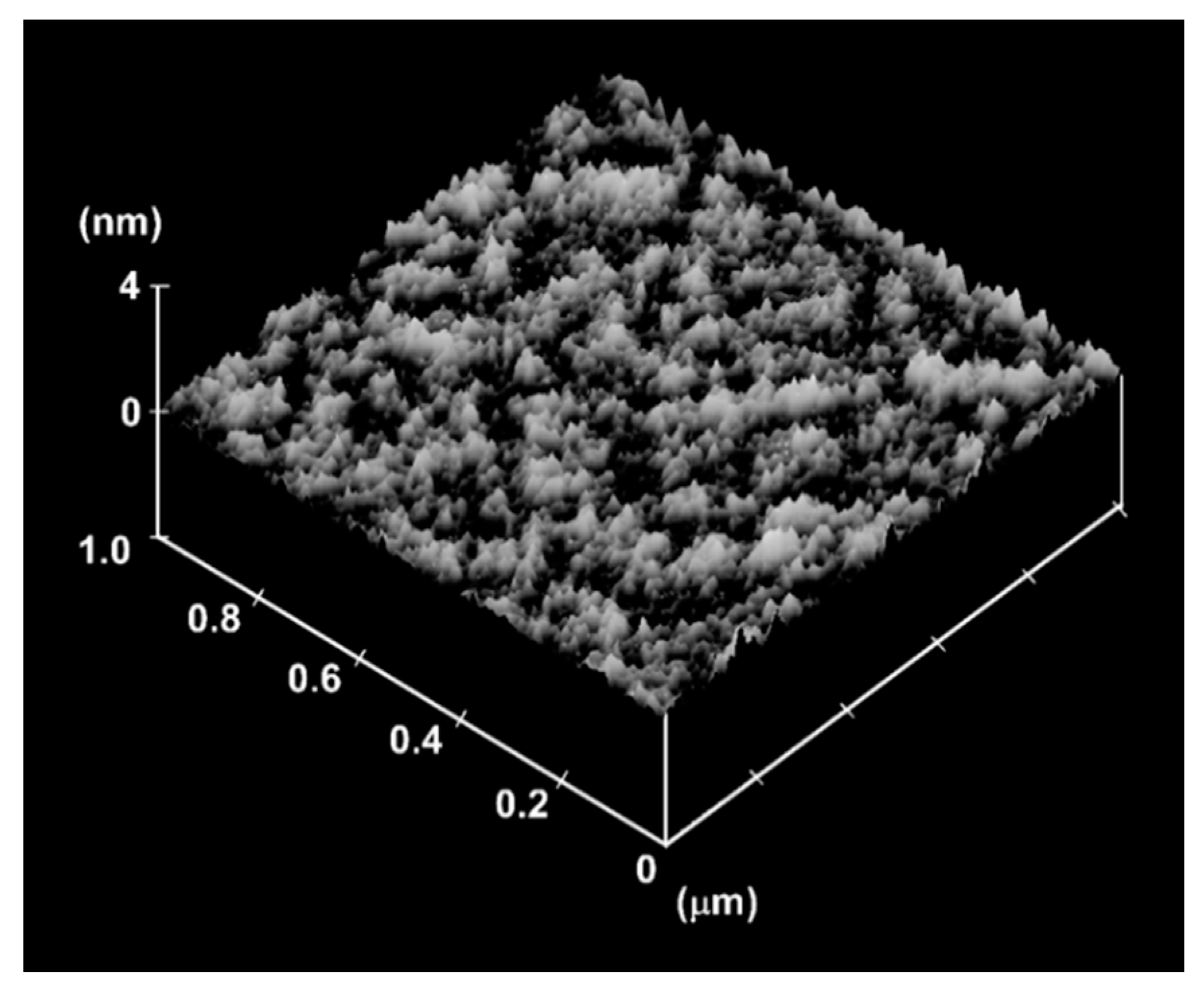
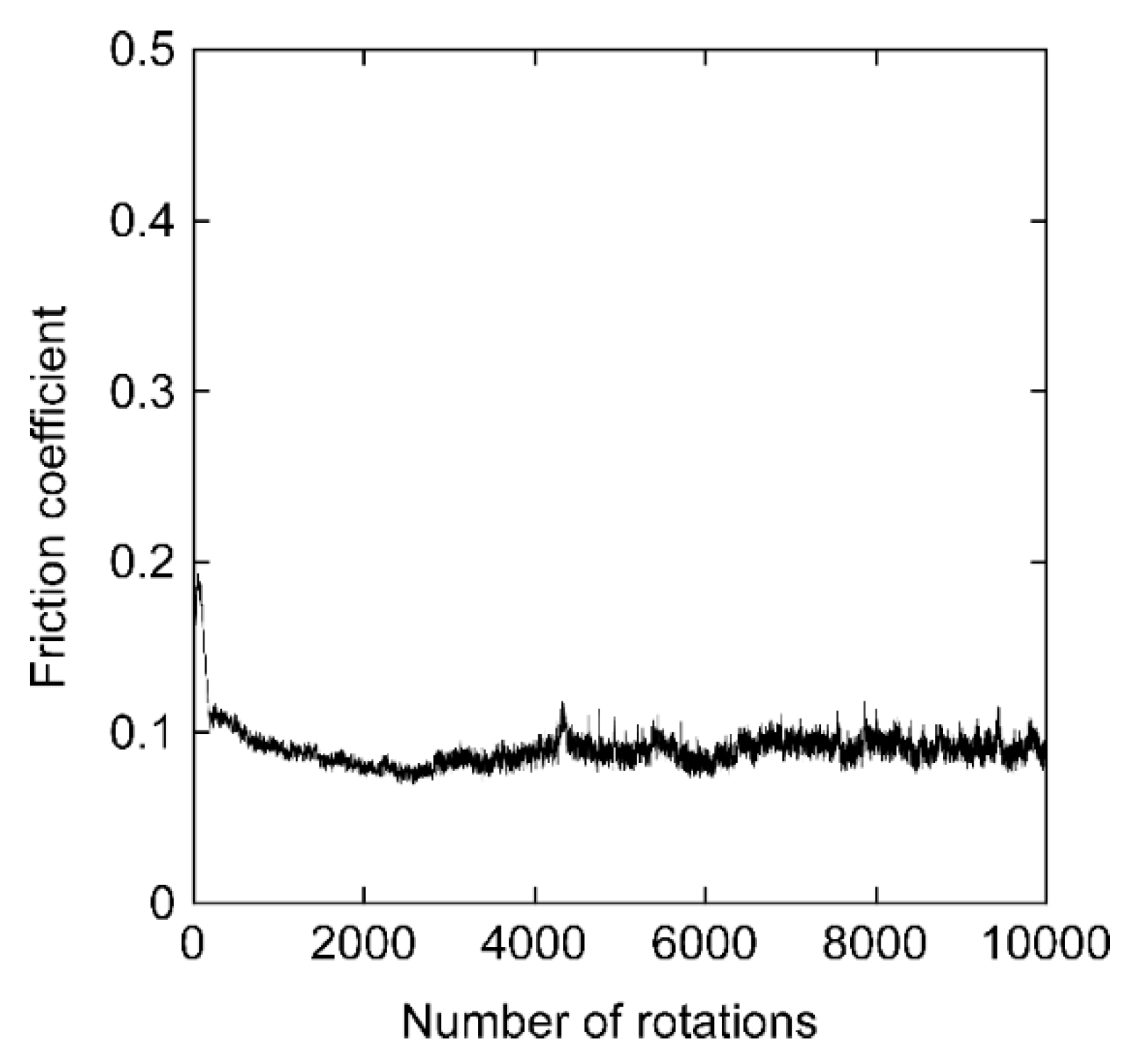
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Demichelis, F.; Pirri, C.F.; Tagliaferro, A.; Benedetto, G.; Boarino, L.; Spagnolo, R.; Dunlop, E.; Haupt, J.; Gissler, W. Mechanical and thermophysical properties of diamond-like carbon (DLC) films with different sp3sp2 ratios. Diam. Relat. Mater. 1993, 2, 890–892. [Google Scholar] [CrossRef]
- Savvides, N.; Bell, T.J. Hardness and elastic modulus of diamond and diamond-like carbon films. Thin Solid Films 1993, 228, 289–292. [Google Scholar] [CrossRef]
- Hatada, R.; Flege, S.; Ashraf, M.N.; Timmermann, A.; Schmid, C.; Ensinger, W. The Influence of preparation conditions on the structural properties and hardness of diamond-like carbon films, prepared by plasma source ion implantation. Coatings 2020, 10, 360. [Google Scholar] [CrossRef]
- Wei, C.; Yang, J.-F. A finite element analysis of the effects of residual stress, substrate roughness and non-uniform stress distribution on the mechanical properties of diamond-like carbon films. Diam. Relat. Mater. 2011, 20, 839–844. [Google Scholar] [CrossRef]
- Pauleau, Y. Residual stresses in dlc films and adhesion to various substrates. In Tribology of Diamond-Like Carbon Films: Fundamentals and Applications; Donnet, C., Erdemir, A., Eds.; Springer: Boston, MA, USA, 2008; pp. 102–136. ISBN 978-0-387-49891-1. [Google Scholar]
- Marques, F.C.; Lacerda, R.G.; Champi, A.; Stolojan, V.; Cox, D.C.; Silva, S.R.P. Thermal expansion coefficient of hydrogenated amorphous carbon. Appl. Phys. Lett. 2003, 83, 3099–3101. [Google Scholar] [CrossRef]
- Friedmann, T.A.; Sullivan, J.P.; Knapp, J.A.; Tallant, D.R.; Follstaedt, D.M.; Medlin, D.L.; Mirkarimi, P.B. Thick stress-free amorphous-tetrahedral carbon films with hardness near that of diamond. Appl. Phys. Lett. 1997, 71, 3820–3822. [Google Scholar] [CrossRef]
- Zhang, W.; Tanaka, A.; Wazumi, K.; Koga, Y.; Xu, B.S. The effect of annealing on mechanical and tribological properties of diamond-like carbon multilayer films. Diam. Relat. Mater. 2004, 13, 2166–2169. [Google Scholar] [CrossRef]
- Sánchez-López, J.C.; Fernández, A. Doping and alloying effects on DLC coatings. In Tribology of Diamond-like Carbon Films: Fundamentals and Applications; Donnet, C., Erdemir, A., Eds.; Springer: Boston, MA, USA, 2008; pp. 311–338. ISBN 978-0-387-49891-1. [Google Scholar]
- Bouabibsa, I.; Lamri, S.; Sanchette, F. Structure, mechanical and tribological properties of Me-Doped diamond-like carbon (DLC) (Me = Al, Ti, or Nb) hydrogenated amorphous carbon coatings. Coatings 2018, 8, 370. [Google Scholar] [CrossRef]
- Zou, C.W.; Wang, H.J.; Feng, L.; Xue, S.W. Effects of Cr concentrations on the microstructure, hardness, and temperature-dependent tribological properties of Cr-DLC coatings. Appl. Surf. Sci. 2013, 286, 137–141. [Google Scholar] [CrossRef]
- Cui, J.; Qiang, L.; Zhang, B.; Ling, X.; Yang, T.; Zhang, J. Mechanical and tribological properties of Ti-DLC films with different Ti content by magnetron sputtering technique. Appl. Surf. Sci. 2012, 258, 5025–5030. [Google Scholar] [CrossRef]
- Corbella, C.; Pascual, E.; Oncins, G.; Canal, C.; Andújar, J.L.; Bertran, E. Composition and morphology of metal-containing diamond-like carbon films obtained by reactive magnetron sputtering. Thin Solid Films 2005, 482, 293–298. [Google Scholar] [CrossRef]
- Conrad, J.R.; Dodd, R.A.; Worzala, F.J.; Qiu, X. Plasma source ion implantation: A new, cost-effective, non-line-of-sight technique for ion implantation of materials. Surf. Coat. Technol. 1988, 36, 927–937. [Google Scholar] [CrossRef]
- Anders, A. (Ed.) Handbook of Plasma Immersion Ion Implantation and Deposition; John Wiley & Sons: New York, NY, USA, 2000; ISBN 0-471-24698-0. [Google Scholar]
- Jeon, Y.; Park, Y.S.; Kim, H.J.; Hong, B.; Choi, W.S. Tribological properties of ultrathin DLC films with and without metal interlayers. J. Korean Phys. Soc. 2007, 51, 1124–1128. [Google Scholar] [CrossRef]
- Deng, J.; Braun, M. DLC multilayer coatings for wear protection. Diam. Relat. Mater. 1995, 4, 936–943. [Google Scholar] [CrossRef]
- Won, Y.J.; Ki, H. Effect of film gradient profile on adhesion strength, residual stress and effective hardness of functionally graded diamond-like carbon films. Appl. Surf. Sci. 2014, 311, 775–779. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Walck, S.D.; Zabinski, J.S. Architecture of multilayer nanocomposite coatings with super-hard diamond-like carbon layers for wear protection at high contact loads. Wear 1997, 203–204, 516–527. [Google Scholar] [CrossRef]
- Miyake, S.; Shindo, T.; Suzuki, M. Nanomechanical and boundary lubrication properties of titanium carbide and diamond-like carbon nanoperiod multilayer and nanocomposite films. Surf. Coat. Technol. 2013, 221, 124–132. [Google Scholar] [CrossRef]
- Pujada, B.R.; Tichelaar, F.D.; Janssen, G.C.A.M. Hardness of and stress in tungsten carbide–diamond like carbon multilayer coatings. Surf. Coat. Technol. 2008, 203, 562–565. [Google Scholar] [CrossRef]
- Guo, C.Q.; Pei, Z.L.; Fan, D.; Gong, J.; Sun, C. Microstructure and tribomechanical properties of (Cr, N)-DLC/DLC multilayer films deposited by a combination of filtered and direct cathodic vacuum arcs. Diam. Relat. Mater. 2015, 60, 66–74. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, Y.J.; Jiang, F.; Leng, Y.X.; Sun, H.; Huang, N. The microstructure and mechanical properties of multilayer diamond-like carbon films with different modulation ratios. Appl. Surf. Sci. 2013, 264, 207–212. [Google Scholar] [CrossRef]
- Logothetidis, S.; Gioti, M.; Charitidis, C.; Patsalas, P. A new process for the development of hard and stable sputtered amorphous carbon films. Vacuum 1999, 53, 61–65. [Google Scholar] [CrossRef]
- Anders, S.; Callahan, D.L.; Pharr, G.M.; Tsui, T.Y.; Singh Bhatia, C. Multilayers of amorphous carbon prepared by cathodic arc deposition. Surf. Coat. Technol. 1997, 94–95, 189–194. [Google Scholar] [CrossRef]
- Zhang, W.; Tanaka, A.; Xu, B.S.; Koga, Y. Study on the diamond-like carbon multilayer films for tribological application. Diam. Relat. Mater. 2005, 14, 1361–1367. [Google Scholar] [CrossRef]
- Lin, Y.; Zia, A.W.; Zhou, Z.; Shum, P.W.; Li, K.Y. Development of diamond-like carbon (DLC) coatings with alternate soft and hard multilayer architecture for enhancing wear performance at high contact stress. Surf. Coat. Technol. 2017, 320, 7–12. [Google Scholar] [CrossRef]
- Li, F.; Zhang, S.; Kong, J.; Zhang, Y.; Zhang, W. Multilayer DLC coatings via alternating bias during magnetron sputtering. Thin Solid Films 2011, 519, 4910–4916. [Google Scholar] [CrossRef]
- Lu, X.; Li, M.; Tang, X.; Lee, J. Micromechanical properties of hydrogenated diamond-like carbon multilayers. Surf. Coat. Technol. 2006, 201, 1679–1684. [Google Scholar] [CrossRef]
- Hatada, R.; Flege, S.; Ensinger, W.; Baba, K. Deposition of diamond-like carbon films on insulating substrates by plasma source ion implantation. Surf. Coat. Technol. 2020, 385, 125426. [Google Scholar] [CrossRef]
- Walter, K.C.; Nastasi, M.; Munson, C. Adherent diamond-like carbon coatings on metals via plasma source ion implantation. Surf. Coat. Technol. 1997, 93, 287–291. [Google Scholar] [CrossRef]
- Baba, K.; Hatada, R.; Nakao, S.; Miyagawa, S.; Miyagawa, Y. Miyagawa Formation of diamond like carbon films by plasma source ion implantation from CH4, C2H2 and C6H6. In Proceedings of the 1998 International Conference on Ion Implantation Technology, Proceedings (Cat. No.98EX144), IEEE, Kyoto, Japan, 22–26 June 1998; 2, pp. 1214–1217. [Google Scholar]
- Baba, K.; Hatada, R. Deposition of diamond-like carbon films by plasma source ion implantation with superposed pulse. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 206, 708–711. [Google Scholar] [CrossRef]
- Hatada, R.; Flege, S.; Ensinger, W.; Hesse, S.; Tanabe, S.; Nishimura, Y.; Baba, K. Preparation of Aniline-Based Nitrogen-Containing Diamond-Like Carbon Films with Low Electrical Resistivity. Coatings 2020, 10, 54. [Google Scholar] [CrossRef]
- Muguruma, T.; Iijima, M.; Kawaguchi, M.; Mizoguchi, I. Effects of sp2/sp3 Ratio and Hydrogen Content on In Vitro Bending and Frictional Performance of DLC-Coated Orthodontic Stainless Steels. Coatings 2018, 8, 199. [Google Scholar] [CrossRef]
- Stoney, G.G.; Parsons, C.A. The tension of metallic films deposited by electrolysis. Proc. R. Soc. Lond. Ser. A 1909, 82, 172–175. [Google Scholar] [CrossRef]
- Gao, Y. A new secondary ion mass spectrometry technique for III-V semiconductor compounds using the molecular ions CsM+. J. Appl. Phys. 1988, 64, 3760–3762. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Chun, S.-Y.; Chayahara, A.; Horino, Y. Development of plasma-based ion implantation (PBII) techniques at Osaka National Research Institute (ONRI). Surf. Coat. Technol. 2001, 136, 32–35. [Google Scholar] [CrossRef]
- Ensinger, W. Formation of carbides and diamond-like carbon films by hydrocarbon plasma immersion ion implantation. In Plasma Surface Engineering Research and Its Practical Applications; Wei, R., Ed.; Research Signpost: Trivandrum, India, 2008; pp. 135–178. ISBN 978-81-308-0257-2. [Google Scholar]
- Choi, J.; Ishii, K.; Kato, T.; Kawaguchi, M.; Lee, W. Structural and mechanical properties of DLC films prepared by bipolar PBII&D. Diam. Relat. Mater. 2011, 20, 845–848. [Google Scholar] [CrossRef]
- Trava-Airoldi, V.J.; Bonetti, L.F.; Capote, G.; Fernandes, J.A.; Blando, E.; Hübler, R.; Radi, P.A.; Santos, L.V.; Corat, E.J. DLC film properties obtained by a low cost and modified pulsed-DC discharge. Thin Solid Films 2007, 516, 272–276. [Google Scholar] [CrossRef]
- Tai, F.C.; Lee, S.C.; Wei, C.H.; Tyan, S.L. Correlation between ID⁄IG ratio from visible Raman spectra and sp2/sp3 ratio from XPS spectra of annealed hydrogenated DLC film. Mater. Trans. 2006, 47, 1847–1852. [Google Scholar] [CrossRef]
- Grill, A. Review of the tribology of diamond-like carbon. Wear 1993, 168, 143–153. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baba, K.; Hatada, R.; Flege, S.; Ensinger, W. Diamond-Like Carbon Films with Low Internal Stress by a Simple Bilayer Approach. Coatings 2020, 10, 696. https://doi.org/10.3390/coatings10070696
Baba K, Hatada R, Flege S, Ensinger W. Diamond-Like Carbon Films with Low Internal Stress by a Simple Bilayer Approach. Coatings. 2020; 10(7):696. https://doi.org/10.3390/coatings10070696
Chicago/Turabian StyleBaba, Koumei, Ruriko Hatada, Stefan Flege, and Wolfgang Ensinger. 2020. "Diamond-Like Carbon Films with Low Internal Stress by a Simple Bilayer Approach" Coatings 10, no. 7: 696. https://doi.org/10.3390/coatings10070696
APA StyleBaba, K., Hatada, R., Flege, S., & Ensinger, W. (2020). Diamond-Like Carbon Films with Low Internal Stress by a Simple Bilayer Approach. Coatings, 10(7), 696. https://doi.org/10.3390/coatings10070696



