1. Introduction
Titanium alloy is an aerospace structural material with excellent high specific strength [
1]. In the process of the high-speed rotation of titanium alloy compressor blades of aero-engines, the blade tip is prone to failure due to scraping between the Al-Si polymer sealing coating of the engine casing and the titanium alloy blade tip [
2]. In order to enhance the wear resistance of the blade tip and maintain a reasonable gap between the blade tip and the engine casing, preparation technology for the surface coating is used to improve the wear resistance of the titanium alloy blade tip. At present, the coating preparation technologies that can be used to improve the wear resistance of titanium alloy materials mainly include thermal spraying, cold spraying, physical vapor deposition (PVD), micro-arc oxidation (MAO), laser cladding, electroplating, etc.
However, the bonding mechanisms of thermal spraying and cold spraying coatings with a metal substrate are primarily mechanical interlockings, which are prone to cause coat peeling under high temperatures and heavy loads due to the poor adhesion [
3,
4]. The coating prepared by PVD, which has excellent adhesion, is only a submicron-thin film [
5]. Numerous pores will be formed in the coating owing to the discharge during the process of MAO [
6]. The serious environmental pollution from the electroplating technology limits its further application [
7]. The laser cladding (LC) technology characteristic of metallurgical melting not only improves the surface performance of related materials but also guarantees the bonding strength between the coating and substrate, therefore, LC is suitable for preparing a hard particle reinforced wear-resistant coating [
8].
Metal matrix composites can be easily prepared on titanium alloy surfaces using LC. There are primarily two types of metal matrix composite coatings, which include ceramic particles directly added into metal matrix materials and ceramic particles formed by an in-situ reaction during the laser cladding process [
9]. For various engineering applications, the former is produced by the introduction of ceramic feedstocks, including carbide, nitride, boride, and oxide ceramic, into the laser molten pool. The latter is prepared by an in-situ reaction to form hard phases, such as TiC, TiN, TiB, WC, Al
2O
3, and other reinforced phases in the laser-generated melt pool [
9]. However, the hard particles of the composite coating require excellent machinability and abrasion resistance during the scraping process between the blade tip and casing sealing coating.
One hard synthetic material is cubic boron nitride (cBN) with a high hardness for mechanical cutting and grinding, similar to that of diamond. The thermal stability of cBN particles is much better than that of diamond [
10]. In the atmosphere, diamond will be prone to generate a graphitization phase transition as soon as the temperature reaches 600 °C, while cBN particles do not undergo phase transition until 1300 °C [
11,
12,
13]. Therefore, cBN is suitable for preparing a wear-resistant composite coating on a titanium alloy blade tip using LC, owing to its outstanding machinability and thermal stability [
14].
At present, the preparation of boron nitride reinforced composite coatings by LC is rarely studied. In industrial applications, boron nitride is typically divided into cubic boron nitride (cBN) and hexagonal boron nitride (hBN) [
14]. The majority of research with respect to hBN is focused on self-lubricating coatings. Lu et al. investigated the Ni60/h-BN self-lubricating coating prepared by laser cladding technology on 0Cr18Ni9 stainless steel substrates [
15]. However, the preparation of cBN reinforced composite coatings using laser cladding is rarely researched. Gupta et al. [
16] prepared boron carbide and cubic boron nitride reinforced Ti6Al4V matrix composites using continuous wave SPI fiber laser direct metal laser-sintering (DMLS) technology, and the results indicated that the Ti6Al4V-B
4C-cBN metal matrix composites showed good affinity with each other. This technology can also be used to prepare various intermetallic composite coatings.
Pacella et al. [
17] suggested that allotropic transformations of cBN into amorphous boron nitride and hBN were identified below the ablated surface and down to depths exceeding 300 nm in high laser thermal radiation. Thepsonthi et al. [
13] investigated the performance of cBN coated and uncoated micro-end mills in terms of the surface roughness, burr formation, and tool wear, aiming to improve the performance of carbide micro-end mills by applying a cBN coating. Yedave et al. [
18] investigated the synthesis and performance of a novel cBN-TiN composite coating, which was prepared in a two-step process, including an electrostatic spray coating of cBN particles and chemical vapor infiltration of TiN.
While the chemical and thermal stability of cBN particles are much better than that of diamond, it is difficult for cBN particles to maintain their original properties without phase transition and decomposition. In this paper, cBN/Ti6Al4V and NicBN/Ti6Al4V composite coatings were prepared by LC on a Ti6Al4V alloy substrate. The microstructure, phase composition, cBN particle evolution, and interface evolution between cBN and the Ti6Al4V matrix of the cBN/Ti6Al4V and NicBN/Ti6Al4V composite coatings were investigated, and the abrasion resistance of the composite coatings was evaluated.
2. Materials and Methods
2.1. Experimental Material
Specimens with dimensions of 100 × 50 × 10 mm
3 were machined from a Ti6Al4V substrate. The oxide layer was removed before laser cladding and the grease was cleaned with acetone. As shown in
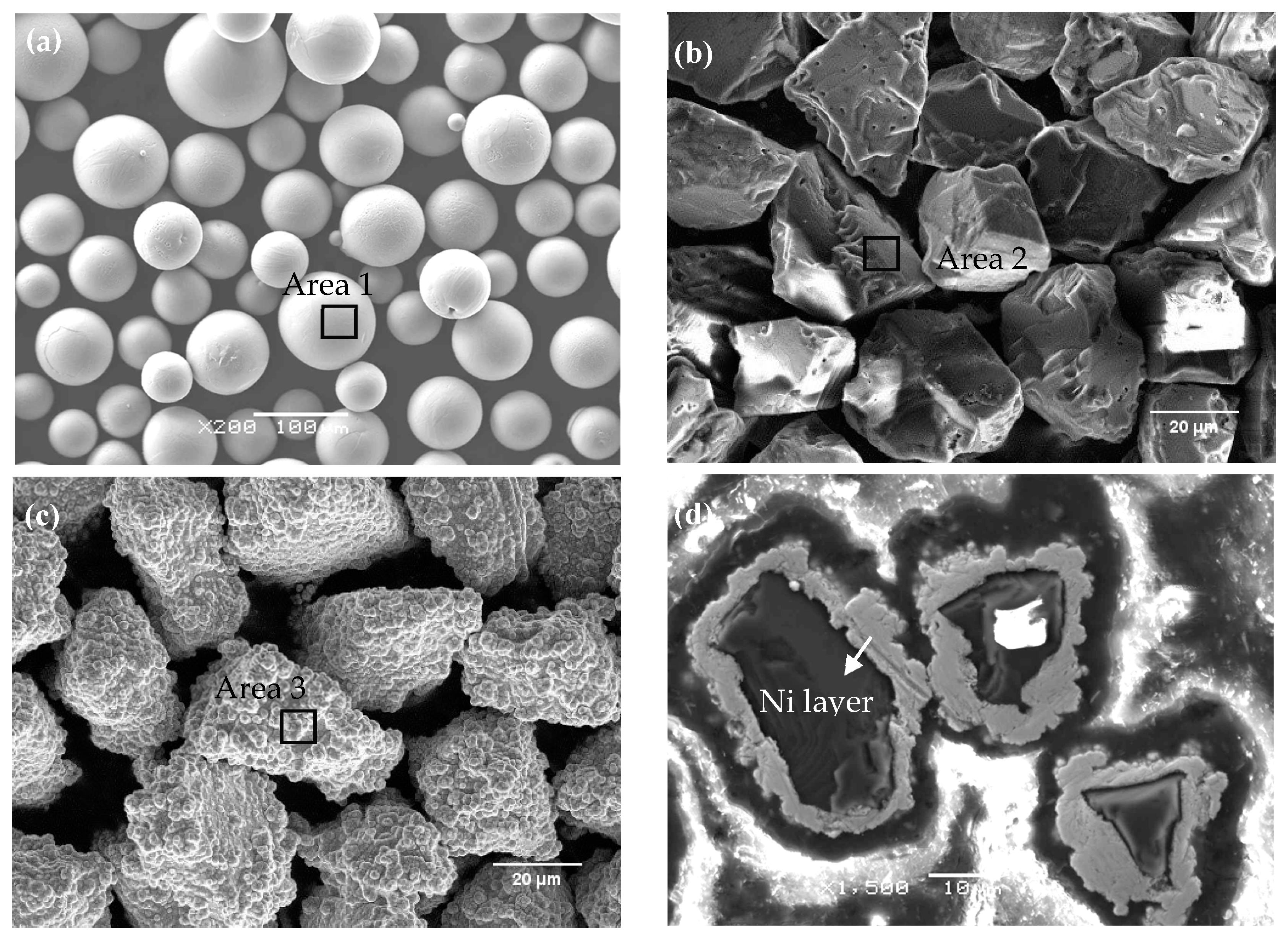
Figure 1a, commercially available Ti6Al4V alloy powder (Sino-Euro Materials Technologies of Xi′an Co., Ltd., Xi′an, China), with particle sizes ranging from 10 to 45 µm, was prepared by high-speed rotating atomization. As shown in
Figure 1b,c, the commercially available cubic boron nitride (cBN) powder and nickel-plated cBN powder (NicBN) (Mingshan New Materials Technologies of Nanjing Co., Ltd., Nanjing, China), with particle sizes ranging from 20 to 35 µm, were produced using the high temperature and high pressure method. The NicBN powder with 60% increasing particle weight was produced by chemical Ni-plating.
Figure 1d is the cross-section view of NicBN. As demonstrated in
Figure 1, the cBN and NicBN powder showed irregular shapes and the Ni-plating thickness of the NicBN particle was approximately 2–5 µm. As shown in
Table 1, the surface elements of Ti6Al4V, cBN, and NicBN particles were analyzed with energy dispersive spectroscopy (EDS). The results showed that the contents of the B and N elements on the surface of the cBN particles were 45.57 and 54.43 wt.%, and the contents of the P and Ni elements on the surface of the NicBN particles were 7.97 and 92.03 wt.%, respectively.
2.2. Preparation of Composite Coating
The pulsed Nd: YAG laser system (JHM-1GY-400D) was used to prepare the cBN/Ti6Al4V and NicBN-Ti6Al4V composite coatings in this study. The effective energy (
Econ) was used as an indicator to examine the effects of the processing parameters on the cladding quality and formability [
19]. In the continuous laser cladding process, this energy was responsible for heating/melting the substrate surface and powder stream and was calculated through Equation (1) [
19]. In the pulse laser cladding process, the effective energy can be expressed by Equation (2).
Here, P is the laser power (W), V is the scanning speed (mm/s), w is the pulse width (ms), and D is the laser spot size (mm). The pulse laser cladding track is composed of a large number of pulse cladding spots. The laser duration at each cladding spot is the pulse width, which is effective to reduce the immersed time for the cBN particles in the molten pool.
The particle volume ratios of cBN or NicBN vs. Ti6Al4V for producing the composite coatings raw material were the same (30 vol.% cBN + 70 vol.% Ti6Al4V and 30 vol.% NicBN + 70 vol.% Ti6Al4V). Both the cBN/Ti6Al4V and NicBN/Ti6Al4V composite coatings were prepared using the same process parameters. During the LC process, the parameters were 5 ms of pulse width, 150 A of current, 18 Hz of pulse frequency, 4 mm/s scanning speed, and 40% lap rate. The LC experiment was performed in a closed protective box filled with Ar gas. The box was made of high-temperature silica glass with a thickness of 3 mm. The laser beam passed through the silica glass to melt the composite powder. The schematic diagram is shown in
Figure 2. Before the experiment, Ti6Al4V alloy powder was dried at 110 °C in a vacuum oven for 60 min.
2.3. Characterization Methods
The cross-sectional coating specimens were cut by wire electrical discharge machining. The coating specimens were ground and polished like a mirror, and then etched for 10 s with a corrosive solution of 1 mL HF: 4 mL HNO3: 45 mL H2O. The composite coating specimens were examined using scanning electron microscopy (SEM, JSM-6460, JEOL, Tokyo, Japan) and X-ray diffraction (XRD, D8 Advance, Bruker, Germany) to analyze the microstructure of the composite coating, the distribution of cBN particles, and the phase composition. We investigated the composite coating and worn surface using energy dispersive spectroscopy (EDS, NanoXflash Detector 5010, Bruker, Germany). We used X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo Fisher Scientific, Waltham, MA, USA) to distinguish the binding energy of chemical elements. The XPS analysis was operated at 100.0 and 30.0 eV to generate monochromatic Al K alpha radiation; the spot sizes of the radiation were 500 and 650 μm; the energy step sizes were 1.0 and 0.1 eV.
The wear resistance of the composite coating was tested and the worn track morphology was evaluated using the ball-on-disc wear test and step profiler (WTM-2E) at room temperature, respectively. The pin was a Si3N4 ceramic ball with a 4 mm diameter. The disk was tested composite coatings. The test was performed under a load of 4.9 N at a rotational speed of 1000 rpm. The sliding time duration was 120 min and the radius of the wear track was 3 mm.
4. Discussion
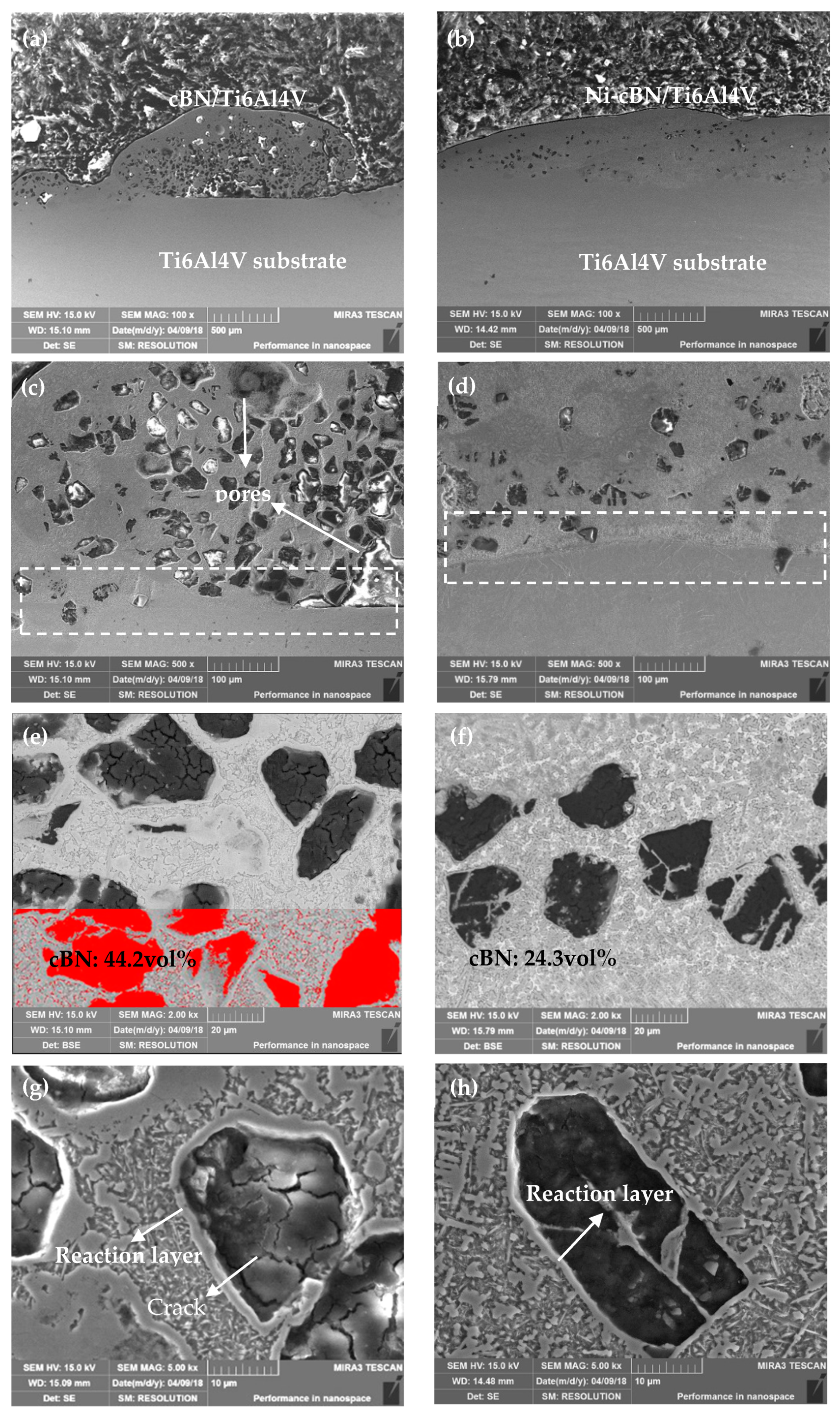
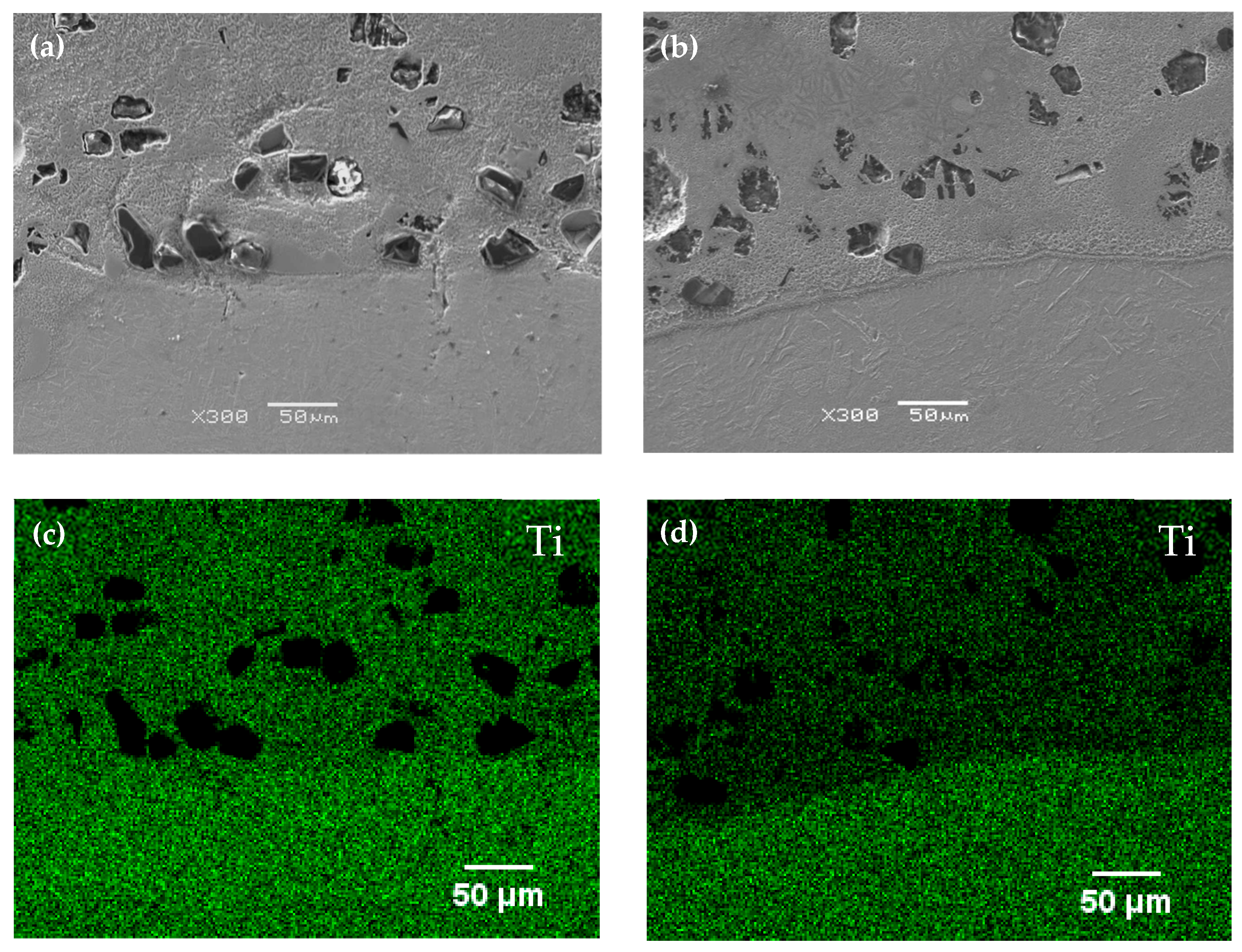
According to the microstructure of the cBN/Ti6Al4V and NicBN/Ti6Al4V composite coatings, Ni plating of cBN particles were beneficial for improving the quality of composite coatings and prevent thermal cracking defects in cBN particles in the laser molten pool. In addition, Ni plating of cBN particles avoided direct contact between the laser high-energy beam and cBN particles, which reduced the thermal impact on the cBN particles. The cBN particles of the composite coatings were sharp and angular, which indicates that the raw cBN particles were well retained in the LC composite coatings. However, a reaction layer with a thickness of 2–5 µm was formed on the bonding interface between the Ti6Al4V alloy matrix and cBN particles. The EDS results in the reaction layer indicated that Ti elements from the Ti6Al4V alloy and N elements from cBN could react to form a TiN reaction layer at the interface. Wang et al. also proved that the Ti elements of Co-based active filler metal produced a continuous reaction layer with a uniform thickness at the metal/cBN interface, when the brazing temperature was between 1473 and 1523 K [
20].
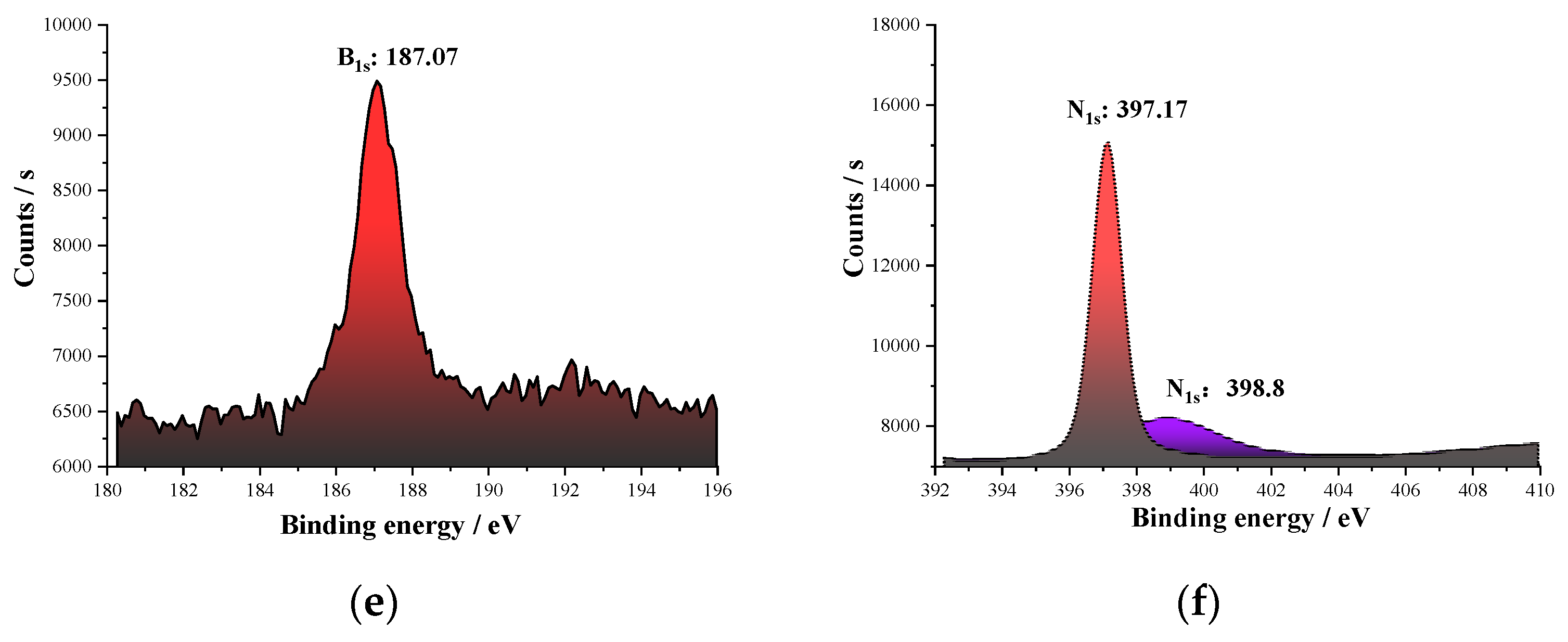
According to the XRD results of the NicBN/Ti6Al4V composite coating, there were Ti solid solution, TiN, TiBx, and BN phases in the composite coating. This demonstrates that the decomposition of the cBN particles in the laser molten pool were converted into TiBx and TiN phases. Compared with the XPS results of the original cBN particles, the binding energy of B in the composite coating was 187.07 eV, indicating that part of the B from the cBN particle was converted to the TiB phase; the standard binding energy of N element in the BN compound was 398 eV. The binding energies of N in cBN particles of the composite coating were 397.13 and 398.8 eV, which indicates that part of nitrogen is prone to react to form TiN. Wang et al. and Gupta et al. reported that the formation of TiN and TiB intermetallic compounds took place between reinforcement and the matrix composition and also contributed to the creation of the metallurgical bonding between the particles of Ti6Al4V/cBN [
16,
20].
B and N atoms in the cBN and Ti atoms in the Ti6Al4V alloy matrix clustered at the interface. The atomic radius of B and N were 0.082 and 0.075 nm, respectively, whereas the diffusion coefficients of B and N in Ti were 4.8 × 10
−7 exp (−5600/T) cm
2/s and 4.5 × 10
−3 exp(−6700/T) cm
2/s, respectively. The atomic radius of N was lower than that of B, whereas the diffusion coefficient of N in Ti was higher when compared with B. The N atom in the cBN particle will first react with the Ti atom. The remaining B atom is prone to acquire Ti from TiN and the Ti6Al4V alloy matrix, as B would react with TiN or the B would directly react with Ti6Al4V at the interface near the cBN [
16,
20,
21,
22]. The reaction at the interface is
According to the results, the formation mechanism of the interfacial reaction layer of cBN particles in the composite coating is shown in
Figure 12. The interfacial reaction of the cBN particles in the composite coating occurred in the laser molten pool. The active Ti atom from the Ti6Al4V alloy matrix easily acquired the N atom from the cBN particles, and then the Ti and N elements reacted to generate the TiN transition layer at the interface. Li et al. [
23] also proved that BN particles reacted with molten titanium to form TiN when the surface temperature was lower than the melting point of BN. The TiN reaction layer at the interface can act like a shield to effectively prevent cBN particles from further decomposing.
According to the wear results of the composite coatings, the friction coefficient of the NicBN/Ti6Al4V composite coating was lower than that of the cBN/Ti6Al4V composite coating. The SEM images of both composite coatings demonstrated that the cBN particles in the cBN/Ti6Al4V composite coating were severely cracked and fell off under the rolling action of the Si3N4 ball. On the contrary, the Ni coated cBN particle in the NicBN/Ti6Al4V composite coating exhibited a better resistance to damage.
Kuang et al. also found that the fracture evolution from strong to weak bonding strength of abrasive cBN particles was from trans-granular to inter-granular for cBN abrasives [
24]. The thermal conductivity (15.24 W/(m K)) of pure Ti is approximately one-third that of pure Fe. The friction heat accumulated in the composite coatings during the rotating sliding process helped to soften and oxidize the worn track. The cracked cBN debris under the action of rotating Si
3N
4 ball consequently aggravated the abrasive wear between the composite coating and the ball as a third body. Thus, the wear mechanisms of the composite coating were primarily adhesive wear, oxidation wear, and abrasive wear. The above results demonstrated that the Ni plating on the surface of the cBN particles and the TiN reaction layer could effectively protect the cBN particles from the thermal defects and retain the raw properties of the cBN particles. This is beneficial for improving the wear resistance of the composite coatings.
5. Conclusions
The denser NicBN/Ti6Al4V composite coating on the Ti6Al4V substrate was achieved in comparison with the cBN/Ti6Al4V composite coating using the LC process. The cBN particles retained the original shape in the composite coating. Thermal cracks were found on the surface of the cBN particles of the cBN/Ti6Al4V composite coating. The cBN particles in both composite coatings were coated with a 2–5 µm reaction layer between the cBN particles and Ti6Al4V matrix. The XRD, EDS, and XPS results showed that the active Ti element from the Ti6Al4V alloy matrix and the N element from the surface of the cBN particles reacted to generate the TiN transition layer at the interface. The TiN transition layer effectively prevented the further decomposition of the cBN particles like a shield.
The NicBN/Ti6Al4V composite coating presented better resistance to damage in comparison with the cBN/Ti6Al4V composite coating in the dry abrasive process, due to the Ni plating on the surface of the cBN particles, which effectively reduced the cracking and fall off of cBN particles under the rolling action of the Si3N4 ball. The wear mechanisms of the composite coating were primarily adhesive wear, oxidation wear, and abrasive wear. Ni plating on the surface of the cBN particles and the TiN reaction layer formed in the laser cladding process demonstrated a protective effect in preventing thermal defects and retaining the raw properties of the cBN particles. The discovery of the TiN reaction layer at the interface near the cBN particles in both the composite coatings can provide a new method to restrain the decomposition of cBN particles in the LC process.