Investigation of the Surface Integrity of Q345 Steel After Nd:YAG Laser Cleaning of Oxidized Mining Parts
Abstract
:1. Introduction
2. Materials and Experimental Methods
2.1. Experimental Setup
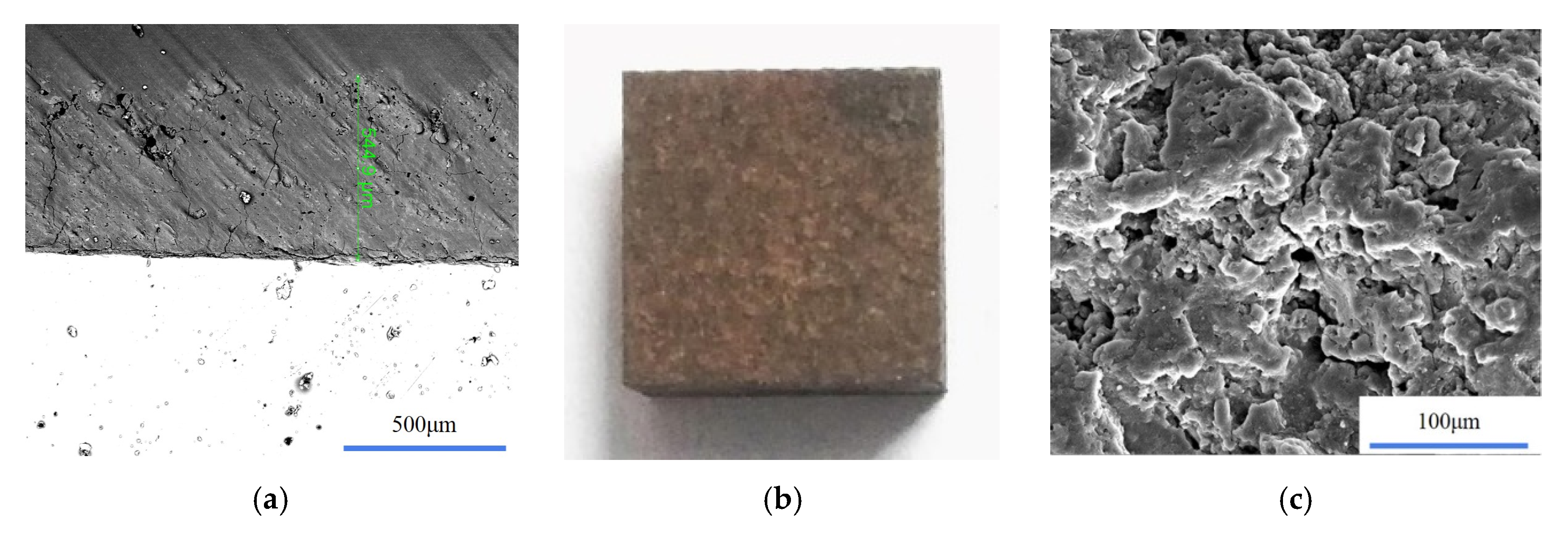
2.2. Sample Preparation
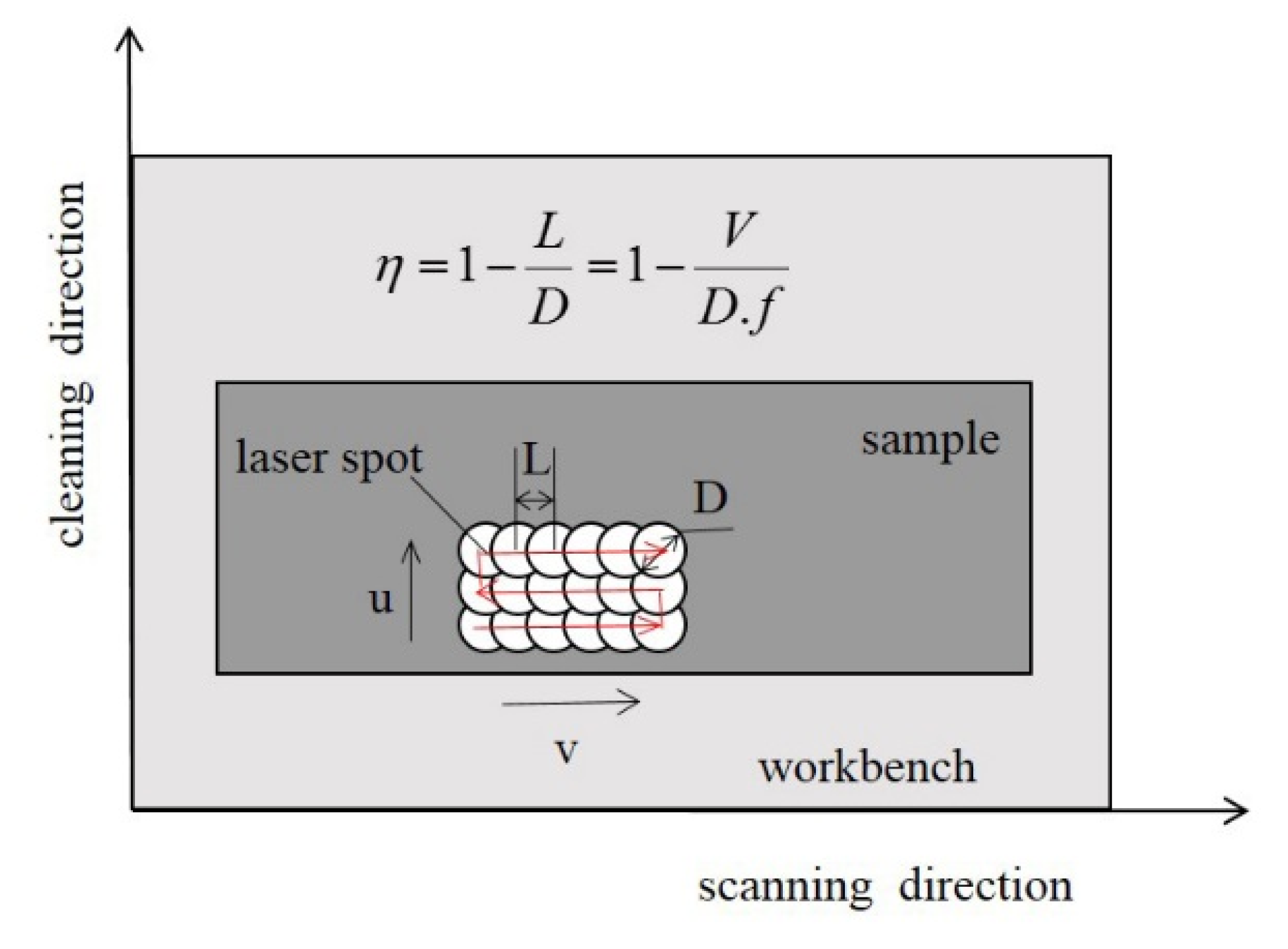
2.3. Experimental and Analytical Methods
3. Results and Discussion
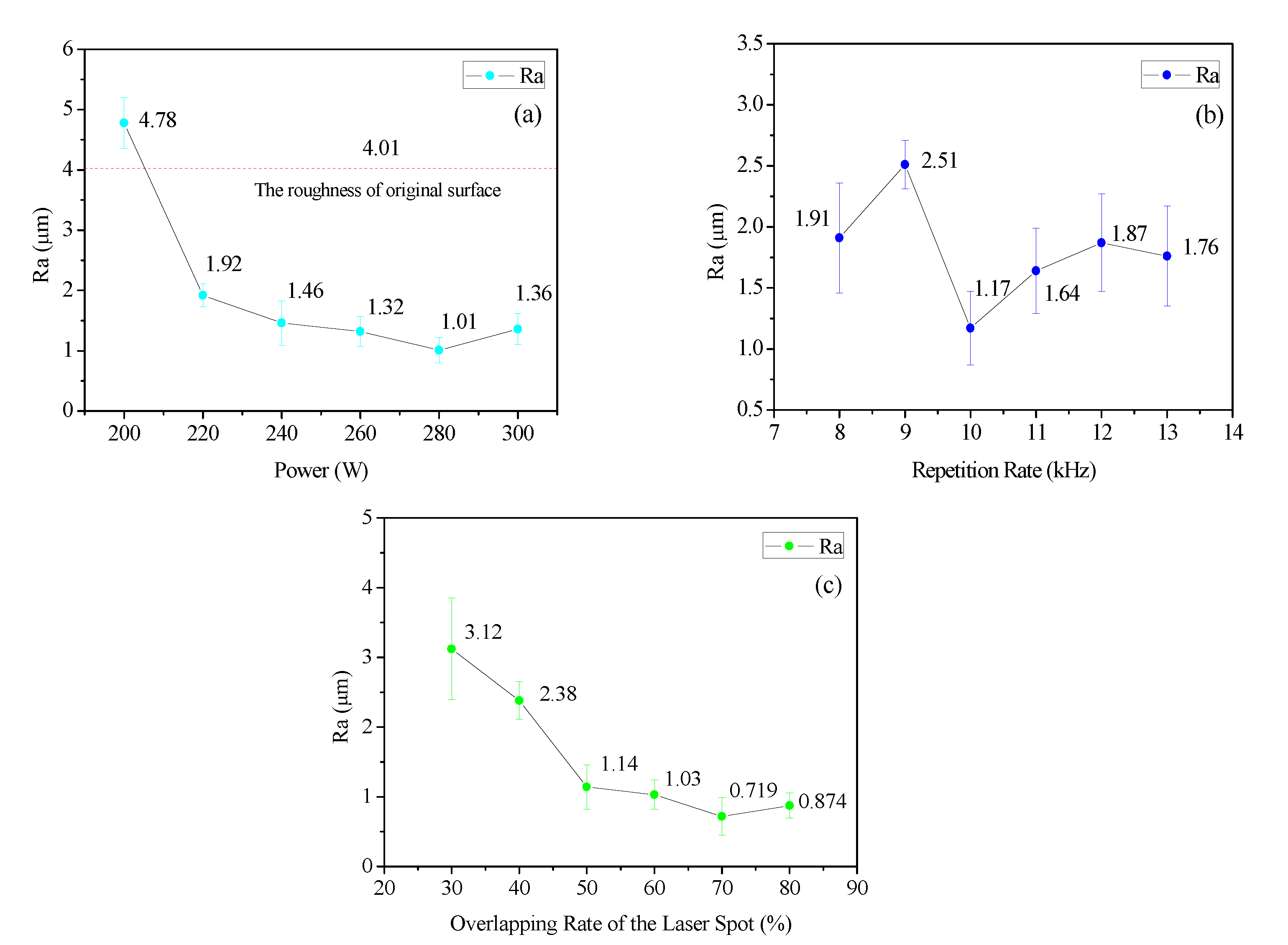
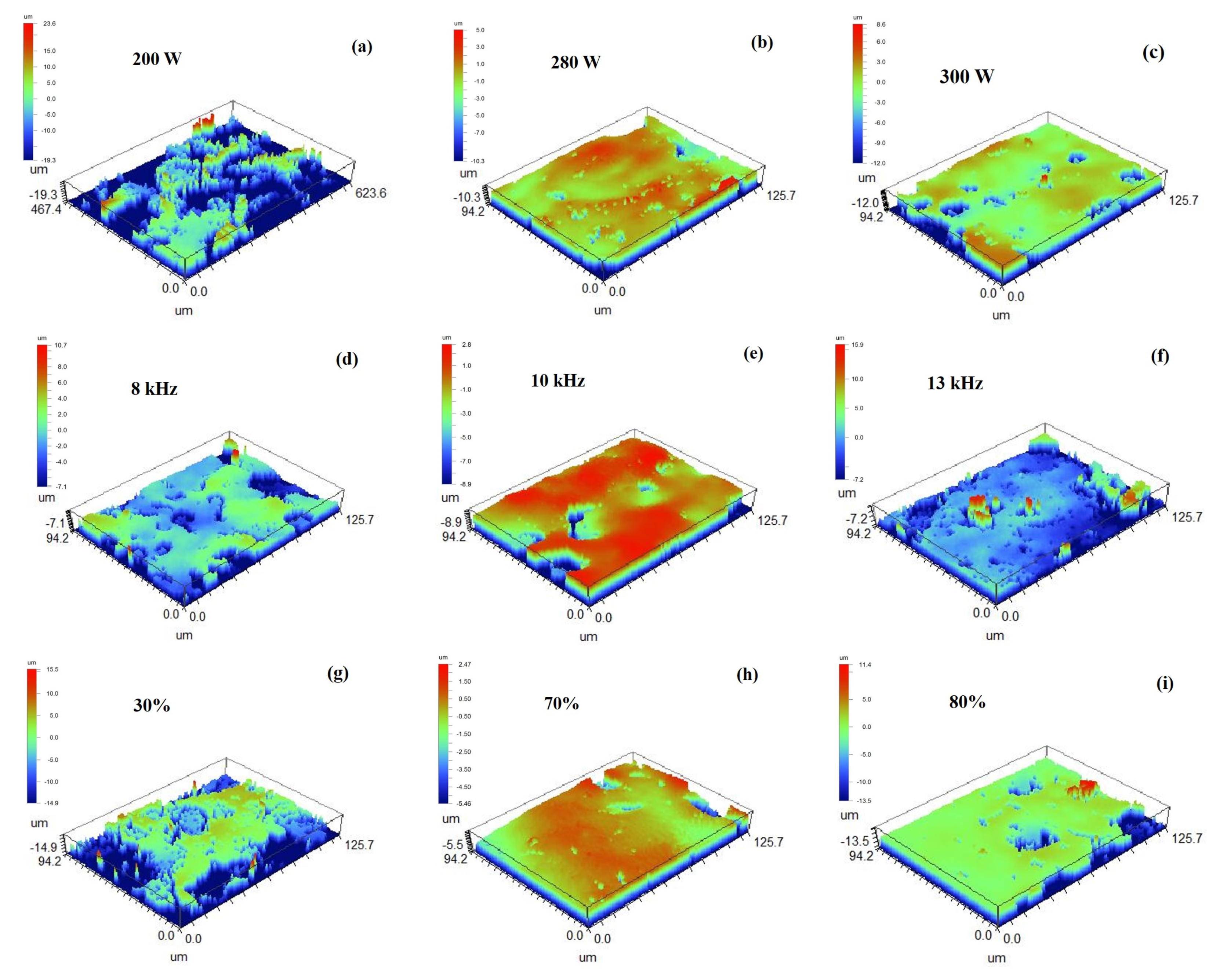
3.1. Surface Topography
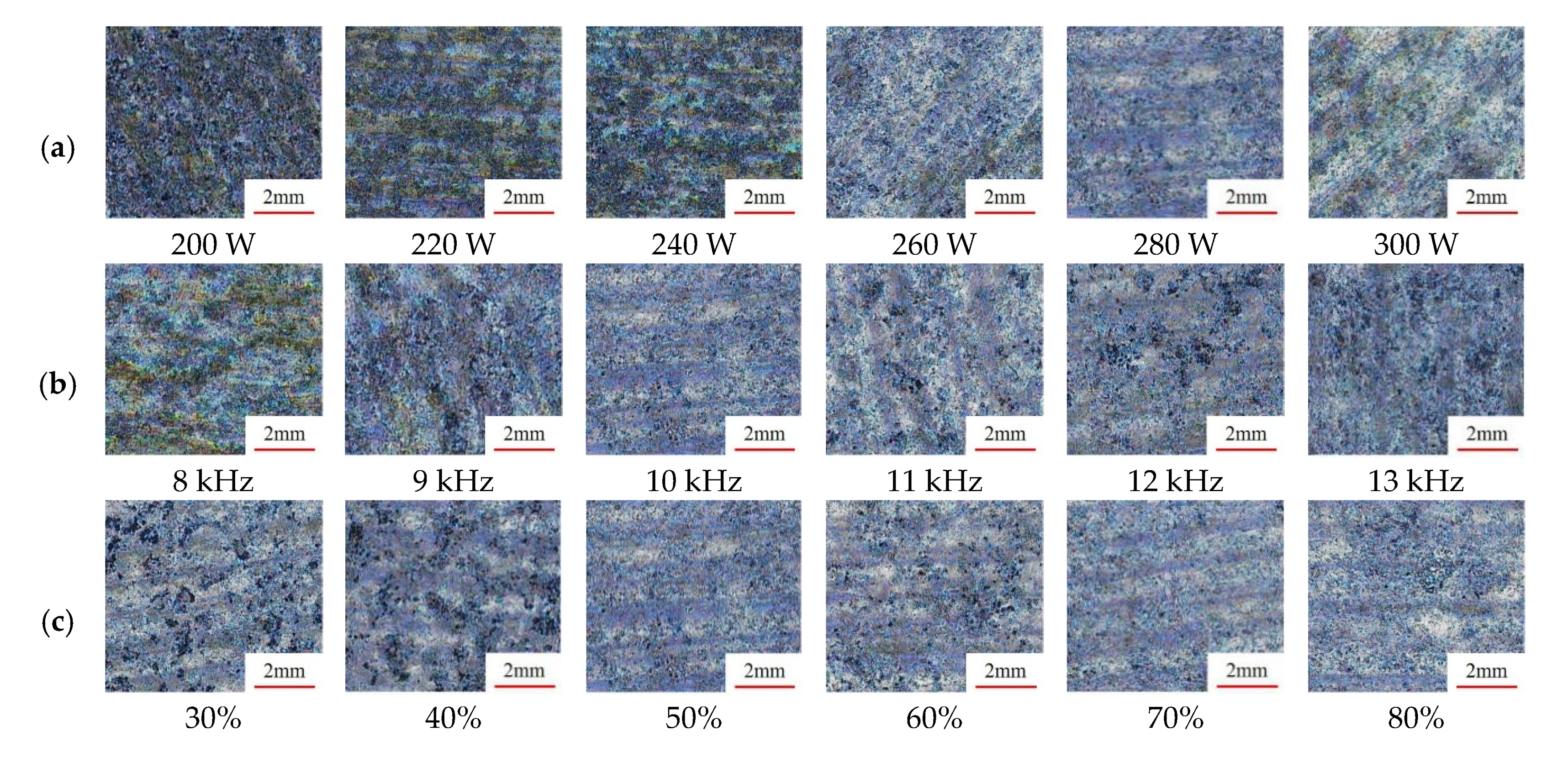
3.1.1. Macroscopic Morphology
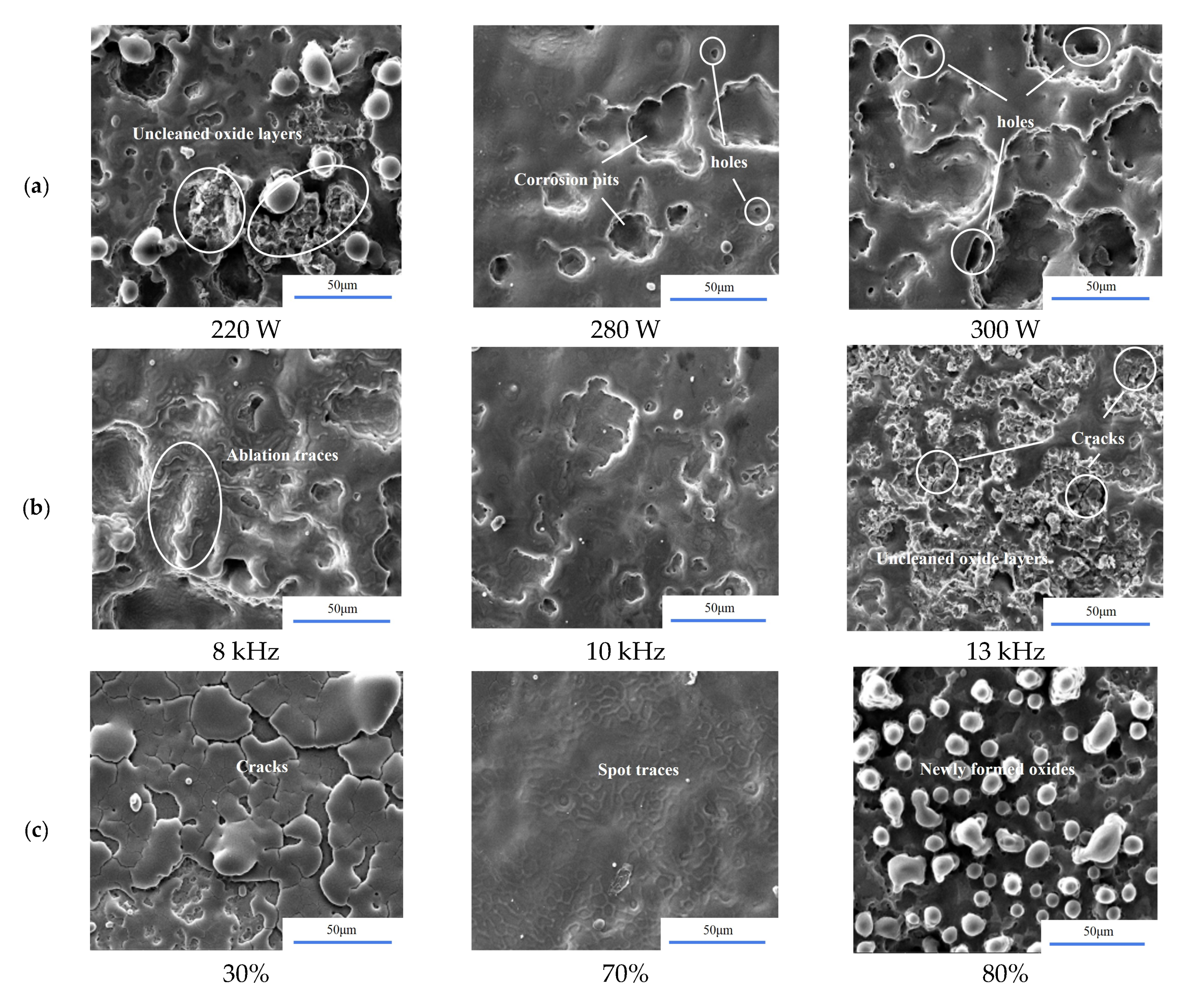
3.1.2. Microscopic Morphology
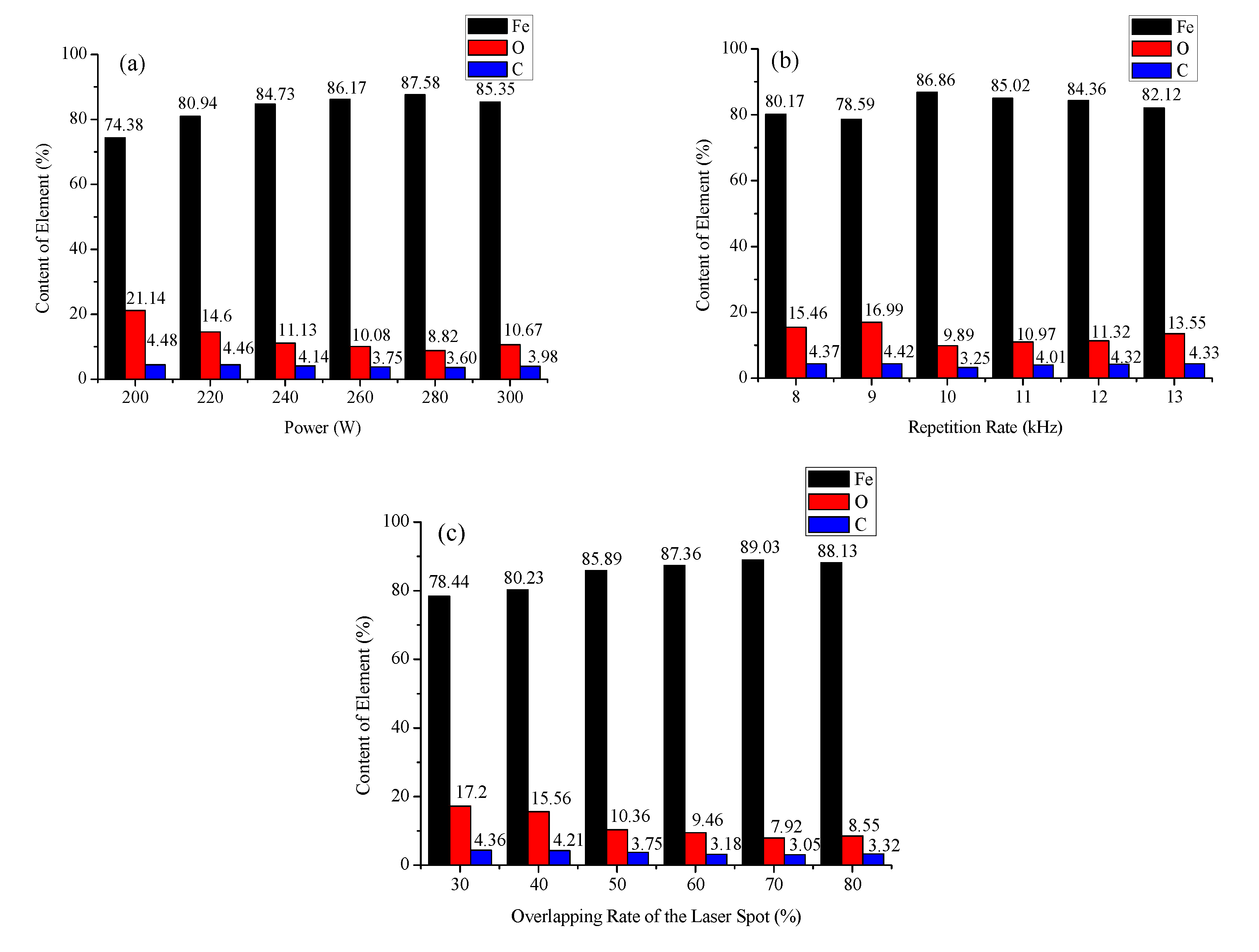
3.2. Element Content
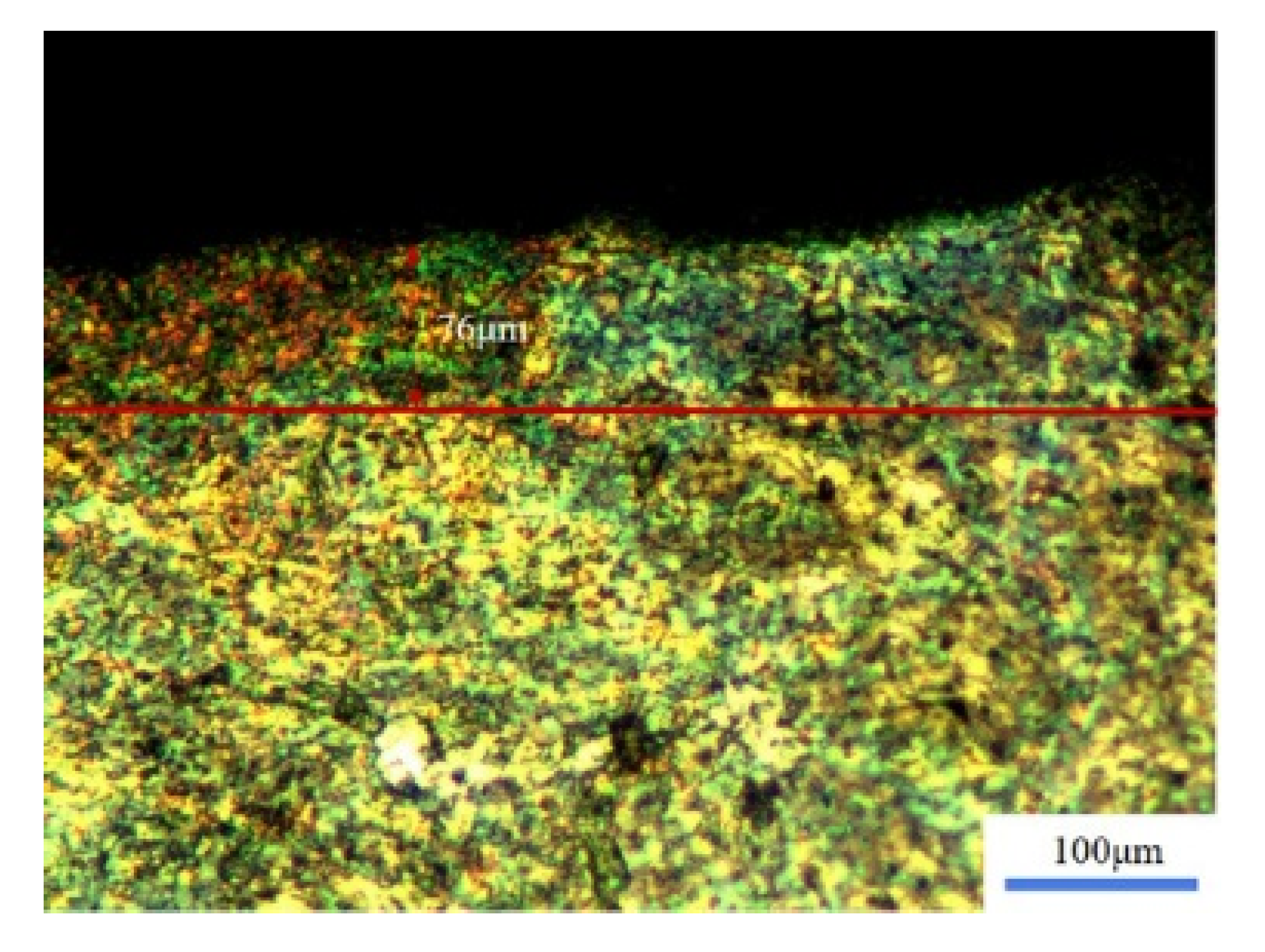
3.3. Microhardness
3.4. Corrosion Resistance
4. Conclusions
- (1)
- The laser cleaning technology had a good removal effect on the oxide layer and other attachments from the surface of the mining parts. When the laser power was 280 W, the repetition rate was 10 kHz (pulse duration 84 ns), and the overlapping rate of the laser spots was 70%, the surface oxygen content was the lowest and the removal effect was the best.
- (2)
- For the 3D morphology on the surface of the sample before and after cleaning, the surface roughness gradually decreased as the heat input per unit of area increased, but when the heat input was too high, ablation pits and new oxides appeared on the surface of the sample, which led to a slight upward trend in the surface roughness. In this process, two main cleaning mechanisms existed, i.e., an ablation effect and a thermal stress effect, to remove oxides during the cleaning process.
- (3)
- The laser cleaning had a significant effect on the microhardness of the sample’s surface. Under the action of laser cleaning, the surface grain was refined and the resistance to dislocations generated between the grain boundaries increased. What is more, the impact effect of the laser spots under a high repetition rate or high pulse duration had the largest influence in the improvement of the hardness. Compared to the original sample, when increasing the rate of the surface hardness value, it was able to reach 60%.
- (4)
- After laser cleaning, the corrosion resistance of the surface of the mining sample was obviously improved. With an increase in the laser power and the overlapping rate of the laser spots, the surface’s anti-corrosion performance gradually improved. When the power and overlapping rate were too high, a new oxide layer and ablation pits appeared on the surface of the mining parts, which resulted in a lower corrosion resistance. The optimal corrosion resistance was improved by more than 62.9% compared to the original sample.
Author Contributions
Funding
Conflicts of Interest
References
- Choi, J.P.; Lee, S.J. Efficient chip breaker design by predicting the chip breaking performance. Int. J. Adv. Manuf. Technol. 2001, 17, 489–497. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Liang, C.C.; Teng, T.L.; Nguyen, A.T. A numerical study on high-speed water jet impact. Ocean Eng. 2013, 72, 98–106. [Google Scholar] [CrossRef]
- Watkins, K.G. Mechanisms of laser cleaning. In Proceedings of the High-Power Lasers in Manufacturing, Osaka, Japan, 7 February 2000; pp. 165–174. [Google Scholar]
- Ristic, S.; Polic, S. Laser cleaning of textile artifacts with metal threads: Process parameter optimization. Sci. Tech. Rev. 2014, 64, 45–52. [Google Scholar]
- Ke, L.; Zhu, H.; Lei, W.; Cheng, Z. Laser cleaning of rust on ship steel using TEA CO2 pulsed laser. In Proceedings of the Photonics and Optoelectronics Meetings (POEM) 2009: Industry Lasers and Applications, Wuhan, China, 21 October 2009; p. 75150G. [Google Scholar]
- Kayahana, E.; Candana, L.; Arasb, M.; Gundogdu, O. Surface cleaning of metals using low power fiber lasers. Acta. Phys. Pol. A 2018, 134, 371–373. [Google Scholar] [CrossRef]
- Iacopo, O.; Salvatore, S. Dependence of Nd:YAG laser derusting and passivation of iron artifacts on pulse duration. In Proceedings of the Fundamentals of Laser-Assisted Micro- and Nanotechnologies 2013, Petersburg, Russia, 24–28 June 2013; p. 906513. [Google Scholar]
- Ali, S.N.; Taha, Z.A.; Mansour, T.S. Laser cleaning using q-switched Nd:YAG laser of low carbon steel alloys. Adv. Condens. Matter. Phys. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.N.; Wong, W.Y.; Walvekar, R.; Kadhum, A.A.H.; Khalid, M. Parametric optimization of pulsed laser ablation on stainless steel for improving corrosion resistance by Taguchi method. Mater. Res. Express 2018, 6, 26533. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, X.; Huang, W. Parameters and surface performance of laser removal of rust layer on A3 steel. Surf. Coat. Technol. 2003, 166, 10–16. [Google Scholar] [CrossRef]
- Choubey, A.; Vishwakarma, S.C.; Vachhani, D.M.; Singh, R.; Misra, P.; Jain, R.K.; Arya, R.; Upadhyaya, B.N.; Oak, S.M. Study and development of 22 kW peak power fiber coupled short pulse Nd:YAG laser for cleaning applications. Opt. Lasers Eng. 2014, 62, 69–79. [Google Scholar] [CrossRef]
- Salimbeni, R.; Pini, R.; Siano, S. A variable pulse width Nd:YAG laser for conservation. J. Cult. Herit. 2003, 4, 72–76. [Google Scholar] [CrossRef]
- Lv, X.; Pan, Y.; Jia, Z.; Li, Z.; Ni, X. Surface damage induced by a combined millisecond and nanosecond laser. Appl. Opt. 2017, 56, 5060–5067. [Google Scholar] [CrossRef]
- Hatcher, G.D.; Ijomah, W.L.; Windmill, J.F.C. Design for remanufacture: A literature review and future research needs. J. Cleaner Prod. 2011, 19, 2004–2014. [Google Scholar] [CrossRef]
- Ma, X.L.; Nie, X.H.; Zhao, J.N.; Han, Y.F.; Pranav, S.; Wang, Y.; Guo, J. Coloring stability analysis of nanosecond pulsed laser induced surface coloring on stainless steel. Opt. Lasers Technol. 2020, 123, 105936. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, S.; Cheng, W.; Wang, G.; Liu, W.; Ren, Y. Investigation on the surface properties of 5A12 Aluminum Alloy after Nd: YAG laser cleaning. Coatings 2019, 9, 578. [Google Scholar] [CrossRef] [Green Version]
- Anashkina, E.A.; Andrianov, A.V.; Leuchs, G. Single-shot reconstruction of a subpicosecond pulse from a fiber laser system via processing strongly self-phase modulated spectra. Results Phys. 2020, 16, 102848. [Google Scholar] [CrossRef]
- Mo, J.; Chen, Y. Silver jewelry microanalysis with dual-pulse laser-induced breakdown spectroscopy: 266 + 1064 nm wavelength combination. Appl. Opt. 2014, 53, 7516–7522. [Google Scholar] [CrossRef]
- Shen, Z.; Ding, T.; Ye, X.; Wang, X.; Ma, B.; Cheng, X.; Liu, H.; Ji, Y.; Wang, Z. Influence of cleaning process on the laser induced damage threshold of substrates. Appl. Opt. 2011, 50, C433–C440. [Google Scholar] [CrossRef]
- Peng, Y.; Wen, Y.; Zhang, D.; Luo, S.; Chen, L.; Zhu, Y. Optimal proportional relation between laser power and pulse number for the fabrication of surface-microstructured silicon. Appl. Opt. 2011, 50, 4765–4768. [Google Scholar] [CrossRef]
- Shi, T.; Wang, C.; Mi, G.; Yan, F. A study of microstructure and mechanical properties of aluminum alloy using laser cleaning. J. Manuf. Process. 2019, 42, 60–66. [Google Scholar] [CrossRef]
- Vrancken, B.; Thijs, L.; Kruth, J.P.; Van Humbeeck, J. Heat treatment of Ti6Al4V produced by selective laser melting: Microstructure and mechanical properties. J. Alloys Compd. 2012, 541, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Xu, R. A new method for detecting jet impact force of laser cavity. Key Eng. Mater. 2013, 552, 463–469. [Google Scholar] [CrossRef]
- Ndiithi, N.J.; Kang, M.; Zhu, J.; Lin, J.; Nyambura, S.M.; Liu, Y.; Huang, F. Microstructural and corrosion behavior of high velocity arc sprayed FeCrAl/Al composite coating on Q235 steel substrate. Coatings 2019, 9, 542. [Google Scholar] [CrossRef] [Green Version]
- Conradi, M.; Sever, T.; Gregorčič, P.; Kocijan, A. Short- and long-term wettability evolution and corrosion resistance of uncoated and polymer-coated laser-textured steel surface. Coatings 2019, 9, 592. [Google Scholar] [CrossRef] [Green Version]
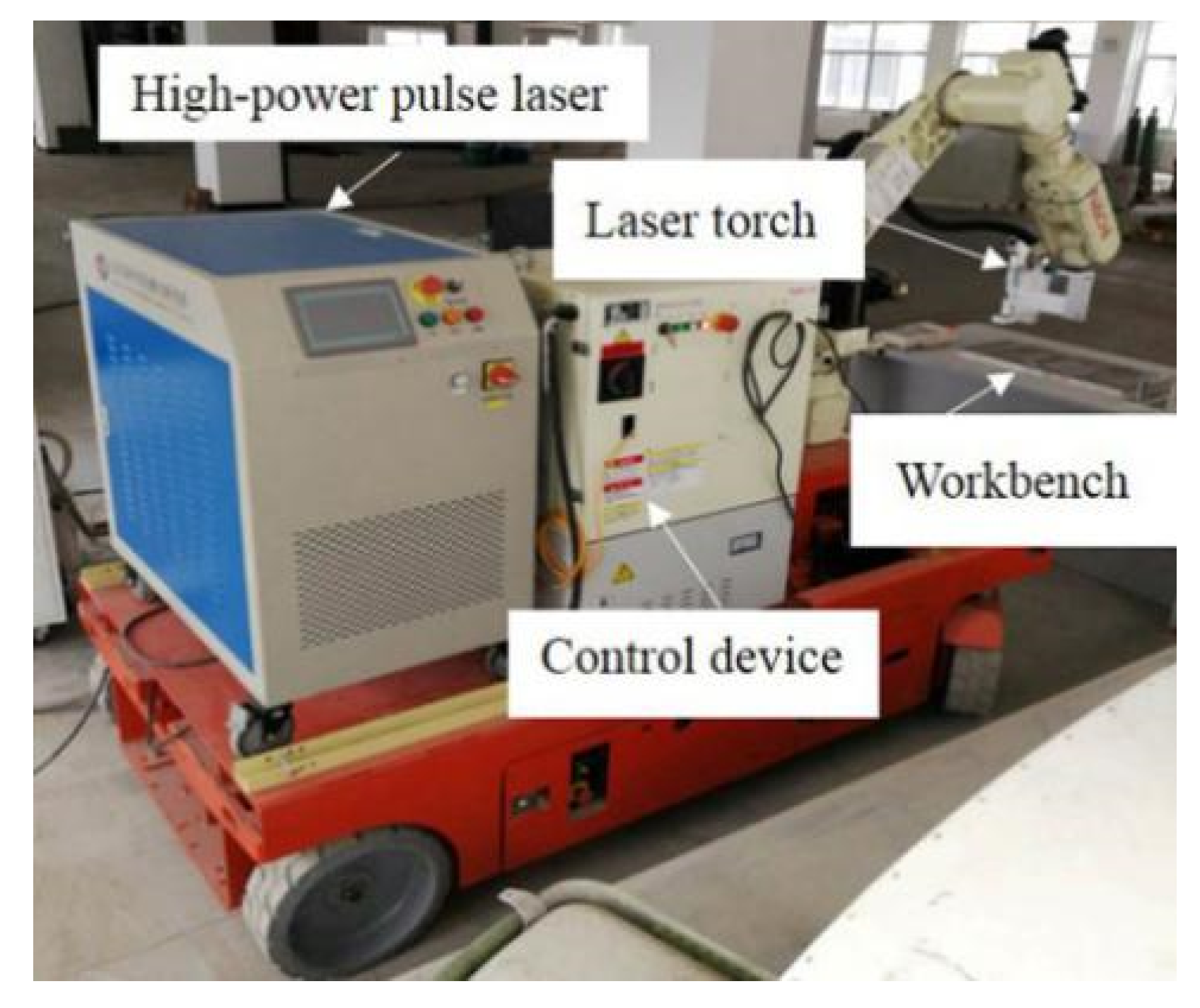
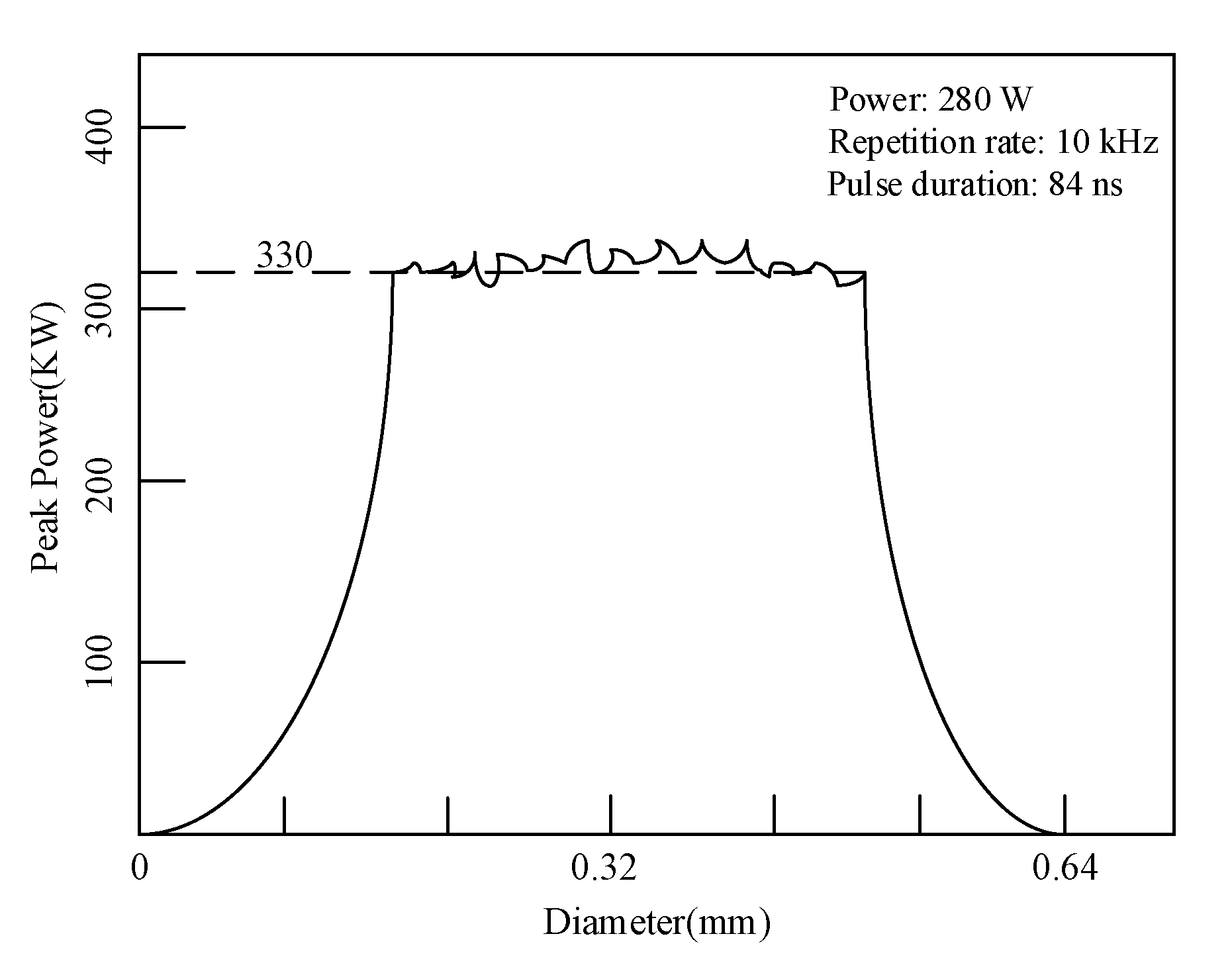




| Nd:YAG Laser | Wavelength/nm | Pulse Duration/ns | Average Power/W | Repetition Rate/kHz | Spot Diameter/mm | Max Peak Power/kW |
|---|---|---|---|---|---|---|
| Parameters | 1064 | 50–140 | 30–300 | 7–15 | 0.64 | 530 |
| Matrix Component | Fe | Mn | Si | C | Cr | P |
|---|---|---|---|---|---|---|
| wt.% | 97.814 | 1.220 | 0.470 | 0.195 | 0.067 | 0.028 |
| Deposit Component | Fe | O | C | Ti | K | Mn |
| wt.% | 40.690 | 33.820 | 21.450 | 1.140 | 0.800 | 0.720 |
| Power/W | As-received | 200 | 220 | 240 | 260 | 280 | 300 |
| (mV) | −1065 | −1066 | −1063 | −1046 | −1053 | −1043 | −1011 |
| (μA/cm2) | 1034 | 838.9 | 656.3 | 609.2 | 510.5 | 383.9 | 928.7 |
| Repetition Rate/kHz | As-received | 8 | 9 | 10 | 11 | 12 | 13 |
| (mV) | −1065 | −1067 | −1062 | −1043 | −1065 | −1064 | −1078 |
| (μA/cm2) | 1034 | 1021 | 672.6 | 383.9 | 773 | 724.7 | 749.8 |
| Overlapping Rate/% | As-received | 30 | 40 | 50 | 60 | 70 | 80 |
| (mV) | −1065 | −1065 | −1050 | −1043 | −1055 | −1049 | −1085 |
| (μA/cm2) | 1034 | 1342 | 1123 | 383.9 | 483.0 | 487.7 | 997.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Wang, L.; Li, J.; Jia, X.; Wang, X.; Ren, Y.; Zhou, Y. Investigation of the Surface Integrity of Q345 Steel After Nd:YAG Laser Cleaning of Oxidized Mining Parts. Coatings 2020, 10, 716. https://doi.org/10.3390/coatings10080716
Ma M, Wang L, Li J, Jia X, Wang X, Ren Y, Zhou Y. Investigation of the Surface Integrity of Q345 Steel After Nd:YAG Laser Cleaning of Oxidized Mining Parts. Coatings. 2020; 10(8):716. https://doi.org/10.3390/coatings10080716
Chicago/Turabian StyleMa, Mingliang, Liming Wang, Jianfeng Li, Xiujie Jia, Xing Wang, Yuan Ren, and Yuansheng Zhou. 2020. "Investigation of the Surface Integrity of Q345 Steel After Nd:YAG Laser Cleaning of Oxidized Mining Parts" Coatings 10, no. 8: 716. https://doi.org/10.3390/coatings10080716
APA StyleMa, M., Wang, L., Li, J., Jia, X., Wang, X., Ren, Y., & Zhou, Y. (2020). Investigation of the Surface Integrity of Q345 Steel After Nd:YAG Laser Cleaning of Oxidized Mining Parts. Coatings, 10(8), 716. https://doi.org/10.3390/coatings10080716






