Effects of Zirconium and Yttrium Oxide on Mechanical and Oxidation Properties of Mo–3Si–1B–1Zr–1Y2O3 (wt.%) Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Microstructure Analysis
3.2. Mechanical Properties of Mo–3Si–1B–1Zr–1Y2O3 Alloy
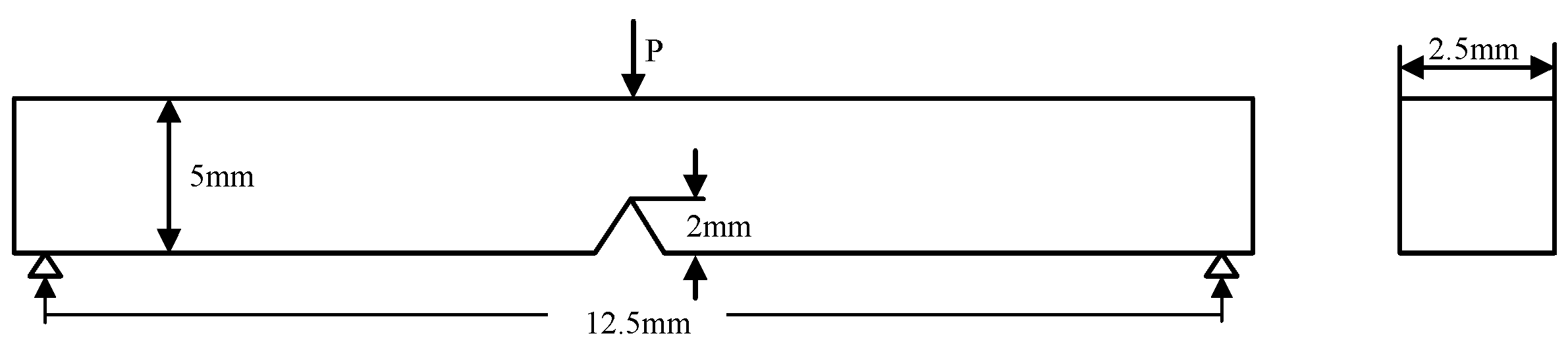
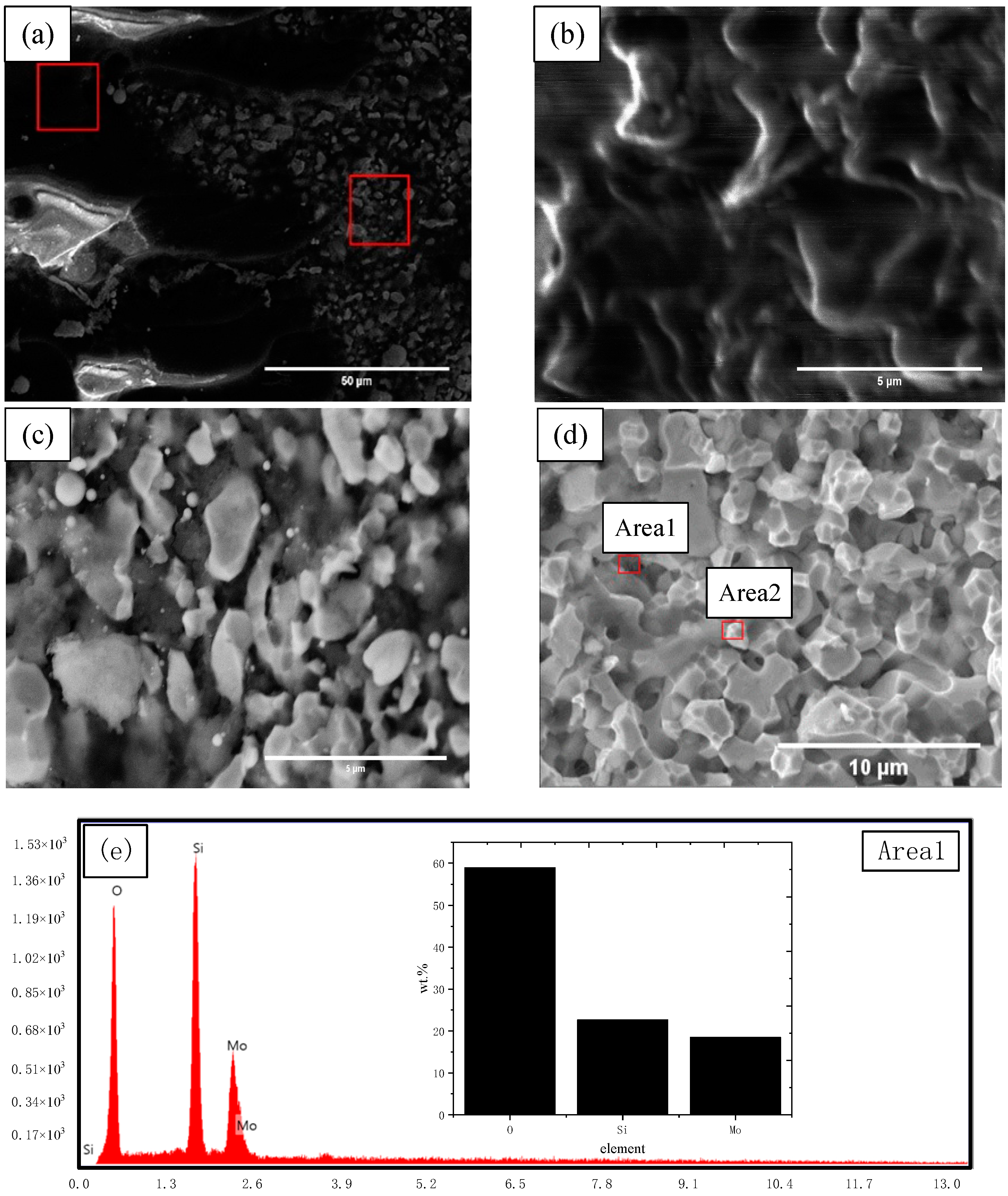
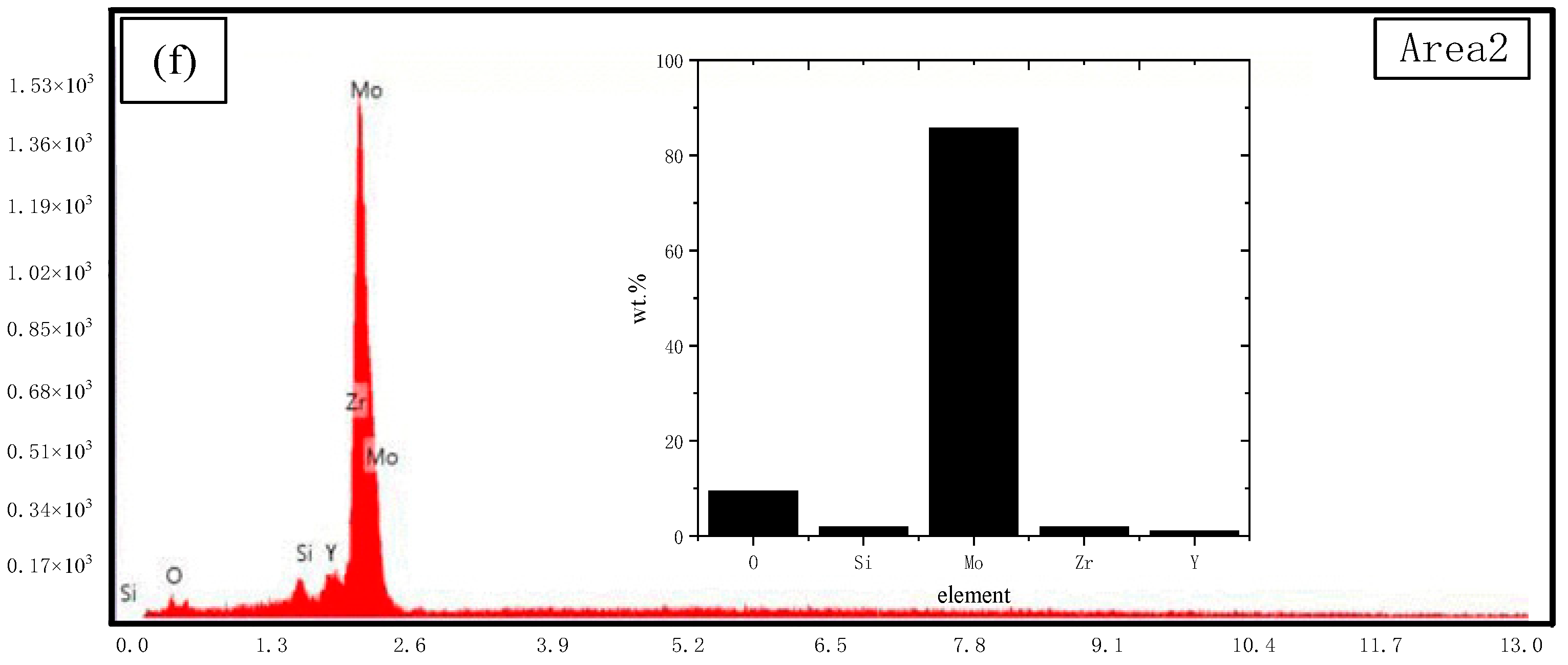
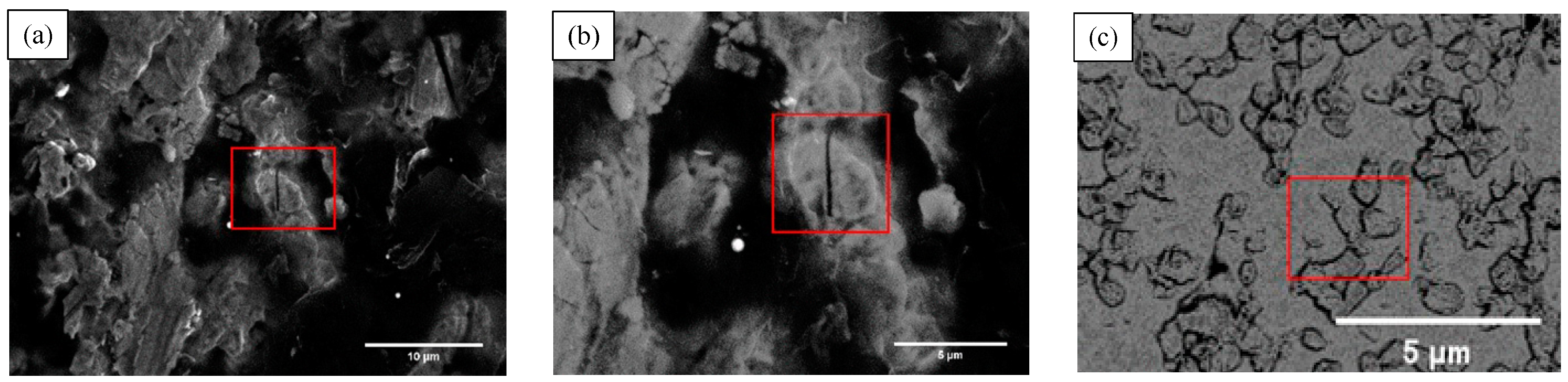
3.2.1. Fracture Toughness and Fracture Surface Features
3.2.2. Crack Growth Behavior
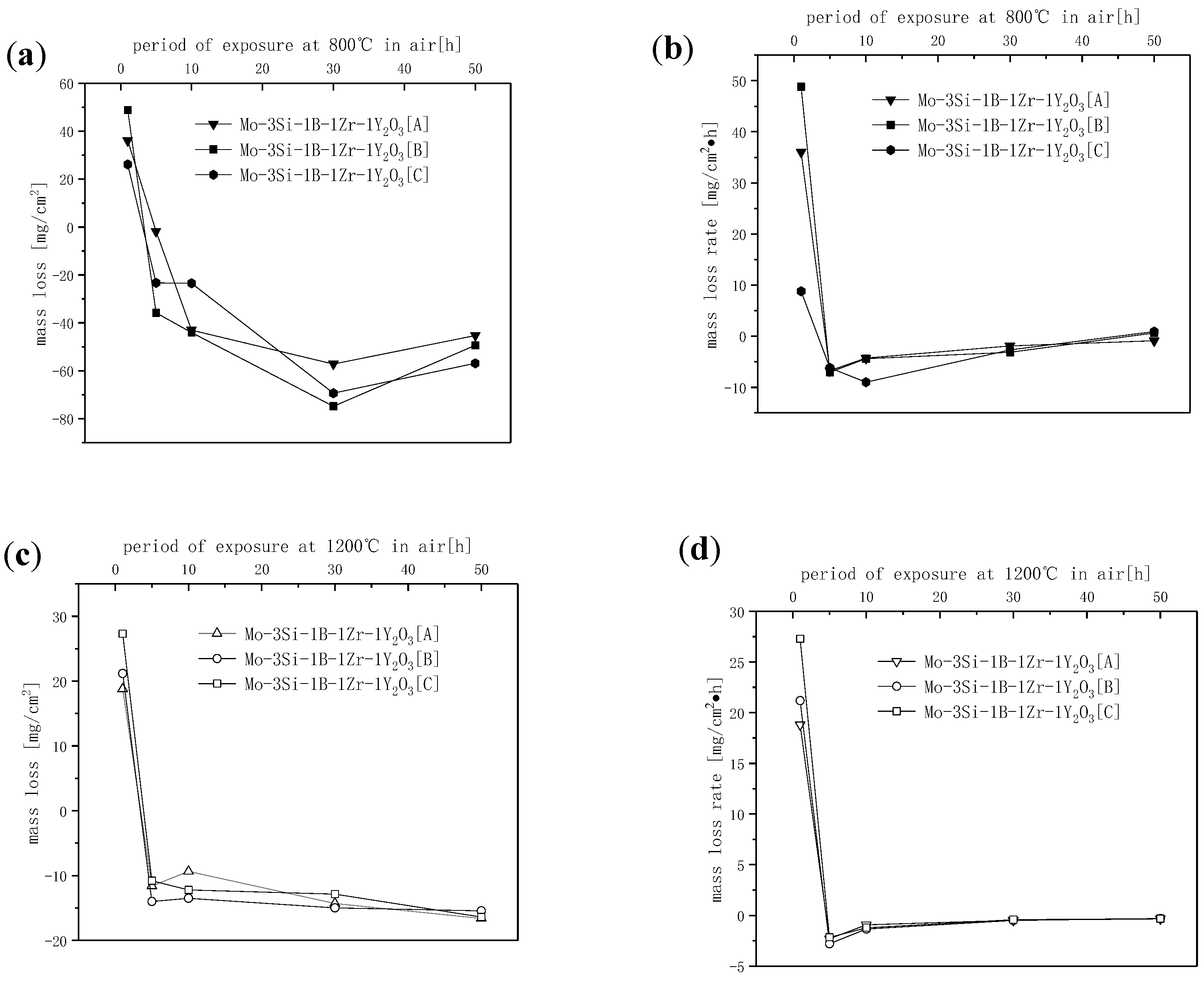
3.3. Oxidation Resistance
3.3.1. Oxidation Kinetics Process Analysis at 800 and 1200 °C
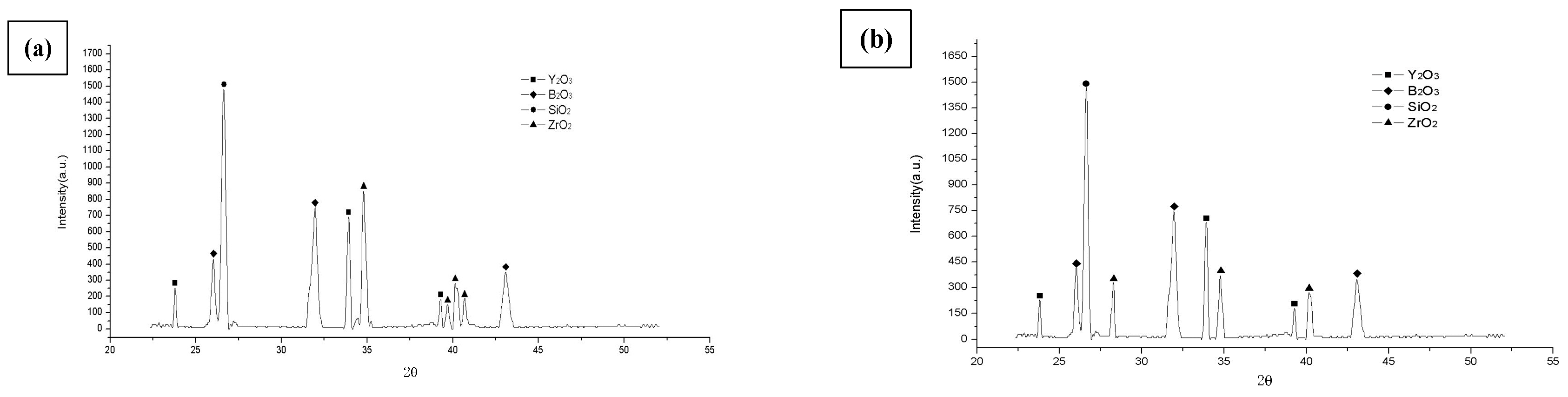
3.3.2. XRD Results
4. Discussion
5. Conclusions
- Improved PM (vibrating sintering technique) manufacturing process enabled Mo–3Si–1B–1Zr–1Y2O3 alloy to produce fine-grained homogenous microstructures with a continuous α-Mo matrix. It was found that the α-Mo phase accounted for 59%, and Mo5SiB2 and Mo3Si phases accounted for 41%. The fracture toughness value reached an average of 13.5 MPa·m1/2.
- The fracture surface characteristics were shown as a mixed type of intergranular fracture and transgranular fracture. By EDS analysis, Zr and Y elements were detected at the position of the grain boundaries. On the fracture surface, the crack hindered and terminated in the α-Mo phase.
- In the temperature oxidation experiment at 800 °C, the Mo–3Si–1B–1Zr–1Y2O3 alloy showed good mid-temperature oxidation resistance, which was due to the transformation of the t-ZrO2 into the m-ZrO2, which caused the volume to shrink, leading to changes in stress and promoting silicon boron glassy phase flow. After 50 h of oxidation, the oxidation weight loss was maintained at about −50.3 mg/cm2. At the same time, the oxidation rate was as low as −1.03 mg/(cm2·h), and the oxidation resistance was improved dozens of times at 800 °C.
- At 1200 °C, ZrO2 changed into t-ZrO2, and no other phase transitions occurred at this temperature. The viscosity improvement brought by high temperature and the self-healing ability of Y2O3 to the glass layer exhibited considerable oxidation performance at 1200 °C.
Author Contributions
Funding
Conflicts of Interest
References
- Nowotny, H.; Dimakopoulu, E.; Kudielka, H. Untersuchungen in den dreistoffsystemen: Molybdän-silizium-bor, wolfram-silizium-borund in dem system: VSi2-TaSi2. Monatshefte für Chemie und verwandte Teile anderer Wissenschaften 1957, 88, 180–192. [Google Scholar] [CrossRef]
- Supatarawanich, V.; Johnson, D.R.; Liu, C.T. Oxidation behavior of multiphase Mo–Si–B alloys. Intermetallics 2004, 12, 721–725. [Google Scholar] [CrossRef]
- Behrani, V.; Thom, A.J.; Kramer, M.J.; Akinc, M. Chlorination treatment to improve the oxidation resistance of Nb–Mo–Si–B alloys. Metall. Mater. Trans. A 2005, 36, 609–615. [Google Scholar] [CrossRef]
- Kumar, N.K.; Das, J.; Mitra, R. Effect of Zr addition on microstructure, hardness and oxidation behavior of arc-melted and spark plasma sintered multiphase Mo–Si–B alloys. Metall. Mater. Trans. A 2019, 50, 2041–2060. [Google Scholar] [CrossRef]
- Behrani, V.; Thom, A.J.; Kramer, M.J.; Akinc, M. Microstructure and oxidation behavior of Nb–Mo–Si–B alloys. Intermetallics 2005, 14, 24–32. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, G.; Wang, T.; Ren, S.; Shi, Y.; Bai, Z.; Cao, Q. Microstructure and oxidation resistance of a multiphase Mo-Si-B ceramic coating on Mo substrates deposited by a plasma transferred arc process. Ceram. Int. 2018, 45, 415–423. [Google Scholar] [CrossRef]
- Li, W.; Zhang, G.; Wang, S.; Li, B.; Sun, J. Ductility of Mo–12Si–8.5B alloys doped with lanthanum oxide by the liquid-liquid doping method. J. Alloys Compd. 2015, 642, 34–39. [Google Scholar] [CrossRef]
- Zhang, G.; He, W.; Li, B.; Zha, Y.; Sun, J. Effect of Si/B ratio on the microstructure and mechanical properties of lanthanum oxide-doped Mo–Si–B alloys. J. Alloys Compd. 2013, 577, 217–221. [Google Scholar] [CrossRef]
- Becker, J.; Betke, U.; Hoffmeister, M.; Krüger, M. Density reduction of Mo–Si–B alloys by vanadium alloying. JOM 2018, 70, 2574–2581. [Google Scholar] [CrossRef]
- Miyamoto, S.; Yoshimi, K.; Ha, S.H.; Kaneko, T.; Nakamura, J.; Sato, T.; Maruyama, K.; Tu, R.; Goto, T. Phase equilibria, microstructure, and high-temperature strength of TiC-added Mo–Si–B alloys. Metall. Mater. Trans. 2014, 45, 1112–1123. [Google Scholar] [CrossRef]
- Jéhanno, P.; Heilmaier, M.; Saage, H.; Heyse, H.; Böning, M.; Kestler, H.; Schneibel, J.H. Super-plasticity of a multiphase refractory Mo-Si-B alloy. Scr. Mater. 2006, 55, 525–528. [Google Scholar] [CrossRef]
- Jéhanno, P.; Heilmaier, M.; Kestler, H. Characterization of an industrially processed mo-based silicide alloy. Intermetallics 2004, 12, 1005–1009. [Google Scholar] [CrossRef]
- Brindley, K.A.; Priddy, M.W.; Neu, R.W. Integrative materials design of three-phase Mo–Si–B alloys. Integr. Mater. Manuf. Innov. 2019, 8, 1–16. [Google Scholar] [CrossRef]
- Hasemann, G.; Ida, S.; Zhu, L.; Iizawa, T.; Yoshimi, K.; Krüger, M. Experimental assessment of the microstructure evolution and liquidus projection in the Mo-rich Mo–Si–B system. Mater. Des. 2020, 185, 108233. [Google Scholar] [CrossRef]
- Yang, T.; Guo, X.P. Mechanical alloying behaviors of Mo–Si–B-based alloy from elemental powders under different milling conditions. Rare Met. 2018, 38, 653–664. [Google Scholar] [CrossRef]
- Majumdar, S.; Dönges, B.; Gorr, B.; Christ, H.J.; Schliephake, D.; Heilmaier, M. Mechanisms of oxide scale formation on yttrium-alloyed Mo–Si–B containing fine-grained microstructure. Corros. Sci. 2015, 90, 76–88. [Google Scholar] [CrossRef]
- Lemberg, J.A.; Ritchie, R.O. Mo–Si–B alloys for ultrahigh-temperature structural applications. Adv. Mater. 2012, 24, 3445–3480. [Google Scholar] [CrossRef]
- Lemberg, J.A.; Middlemas, M.R.; Weingärtner, T.; Gludovatz, B.; Cochran, J.K.; Ritchie, R.O. On the fracture toughness of fine-grained Mo-3Si-1B (wt.%) alloys at ambient to elevated (1300 °C) temperatures. Intermetallics 2012, 20, 141–154. [Google Scholar] [CrossRef]
- Yang, Y.; Bei, H.; Tiley, J.; George, E.P. Re effects on phase stability and mechanical properties of MoSS+Mo3Si+Mo5SiB2 alloys. J. Alloys Compd. 2013, 556, 32–38. [Google Scholar] [CrossRef]
- Makineni, S.K.; Kini, A.R.; Jägle, E.A.; Springer, H.; Raabe, D.; Gault, B. Synthesis and stabilization of a new phase regime in a Mo-Si-B based alloy by laser-based additive manufacturing. Acta Mater. 2018, 151, 31–40. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, W.; Xu, Y.; Lu, Z.; Li, D. The thermal-mechanical behavior of WMoNb high-entropy alloy via selective laser melting (SLM): Experiment and simulation. Int. J. Adv. Manuf. Technol. 2018, 96, 461–474. [Google Scholar] [CrossRef]
- Guo-jun, Z.; Qian, D.; Hao, K.; Rui-hong, W.; Gang, L.; Jun, S. Microstructure and mechanical properties of lanthanum oxide-doped Mo–12Si–8.5B (at.%) alloys. J. Alloys Compd. 2013, 577, 493–498. [Google Scholar] [CrossRef]
- Schmelzer, J.; Rittinghaus, S.K.; Weisheit, A.; Stobik, M.; Paulus, J.; Gruber, K.; Wessel, E.; Heinze, C.; Krügere, M. Printability of gas atomized Mo-Si-B powders by laser metal deposition. Int. J. Refract. Metals Hard Mater. 2019, 78, 123–126. [Google Scholar] [CrossRef]
- Krüger, M.; Schmelzer, J.; Smokovych, I.; Barrilao, J.L.; Hasemann, G. Processing of Mo silicide powders as filler particles in polymer-derived ceramic coatings for Mo-Si-B substrates. Powder Technol. 2019, 352, 381–385. [Google Scholar] [CrossRef]
- Krüger, M.; Franz, S.; Saage, H.; Heilmaier, M.; Schneibel, J.H.; Jéhanno, P.; Böning, M.; Kestler, H. Mechanically Alloyed Mo-Si-B Alloys with a Continuous α-Mo Matrix and Improved Mechanical Properties. Intermetallics 2008, 16, 933–941. [Google Scholar] [CrossRef]
- Schliephake, D.; Azim, M.; von Klinski-Wetzel, K.; Gorr, B.; Christ, H.J.; Bei, H.; George, E.P.; Heilmaier, M. High-temperature creep and oxidation behavior of Mo-Si-B alloys with high Ti contents. Metall. Mater. Trans. A 2014, 45, 1102–1111. [Google Scholar] [CrossRef]

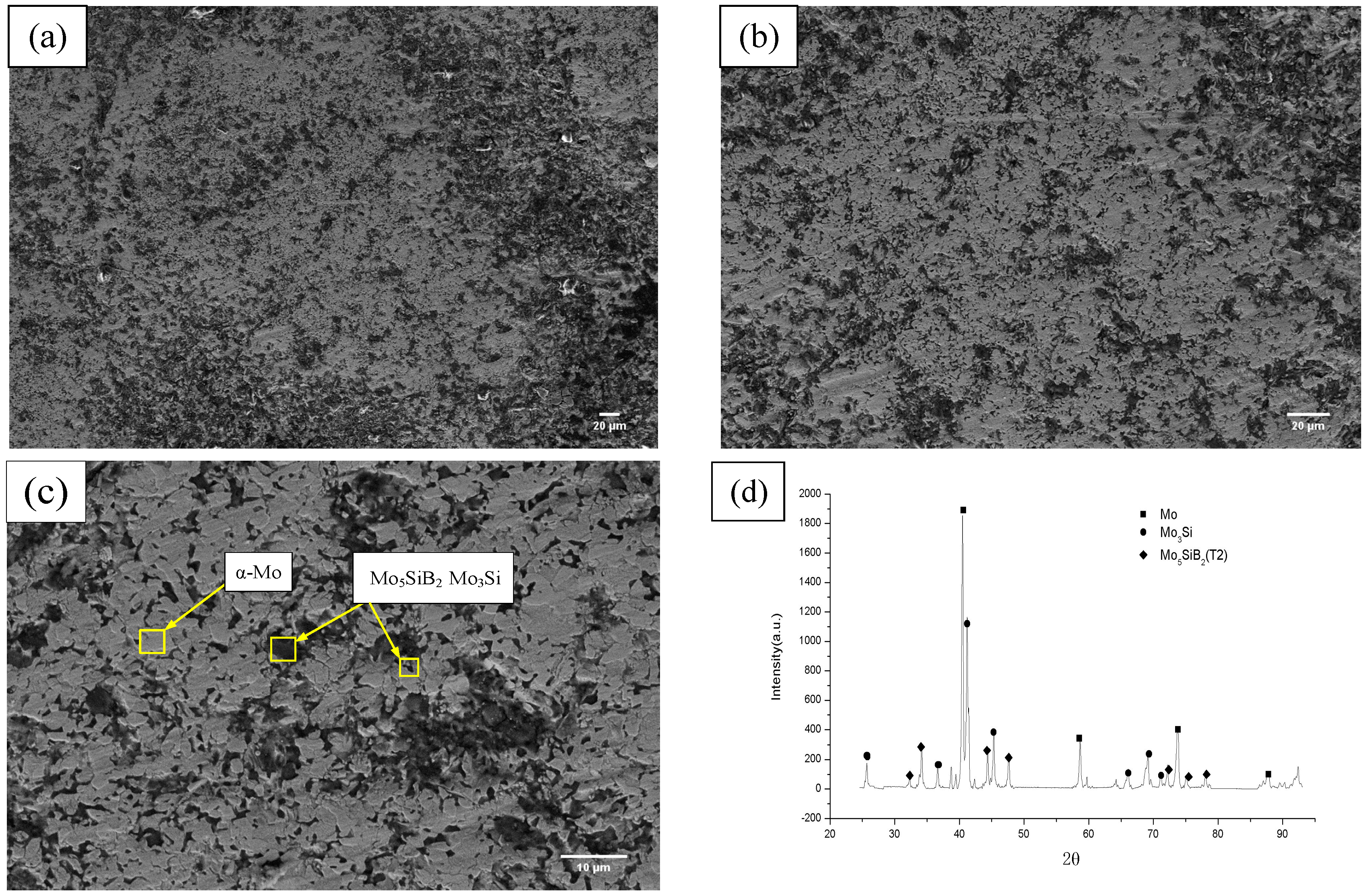

| Phases | XRD Calculated |
|---|---|
| α-Mo | 59% |
| Mo3Si | 31% |
| Mo5SiB2 | 10% |
| Sample Serial Number | Alloy Composition in wt.% | Fracture Toughness (Kq) in MPa·m1/2 |
|---|---|---|
| A | Mo–3Si–1B–1Zr–1Y2O3 | 12.8 |
| B | Mo–3Si–1B–1Zr–1Y2O3 | 14.1 |
| C | Mo–3Si–1B–1Zr–1Y2O3 | 13.7 |
| D | Mo–3Si–1B | 5.91 |
| E | Mo–3Si–1B | 6.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Wang, L.; Wang, C.; Li, Q. Effects of Zirconium and Yttrium Oxide on Mechanical and Oxidation Properties of Mo–3Si–1B–1Zr–1Y2O3 (wt.%) Alloy. Coatings 2020, 10, 833. https://doi.org/10.3390/coatings10090833
Guo Z, Wang L, Wang C, Li Q. Effects of Zirconium and Yttrium Oxide on Mechanical and Oxidation Properties of Mo–3Si–1B–1Zr–1Y2O3 (wt.%) Alloy. Coatings. 2020; 10(9):833. https://doi.org/10.3390/coatings10090833
Chicago/Turabian StyleGuo, Zhenping, Lei Wang, Cheng Wang, and Qiuliang Li. 2020. "Effects of Zirconium and Yttrium Oxide on Mechanical and Oxidation Properties of Mo–3Si–1B–1Zr–1Y2O3 (wt.%) Alloy" Coatings 10, no. 9: 833. https://doi.org/10.3390/coatings10090833




