Recent Developments in Coating Technologies for Adsorption Heat Pumps: A Review
Abstract
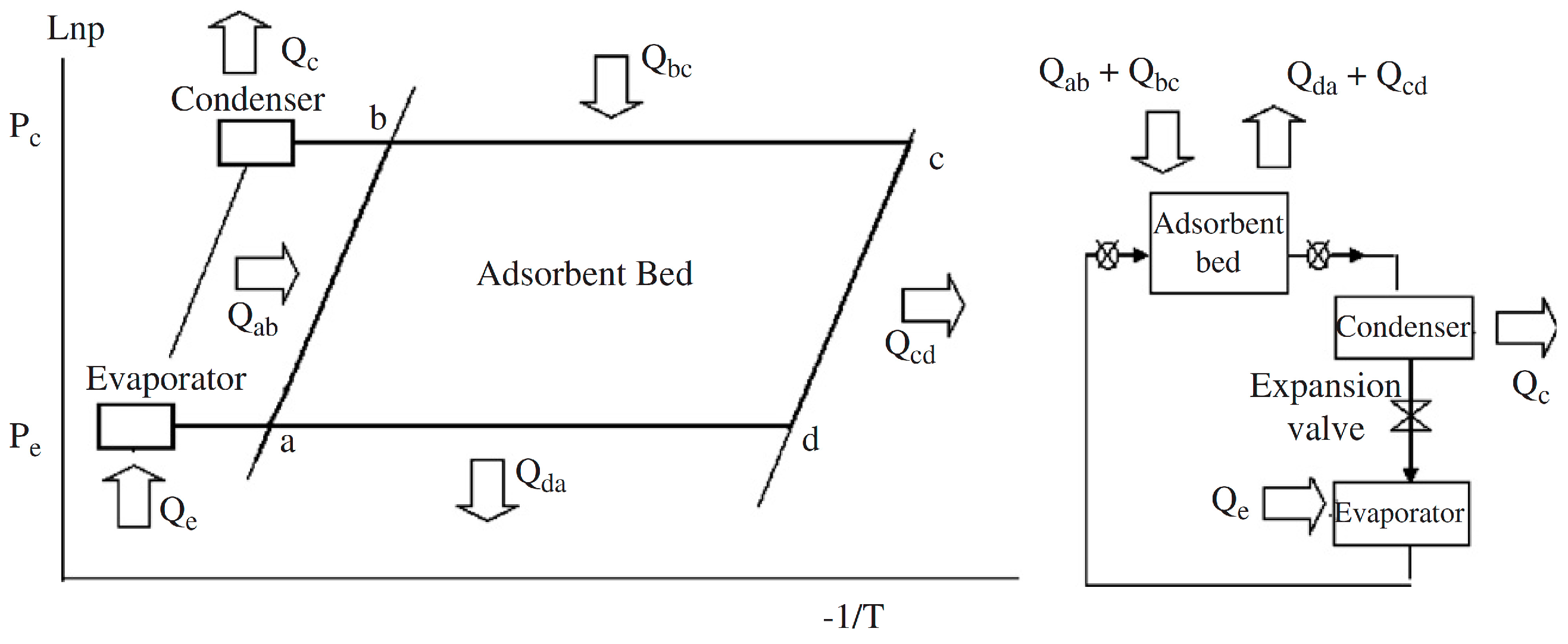
:1. Introduction
2. Adsorbent Coating Technologies
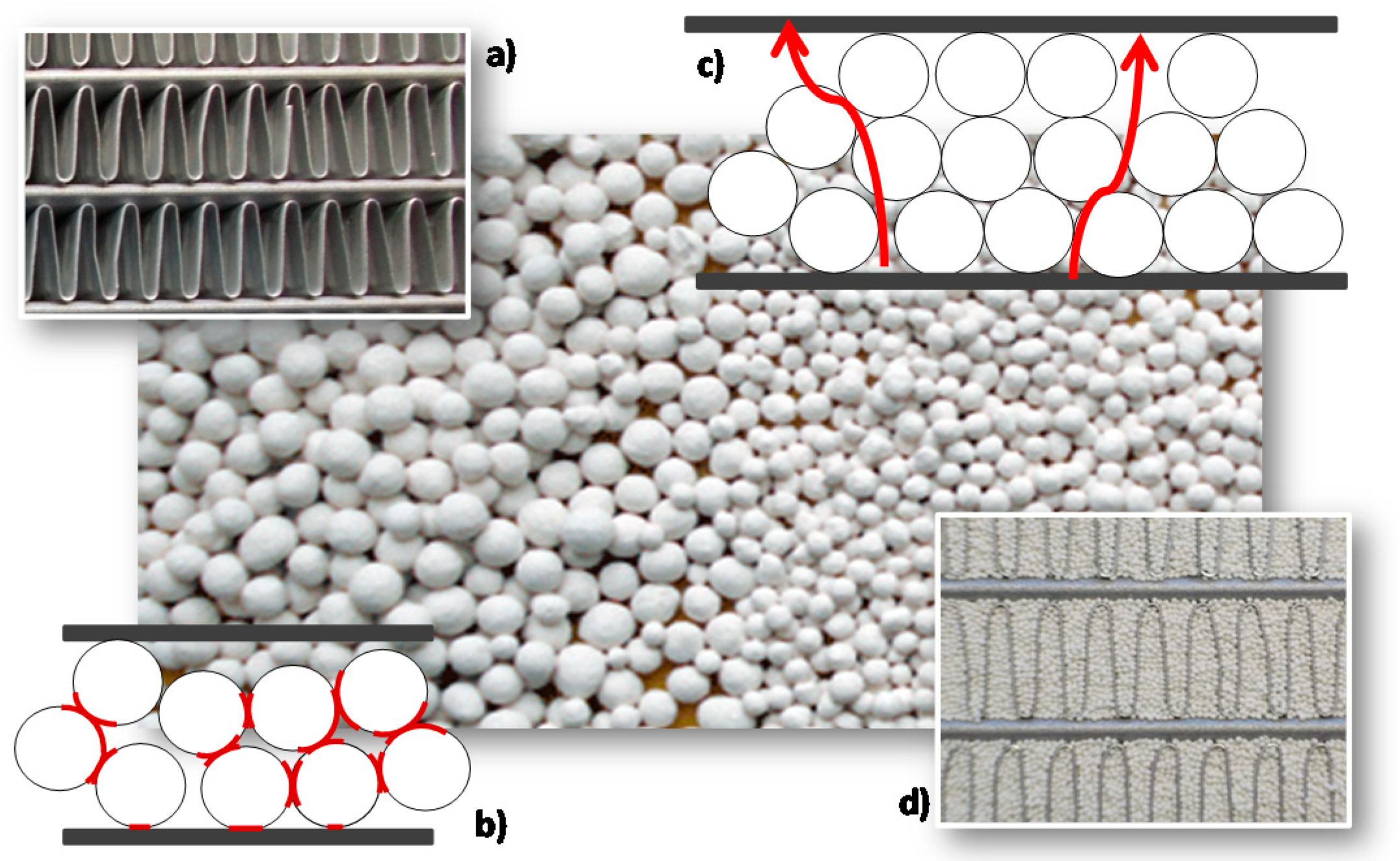
2.1. Consolidated Beds
2.2. In Situ Direct Synthesis
2.3. Binder-Based Coatings
- The dip-coating technique (Figure 6a) is suitable for the production of uniform thickness coatings. The substrate is fully immersed into the composite coating solution, extracted and then dried. This technique requires the high consumption of solution which sometimes makes it economically unaffordable. Moreover, some limits can be represented by the need for constant control of the viscosity of the solution and of immersion/extraction speed. It is suitable for complex geometries but not for thicknesses that are too high because it may require many deposition steps. Furthermore, it often requires a pre-treatment step in order to increase the interfacial adhesion of the coating to the metal substrate.
- Spray coating (Figure 6b), like dip coating, is suitable for complex heat exchange geometries. It requires a lower consumption of solution and is less affected by, but not exempt from, the viscosity concerns observed in the dip-coating process. However, this technique has some issues due to the depth of the flow inside the HEX cavities. Adsorbent materials tend to be deposited mainly on the external surfaces, becoming scarce and not well distributed inside them, leading to an irregular and homogenous coating deposition. However, this technique represents an easy manufacturing technique that could be affordably automated.
- Spin coating (Figure 6c) is a rather cheap and reproducible coating method, but is unsuitable for complex geometries. It can be essentially used on flat surfaces on which it can produce homogeneous coatings, but with a high quantity of waste. A small quantity of coating precursor slurry is applied on the center of a flat substrate and then distributed through the spinner by centrifugal force.
- The “drop-coating” technique (Figure 6d) can be imagined as an evolution of dip-coating, in which the exchanger can be coated by crossing over a liquid solution cascade, followed by single or multiple drying steps. This method has the advantage of being able to be applied on complex surfaces, but requires, as in the dip-coating process, well-defined viscosity control. The main advantage of the process is the low wastage of materials, considering that the solution can be easy collected and re-used. Furthermore, a continuous production of coated adsorber can be applied.
3. Coating Technique Performance Comparison
4. Conclusions and Future Perspectives
Funding
Conflicts of Interest
Abbreviations
| AC | activated carbon |
| Cp | specific heat (kJ/kg 1C) |
| COP | coefficient of performance |
| ∆Ha | heat of adsorption (kJ/kg adsorbate) |
| ∆Hv | heat of vaporization (kJ/kg adsorbate) |
| m | mass of dry adsorbent (kg) |
| mbed | mass of structure of adsorbent bed (kg) |
| P | pressure (kPa) |
| Q | heat transferred (kJ) |
| Qab | heat of isosteric heating process (kJ) |
| Qbc | heat of isobaric desorption process (kJ) |
| Qcd | heat of isosteric cooling process (kJ) |
| Qda | heat of isobaric adsorption process (kJ) |
| Ad-HEx | adsorbent e heat exchanger unit |
| SCP | specific cooling power (W/kg) |
| SHP | specific heating power (W/kg) |
| T | temperature (K) |
| X | mass fraction of adsorbed adsorbate per dry adsorbent (kg adsorbate/kg dry adsorbent) |
| PTFE | polytetrafluoroethylene |
| PVA/PVOH | polyvinyl alcohol |
| PVP | polyvinylpyrrolidone |
| EG | expanded graphite |
| PANI | polyaniline |
| TCR | thermal contact resistance |
| TC | thermal conductivity |
| TG | thermogravimetry |
References
- Buffa, S.; Cozzini, M.; D’antoni, M.; Baratieri, M.; Fedrizzi, R. 5th generation district heating and cooling systems: A review of existing cases in Europe. Renew. Sustain. Energy Rev. 2019, 104, 504–522. [Google Scholar] [CrossRef]
- Martins, F.; Felgueiras, C.; Smitková, M. Fossil Fuel Energy Consumption in European Countries. Energy Procedia 2018, 153, 107–111. [Google Scholar] [CrossRef]
- Velders, G.J.M.; Ravishankara, A.R.; Miller, M.K.; Molina, M.J.; Alcamo, J.; Daniel, J.S.; Fahey, D.W.; Montzka, S.A.; Reimann, S. Preserving montreal protocol climate benefits by limiting HFCs. Science 2012, 335, 922–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurano-Cervelló, P.; Pozo, C.; Mateo-Sanz, J.M.; Jiménez, L.; Guillén-Gosálbez, G. Sustainability efficiency assessment of the electricity mix of the 28 EU member countries combining data envelopment analysis and optimized projections. Energy Policy 2019, 134, 110921. [Google Scholar] [CrossRef]
- Oniversitesi, D.E. Adsorption heat pumps. J. Heat Recover. Syst. 1986, 6, 277–284. [Google Scholar]
- Cacciola, G.; Restuccia, G. Progress on adsorption heat pumps. Heat Recover. Syst. CHP 1994, 14, 409–420. [Google Scholar] [CrossRef]
- Srivastava, N.C.; Eames, I.W. A review of adsorbents and adsorbates in solid-vapour adsorption heat pump systems. Appl. Therm. Eng. 1998, 18, 707–714. [Google Scholar] [CrossRef]
- Maeda, S.; Thu, K.; Maruyama, T.; Miyazaki, T. Critical review on the developments and future aspects of adsorption heat pumps for automobile air conditioning. Appl. Sci. 2018, 8, 2061. [Google Scholar] [CrossRef] [Green Version]
- Freni, A.; Maggio, G.; Sapienza, A.; Frazzica, A.; Restuccia, G.; Vasta, S. Comparative analysis of promising adsorbent/adsorbate pairs for adsorptive heat pumping, air conditioning and refrigeration. Appl. Therm. Eng. 2016, 104, 85–95. [Google Scholar] [CrossRef]
- Demir, H.; Mobedi, M.; Ülkü, S. A review on adsorption heat pump: Problems and solutions. Renew. Sustain. Energy Rev. 2008, 12, 2381–2403. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, S.; Sefidvash, F. Study of Pressure Drop in Fixed Bed Reactor Using a Computational Fluid Dynamics (CFD) Code. ChemEngineering 2018, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Saha, B.B.; Uddin, K.; Pal, A.; Thu, K. Emerging sorption pairs for heat pump applications: An overview. JMST Adv. 2019, 1, 161–180. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.W.; Wang, R.Z.; Oliveira, R.G. A review on adsorption working pairs for refrigeration. Renew. Sustain. Energy Rev. 2009, 13, 518–534. [Google Scholar] [CrossRef]
- Yang, Z.; Gluesenkamp, K.R.; Frazzica, A. Database of Sorption Materials Equilibrium Properties. In Proceedings of the 8th Heat Powered Cycles Conference, Bayreuth, Germany, 16–19 September 2018; pp. 227–235. [Google Scholar]
- Aristov, Y.I. Novel materials for adsorptive heat pumping and storage: Screening and nanotailoring of sorption properties. J. Chem. Eng. Jpn. 2007, 40, 1242–1251. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Thu, K.; Ismail, A.B.; Shahzad, M.W.; Ng, K.C. Performance of adsorbent-embedded heat exchangers using binder-coating method. Int. J. Heat Mass Transf. 2016, 92, 149–157. [Google Scholar] [CrossRef]
- Ge, T.S.; Dai, Y.J.; Wang, R.Z. Performance study of silica gel coated fin-tube heat exchanger cooling system based on a developed mathematical model. Energy Convers. Manag. 2011, 52, 2329–2338. [Google Scholar] [CrossRef]
- Freni, A.; Bonaccorsi, L.; Calabrese, L.; Caprì, A.; Frazzica, A.; Sapienza, A. SAPO-34 coated adsorbent heat exchanger for adsorption chillers. Appl. Therm. Eng. 2015, 82, 1–7. [Google Scholar] [CrossRef]
- Atakan, A.; Fueldner, G.; Munz, G.; Henninger, S.; Tatlier, M. Adsorption kinetics and isotherms of zeolite coatings directly crystallized on fibrous plates for heat pump applications. Appl. Therm. Eng. 2013, 58, 273–280. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, R.Z.; Ge, T.S.; Hu, L.M. Performance study of SAPO-34 and FAPO-34 desiccants for desiccant coated heat exchanger systems. Energy 2015, 93, 88–94. [Google Scholar] [CrossRef]
- Cacciola, G.; Restuccia, G.; Mercadante, L. Composites of activated carbon for refrigeration adsorption machines. Carbon N. Y. 1995, 33, 1205–1210. [Google Scholar] [CrossRef]
- Askalany, A.A.; Henninger, S.K.; Ghazy, M.; Saha, B.B. Effect of improving thermal conductivity of the adsorbent on performance of adsorption cooling system. Appl. Therm. Eng. 2017, 110, 695–702. [Google Scholar] [CrossRef]
- De Lange, M.F.; Verouden, K.J.F.M.; Vlugt, T.J.H.; Gascon, J.; Kapteijn, F. Adsorption-Driven Heat Pumps: The Potential of Metal-Organic Frameworks. Chem. Rev. 2015, 115, 12205–12250. [Google Scholar] [CrossRef] [PubMed]
- Henninger, S.K.; Ernst, S.J.; Gordeeva, L.; Bendix, P.; Fröhlich, D.; Grekova, A.D.; Bonaccorsi, L.; Aristov, Y.; Jaenchen, J. New materials for adsorption heat transformation and storage. Renew. Energy 2017, 110, 59–68. [Google Scholar] [CrossRef]
- Kummer, H.; Jeremias, F.; Warlo, A.; Füldner, G.; Fröhlich, D.; Janiak, C.; Gläser, R.; Henninger, S.K. A Functional Full-Scale Heat Exchanger Coated with Aluminum Fumarate Metal-Organic Framework for Adsorption Heat Transformation. Ind. Eng. Chem. Res. 2017, 56, 8393–8398. [Google Scholar] [CrossRef]
- Jeremias, F.; Fröhlich, D.; Janiak, C.; Henninger, S.K. Advancement of sorption-based heat transformation by a metal coating of highly-stable, hydrophilic aluminium fumarate MOF. RSC Adv. 2014, 4, 24073–24082. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Ge, T.S.; Jiang, Y.; Wang, R.Z. Experimental study on silica gel-LiCl composite desiccants for desiccant coated heat exchanger. Int. J. Refrig. 2015, 51, 24–32. [Google Scholar] [CrossRef]
- Freni, A.; Russo, F.; Vasta, S.; Tokarev, M.; Aristov, Y.I.; Restuccia, G. An advanced solid sorption chiller using SWS-1L. Appl. Therm. Eng. 2007, 27, 2200–2204. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Aristov, Y.I. Composites “salt inside porous matrix” for adsorption heat transformation: A current state-of-the-art and new trends. Int. J. Low-Carbon Technol. 2012, 7, 288–302. [Google Scholar] [CrossRef] [Green Version]
- Girnik, I.S.; Aristov, Y.I. Dynamic optimization of adsorptive chillers: The “AQSOATM-FAM-Z02–Water” working pair. Energy 2016, 106, 13–22. [Google Scholar] [CrossRef]
- Girnik, I.; Yang, T.; Gordeeva, L.; Wang, W.; Ge, T.; Aristov, Y. New adsorption method for moisture and heat exchange in ventilation systems in cold countries: Concept and mathematical simulation. Energies 2020, 13, 1386. [Google Scholar] [CrossRef] [Green Version]
- Mat Wajid, N.; Mompuouo, B.; Omer, S.; Riffat, S.B. Review of Heat and Mass Transfer Enhancement Techniques and Current Advancement for Adsorption Heating/Cooling Systems. Int. J. Low-Carbon Technol. 2019, 14, 461–467. [Google Scholar] [CrossRef]
- Sapienza, A.; Frazzica, A.; Freni, A.; Aristov, Y. Adsorptive Heat Transformation and Storage: Thermodynamic and Kinetic Aspects; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Rouhani, M.; Huttema, W.; Bahrami, M. Effective thermal conductivity of packed bed adsorbers: Part 1-Experimental study. Int. J. Heat Mass Transf. 2018, 123, 1204–1211. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, D.; Tan, Y. Heat transfer enhancement of the adsorber of an adsorption heat pump. Adsorption 1999, 5, 279–286. [Google Scholar] [CrossRef]
- Fayazmanesh, K.; McCague, C.; Bahrami, M. Consolidated adsorbent containing graphite flakes for heat-driven water sorption cooling systems. Appl. Therm. Eng. 2017, 123, 753–760. [Google Scholar] [CrossRef]
- Cho, S.-H.; Kim, J.-N. Modelling of Silica gel/water adsorption-cooling system. Energy 1992, 17, 829–839. [Google Scholar]
- El-Sharkawy, I.I.; Pal, A.; Miyazaki, T.; Saha, B.B.; Koyama, S. A study on consolidated composite adsorbents for cooling application. Appl. Therm. Eng. 2016, 98, 1214–1220. [Google Scholar] [CrossRef] [Green Version]
- Khajehpour, M.; McCague, C.; Shokoya, S.; Bahrami, M. Effect of Conductive Additives on Performance of CaCl2-Silica Gel Sorbent Materials. Heat Powered Cycles Conf. 2018, 2018, 146–190. [Google Scholar]
- Bauer, J.; Herrmann, R.; Mittelbach, W.; Schwieger, W. Zeolite/aluminum composite adsorbents for application in adsorption refrigeration. Int. J. Energy Res. 2009, 33, 1233–1249. [Google Scholar] [CrossRef]
- Eun, T.H.; Song, H.K.; Hun Han, J.; Lee, K.H.; Kim, J.N. Enhancement of heat and mass transfer in silica-expanded graphite composite blocks for adsorption heat pumps: Part I. Characterization of the composite blocks. Int. J. Refrig. 2000, 23, 64–73. [Google Scholar] [CrossRef]
- Eun, T.H.; Song, H.K.; Han, J.H.; Lee, K.H.; Kim, J.N. Enhancement of heat and mass transfer in silica-expanded graphite composite blocks for adsorption heat pumps. Part II. Cooling system using the composite blocks. Int. J. Refrig. 2000, 23, 74–81. [Google Scholar] [CrossRef]
- Wang, L.W.; Metcalf, S.J.; Critoph, R.E.; Thorpe, R.; Tamainot-Telto, Z. Development of thermal conductive consolidated activated carbon for adsorption refrigeration. Carbon N. Y. 2012, 50, 977–986. [Google Scholar] [CrossRef] [Green Version]
- Basile, A.; Cacciola, G.; Colella, C.; Mercadante, L.; Pansini, M. Thermal conductivity of natural zeolite-PTFE composites. Heat Recover. Syst. CHP 1992, 12, 497–503. [Google Scholar] [CrossRef]
- Calabrese, L. Anticorrosion Behavior of Zeolite Coatings Obtained by In Situ Crystallization: A Critical Review. Materials 2018, 12, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, L.; Bonaccorsi, L.; Pietro, D.D.; Proverbio, E. Effect of process parameters on behaviour of zeolite coatings obtained by hydrothermal direct synthesis on aluminium support. Ceram. Int. 2014, 40, 12837–12845. [Google Scholar] [CrossRef]
- Atakan, A.; Atalay-Oral, Ç.; Tatlier, M.; Erciyes, T.; Erdem-Enatalar, A. Post-synthesis treatment for improving zeolite coating stability. Microporous Mesoporous Mater. 2012, 156, 262–269. [Google Scholar] [CrossRef]
- Atalay-Oral, Ç.; Bora, Ş.; Tatlier, M. Effects of Using Different Polymers in Post-Synthesis Treatments of Zeolite a Coatings. Chem. Eng. Commun. 2015, 202, 375–383. [Google Scholar] [CrossRef]
- Tatlier, M.; Kummer, H.; Henninger, S.K. Crystallization of zeolite X coatings on stainless steel by microwave heating. J. Porous Mater. 2015, 22, 347–352. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Calabrese, L.; Proverbio, E. Low temperature single-step synthesis of zeolite Y coatings on aluminium substrates. Microporous Mesoporous Mater. 2011, 144, 40–45. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Bruzzaniti, P.; Calabrese, L.; Freni, A.; Proverbio, E.; Restuccia, G. Synthesis of SAPO-34 on graphite foams for adsorber heat exchangers. Appl. Therm. Eng. 2013, 61, 848–852. [Google Scholar] [CrossRef]
- Wittstadt, U.; Füldner, G.; Andersen, O.; Herrmann, R.; Schmidt, F. A new adsorbent composite material based on metal fiber technology and its application in adsorption heat exchangers. Energies 2015, 8, 8431–8446. [Google Scholar] [CrossRef]
- Erdem-Senatalar, A.; Tatlier, M.; Ürgen, M. Preparation of zeolite coatings by direct heating of the substrates. Microporous Mesoporous Mater. 1999, 32, 331–343. [Google Scholar] [CrossRef]
- Tatlier, M.; Demir, M.; Tokay, B.; Erdem-Şenatalar, A.; Kiwi-Minsker, L. Substrate heating method for coating metal surfaces with high-silica zeolites: ZSM-5 coatings on stainless steel plates. Microporous Mesoporous Mater. 2007, 101, 374–380. [Google Scholar] [CrossRef] [Green Version]
- Jeremias, F.; Henninger, S.K.; Janiak, C. High performance metal-organic-framework coatings obtained via thermal gradient synthesis. Chem. Commun. 2012, 48, 9708–9710. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, L.; Proverbio, E. Synthesis of thick zeolite 4A coatings on stainless steel. Microporous Mesoporous Mater. 2004, 74, 221–229. [Google Scholar] [CrossRef]
- Freni, A.; Bonaccorsi, L.; Proverbio, E.; Maggio, G.; Restuccia, G. Zeolite synthesised on copper foam for adsorption chillers: A mathematical model. Microporous Mesoporous Mater. 2009, 120, 402–409. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Freni, A.; Proverbio, E.; Restuccia, G.; Russo, F. Zeolite coated copper foams for heat pumping applications. Microporous Mesoporous Mater. 2006, 91, 7–14. [Google Scholar] [CrossRef]
- Tatlier, M.; Erdem-Senatalar, A. Effects of metal mass on the performance of adsorption heat pumps utilizing zeolite 4A coatings synthesized on heat exchanger tubes: Effets de la masse metallique sur la performance des pompes a chaleur a adsorption utilisant des revetements en zeolite 4A. Int. J. Refrig. 2000, 23, 260–268. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Calabrese, L.; Proverbio, E.; Frazzica, A.; Freni, A.; Restuccia, G.; Piperopoulos, E.; Milone, C. Synthesis of SAPO-34/graphite composites for low temperature heat adsorption pumps. J. Energy Chem. 2013, 22, 245–250. [Google Scholar] [CrossRef]
- Tatlier, M.; Erdem-Şenatalar, A. Optimization of the cycle durations of adsorption heat pumps employing zeolite coatings synthesized on metal supports. Microporous Mesoporous Mater. 2000, 34, 23–30. [Google Scholar] [CrossRef]
- Schnabel, L.; Tatlier, M.; Schmidt, F.; Erdem-Şenatalar, A. Adsorption kinetics of zeolite coatings directly crystallized on metal supports for heat pump applications (adsorption kinetics of zeolite coatings). Appl. Therm. Eng. 2010, 30, 1409–1416. [Google Scholar] [CrossRef]
- Palomba, V.; Vasta, S.; Giacoppo, G.; Calabrese, L.; Gulli‘, G.; La Rosa, D.; Freni, A. Design of an Innovative Graphite Exchanger for Adsorption Heat Pumps and Chillers. Energy Procedia 2015, 81, 1030–1040. [Google Scholar] [CrossRef] [Green Version]
- Frazzica, A.; Füldner, G.; Sapienza, A.; Freni, A.; Schnabel, L. Experimental and theoretical analysis of the kinetic performance of an adsorbent coating composition for use in adsorption chillers and heat pumps. Appl. Therm. Eng. 2014, 73, 1022–1031. [Google Scholar] [CrossRef]
- van Heyden, H.; Munz, G.; Schnabel, L.; Schmidt, F.; Mintova, S.; Bein, T. Kinetics of water adsorption in microporous aluminophosphate layers for regenerative heat exchangers. Appl. Therm. Eng. 2009, 29, 1514–1522. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.S.; Chen, M.T.; Chung, T.W. Effects of the thickness and particle size of silica gel on the heat and mass transfer performance of a silica gel-coated bed for air-conditioning adsorption systems. Appl. Therm. Eng. 2005, 25, 2330–2340. [Google Scholar] [CrossRef]
- Sharafian, A.; Fayazmanesh, K.; McCague, C.; Bahrami, M. Thermal conductivity and contact resistance of mesoporous silica gel adsorbents bound with polyvinylpyrrolidone in contact with a metallic substrate for adsorption cooling system applications. Int. J. Heat Mass Transf. 2014, 79, 64–71. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Bonaccorsi, L.; Frazzica, A.; Caprì, A.; Freni, A.; Proverbio, E. Development and characterization of silane-zeolite adsorbent coatings for adsorption heat pump applications. Appl. Therm. Eng. 2017, 116, 364–371. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Electrochemical behavior of hydrophobic silane–zeolite coatings for corrosion protection of aluminum substrate. J. Coat. Technol. Res. 2014, 11, 883–898. [Google Scholar] [CrossRef]
- Bendix, P.B.; Henninger, S.K.; Henning, H.-M. Temperature and Mechanical Stabilities and Changes in Porosity of Silicone Binder Based Zeolite Coatings. Ind. Eng. Chem. Res. 2016, 55, 4942–4947. [Google Scholar] [CrossRef]
- Kummer, H.; Fuldner, G.; Henninger, S.K. Versatile siloxane based adsorbent coatings for fast water adsorption processes in thermally driven chillers and heat pumps. Appl. Therm. Eng. 2015, 85, 1–8. [Google Scholar] [CrossRef]
- Dawoud, B. Water vapor adsorption kinetics on small and full scale zeolite coated adsorbers; A comparison. Appl. Therm. Eng. 2013, 50, 1645–1651. [Google Scholar] [CrossRef]
- Vasta, S.; Giacoppo, G.; Barbera, O.; Calabrese, L.; Bonaccorsi, L.; Freni, A. Innovative zeolite coatings on graphite plates for advanced adsorbers. Appl. Therm. Eng. 2014, 72, 153–159. [Google Scholar] [CrossRef]
- Ammann, J.; Michel, B.; Ruch, P.W. Characterization of transport limitations in SAPO-34 adsorbent coatings for adsorption heat pumps. Int. J. Heat Mass Transf. 2019, 129, 18–27. [Google Scholar] [CrossRef]
- Jiang, Y.; Ge, T.S.; Wang, R.Z.; Hu, L.M. Experimental investigation and analysis of composite silica-gel coated fin-tube heat exchangers. Int. J. Refrig. 2015, 51, 169–179. [Google Scholar] [CrossRef]
- McCague, C.; Huttema, W.; Fradin, A.; Bahrami, M. Lab-scale sorption chiller comparison of FAM-Z02 coating and pellets. Appl. Therm. Eng. 2020, 173, 115219. [Google Scholar] [CrossRef]
- Gluesenkamp, K.R.; Frazzica, A.; Velte, A.; Metcalf, S.; Yang, Z.; Rouhani, M.; Blackman, C.; Qu, M.; Laurenz, E.; Rivero-Pacho, A.; et al. Experimentally Measured Thermal Masses of Adsorption Heat Exchangers. Energies 2020, 13, 1150. [Google Scholar] [CrossRef] [Green Version]
- Qian, S.; Gluesenkamp, K.; Hwang, Y.; Radermacher, R.; Chun, H.H. Cyclic steady state performance of adsorption chiller with low regeneration temperature zeolite. Energy 2013, 60, 517–526. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Freni, A.; Proverbio, E. Silicone composite foams for adsorption heat pump applications. Sustain. Mater. Technol. 2017, 12, 27–34. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Bruzzaniti, P.; Freni, A.; Proverbio, E. Morphological and functional aspects of zeolite filled siloxane composite foams. J. Appl. Polym. Sci. 2018, 135, 45683. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Frazzica, A. Experimental evaluation of the hydrothermal stability of a silicone/zeolite composite foam for solar adsorption heating and cooling application. J. Appl. Polym. Sci. 2019, 137, 48311. [Google Scholar] [CrossRef]
- Calabrese, L.; Bruzzaniti, P.; Palamara, D.; Freni, A.; Proverbio, E. New SAPO-34-SPEEK composite coatings for adsorption heat pumps: Adsorption performance and thermodynamic analysis. Energy 2020, 203, 117814. [Google Scholar] [CrossRef]
- Chanda, R.; Selvam, T.; Herrmann, R.; Schwieger, W. Reactive coating process for binder-free zeolite FAU films on metallic aluminum supports. Mater. Lett. 2018, 211, 103–106. [Google Scholar] [CrossRef]
- Aristov, Y. Concept of adsorbent optimal for adsorptive cooling/heating. Appl. Therm. Eng. 2014, 72, 166–175. [Google Scholar] [CrossRef]






| Ref. | Working Pairs | Binder/Additives | Performed Characterizations | Results |
|---|---|---|---|---|
| [41] | Silica gel/water | Expanded graphite | Morphology: SEM Adsorption equilibrium: isobars Gas permeability, µ Effective thermal conductivity, λeff | Adsorption: up to 0.32 g/g at 30 °C and 4.3 kPa µ: 18–32 × 10−12 m2 at 8 MPa of compression λeff: 10–18 W/(m K) |
| [43] | Activated carbon (AC)/ammonia | Expanded graphite | Morphology: SEM Adsorption equilibrium: isobars Gas permeability, µ Effective thermal conductivity, λeff | Adsorption: up to 0.5 g/g at 50 °C and 2114 kPa µ: 1.0–7.8 × 10−10 m2 with 71% of AC inside the consolidate λeff: 10–18 W/(m K) |
| [44] | Zeolite/water | Polytetrafluoroethylene | Thermal conductivity, λ | λ: up to 0.38 W/(m K) |
| [21] | Activated carbon/methanol | Polytetrafluoroethylene | Thermal conductivity, λ | λ: up to 0.18 W/(m K) |
| [38] | Activated carbon/ethanol | Expanded graphite and Poly(vinyl alcohol) | Adsorption equilibrium: isobars Thermal conductivity, λ | Adsorption up to 0.87 g/g at 20 °C and 4.28 kPa λ: up to 0.72 W/(m K) |
| [36] | Silica gel–CaCl2/water | Expanded graphite or copper powders and Polyvinylpyrrolidone | Morphology: SEM Adsorption equilibrium: isotherms Cycling stability Thermal conductivity, λ | Adsorption up to 0.32 g/g at 34.7 °C and 2.5 kPa Stability checked up to 300 cycles λ: up to 0.78 W/(m K) |
| [35] | Zeolite 13X/water | Polyaniline | Adsorption equilibrium: isotherms Thermal conductivity, λ | Adsorption up to 0.30 g/g at 30 °C and 2.5 kPa λ: up to 0.78 W/(m K) |
| [28] | Silica gel–CaCl2/water | Bentonite | Small-scale prototype testing | COP: 0.15–0.30 Specific cooling power: 150–200 W/kg Global heat transfer coefficient: 80 W/(m2 K) |
| Ref. | Working Pairs | Substrate | Performed Characterizations | Results |
|---|---|---|---|---|
| [52] | SAPO-34/water | Aluminum fibers onto aluminum HEX | Adsorption kinetic on small-scale adsorber Adsorption kinetic on full-scale adsorber | Volumetric-Specific Cooling Power per adsorber (VSCP): 55 kW/m3 (small-scale)–59 kW/m3 (full-scale) Mass-Specific Cooling Power per adsorbent mass (MSCP): 0.96 kW/kg (small-scale)–0.77 kW/kg (full-scale) |
| [47] | Zeolite A/water Zeolite X/water | Stainless steel plates | XRD SEM thermal gravimetric analysis (TGA) | Pure adsorbent coating the stainless steel surface Polymeric coating improves the stability of the crystallized layer |
| [62] | Zeolite A/water Zeolite X/water | Stainless steel plates | XRD Adsorption kinetic on small-scale adsorber | Pure adsorbent coating the stainless steel surface Mass-Specific Cooling Power per adsorbent mass (MSCP): 5.5 kW/kg (zeolite X) * |
| [49] | Zeolite X/water | Stainless steel plates | XRD Adsorption equilibrium: isotherm | Pure adsorbent coating the stainless steel surface Adsorption: up to 0.35 g/g at 40 °C and 0.85 p/p0 |
| [50] | Zeolite Y/water | Stainless steel plates | SEM XRD Adhesion DC polarization curves | Good morphology and pure adsorbent coating the stainless steel surface Highest achievable adhesion quality Good protection against corrosion issues |
| [19] | Zeolite A/water | Copper and stainless steel fibrous plates | XRD 3D laser microscope Adsorption kinetic on small-scale adsorber | Pure adsorbent coating the surfaces Good distribution of the coating over the fibers Mass-Specific Cooling Power per adsorbent mass (MSCP): 4-6 kW/kg (varying with substrate) * |
| [26] | Metal–organic framework-MOF (aluminum fumarate)/water | Aluminum sheet | XRD Optical microscope and SEM Adsorption equilibrium: isobar Thermal conductivity, λ | Pure adsorbent coating the surfaces Coating uniform and with a microporous structure Adsorption: up to 0.35 g/g at 40 °C and 5.6 kPa λ: 0.3 W/(m K) |
| [55] | MOF (HKUST)/water | Copper sheet | XRD Optical microscope and SEM FT-IR Thermal conductivity, λ | Pure adsorbent coating the surfaces λ: 1.4 W/(m K) |
| [60] | SAPO34/water | Graphitic supports | XRD SEM Adsorption equilibrium: isobar | Pure adsorbent coating the surfaces Coating well-distributed and adherent on the surface Adsorption: up to 0.25 g/g at 40 °C and 2.5 kPa |
| Ref. | Working Pairs | Binder Type and Amount | Substrate | Performed Characterizations | Results |
|---|---|---|---|---|---|
| [18] | SAPO-34/water | Silane-10 wt.% | Aluminum finned flat-tube HEX | Mechanical characterization Adsorption kinetic small-scale Lab-scale adsorber testing | Pull-off strength: 0.63 MPa; peel detached area: 21.0% (3B index); Damage impact energy: 177.7 mJ; microhardness: 2.5 HV; Effective diffusion coefficient: from 1.97 × 10−10 to 1.70 × 10−11 m2/s Specific Cooling Power: 675 W/kgads Volumetric Cooling Power: 93 W/dm3 Cooling COP: 0.24 |
| [64] | SAPO-34/water | Bentonite and carbon fibers-20 wt.% | Aluminum plate | XRD Adsorption equilibrium: isobars Large Pressure Jump kinetic | Crystalline structure confirmed after the synthesis Adsorption up to 0.235 g/g at 30 °C and 1.2 kPa Effective diffusion coefficient: from 1 × 10−3 to 3.5 × 10−3 m2/s Effective thermal conductivity: up to 0.8 W/(m K) |
| [65] | AlPO-18/water | Polyvinyl alcohol (PVOH)—10 wt.% | Aluminum plate | SEM Large Pressure Jump kinetic | Clear macroporosity of the coating highlighted. Good distribution of the binder and powders. Fast kinetic: 90% of conversion in less than 2 min for coating thickness < 200 µm |
| [20] | SAPO-34/water FAPO-34/water | Trimethoxypropylsilane—5 wt.% | Aluminum sheet | Nitrogen physisorption SEM Adsorption equilibrium: isotherms Adsorption kinetic | Slight pore volume reduction due to the binder More homogeneous surface for FAPO coating than SAPO one Adsorption kinetic two to three times faster than the same configuration using silica gel as adsorbent SAPO-34 Adsorption up to 0.3 g/g at 20 °C and 0.7 p/p0 FAPO-34 Adsorption up to 0.24 g/g at 20 °C and 0.75 p/p0 |
| [70] | Zeolite Y/water | Different commercial binders and compositions | Aluminum sheet | Nitrogen physisorption Mechanical stability Thermogravimetric analysis (TGA) Temperature-controlled Diffuse Reflectance Infrared Fourier Transform Spectroscopy (T-DRIFTS) | Reduction in pore volume and specific surface area in line with the binder content Crosscut, bend and impact tests performed to evaluate the most performing coating composition Identification of critical solvent losses for binders having curing process at ambient conditions Loss of absorbance for some of the samples at increasing temperature due to the formation of links |
| [71] | SAPO-34/water Zeolite Y/water | Commercial silicone resin—from 3 to 25 wt.% | Aluminum plate | Coating thickness Cycling stability Adsorption equilibrium: isobars Large Pressure Jump kinetic | Average thickness 240 µm for SAPO-34 with 25 wt.% of binder Coating almost destroyed after 500 hydrothermal cycles with 2.5 wt.% of binder. Stable, with color variation, up to 3000 cycles for the one with 25 wt.% of binder. Adsorption capacity reduction in line with the amount of binder. Fast adsorption kinetic independently on the amount of binder. |
| [72] | AQSOA Z02 (commercial SAPO-34)/water | Unknown (commercial product) | Aluminum plate and finned-tube HEX | Large Temperature Jump kinetic Single module testing | Small scale kinetic two times faster for 200 µm thick coating against 500 µm. Coating of 300 µm 1.8 to 3.8 times faster than loose grains configuration. Thermal COP: 1.25 for 500 µm thick coating; 1.17 for 200 µm thick coating. |
| [73] | SAPO-34/water | Silane—15 wt.% | Graphite plates | Adsorption equilibrium: isobars | Adsorption up to 0.225 g/g at 30 °C and 1 kPa |
| [74] | AQSOA Z02 (commercial SAPO-34)/water | Hydroxyethyl ether—5 wt.% | Aluminum sheet | SEM Adsorption equilibrium: isobars Impedance and sorption kinetic | Uniform thickness of the coating Adsorption up to 0.3 g/g at 30 °C and 1.6 kPa Mass transfer identified as main limiting factor during the adsorption regardless the coating thickness |
| [66] | Silica gel/water | Polyvinyl alcohol (PVA)—n.a. | Stainless steel tube | Nitrogen physisorption Breakthrough curve | Slight reduction in pore volume and specific surface area due to the binder Thinner layer with larger grain size shows the best performance in terms of mass transfer resistance |
| [67] | Silica gel/water | Polyvinylpyrrolidone (PVP)—n.a. | Copper plate | SEM Thermal conductivity, λ | Well distributed silica gel grains inside the coating λ: 0.25–0.3 W/(m K) Thermal contact resistance: 1.29–3.80 K/W |
| [27] | Composite silica gel-LiCl/water | Liquid glue—n.a. | Aluminum glue | Nitrogen physisorption Thermal conductivity Sorption kinetic Adsorption equilibrium: isotherms | Pore volume and specific surface area reduction mainly due to LiCl embedding inside the pores λ: 3.3–5.8 W/(m K) Comparable kinetic curve in the first adsorption phase, regardless the salt content, due to the enhanced heat transfer of the coating Adsorption up to 0.65 g/g at 20 °C and 0.8 p/p0 |
| [75] | Silica gel/water | Liquid glue—n.a | Finned-tube HEX | Dehumidification performance | Analysis of different operating conditions parameters (e.g., humidity, temperature, heat transfer flow rate etc.) |
| [25] | Basolite A520 (commercial aluminum fumarate)/water | Polysiloxane—c.a 23 wt.% | Finned-tube HEX | Lab-scale module testing | Average cooling COP: 0.65 @ 16 °C evaporation temperature Volumetric Specific Cooling Power: 101 W/dm3 |
| Reference | Working Pair | Technology | Testing Conditions: Tdes/Tcon/Tads/Tev | Mass SCP (W/kgads) | Volumetric SCP (kW/m3) | COP | Metal-to-Adsorbent Ratio | Coating Thickness (µm) | HEX Volume (dm3) |
|---|---|---|---|---|---|---|---|---|---|
| [18] | SAPO-34/water | Binder based | 90/28/28/15 °C | 675 | 93 | 0.24 (cooling) | 6.07 | 100 | 1.0 |
| [52] | SAPO-34/water | In situ crystallization | 90/21/39.8 °C Pressure jump from 2.6 to 11 mbar | 770 | 59 | - | 5.55 | 45 | 3.0 |
| [28] | SWS 1L/water | Consolidated bed | 95/35/20/12 °C | 150-200 | - | 0.15–0.3 (cooling) | 3.47 | - | 8.6 |
| [72] | AQSOA 02/water | Binder based | 90/35/35/5 °C | - | - | 1.17–1.25 (heating) | 2.58–4.37 | 300–500 | 15.4 |
| [72] | AQSOA 02/water | Binder based | 90/35/35/5 °C | - | - | 1.21–1.23 (heating) | 2.59–2.85 | 150–200 | 1.69 |
| [25] | Aluminum fumarate MOF/water | Binder based | 65/35/35/22–10 °C * 90/30/30/18 °C ** | 1394 | 101 | 0.1–0.7 (cooling) | 6.08 | 300–330 | 6.77 |
| [78] | AQSOA 01/water | Binder based | 70/34–28/34–28/13–7 °C | 50–350 | - | 0.1–0.4 (cooling) | - | - | - |
| [76] | AQSOA 02/water | Binder based | 90/30/30/15 °C | 250–480 | 45–90 | 0.18–0.34 | 3.13 | 330 | 4.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caprì, A.; Frazzica, A.; Calabrese, L. Recent Developments in Coating Technologies for Adsorption Heat Pumps: A Review. Coatings 2020, 10, 855. https://doi.org/10.3390/coatings10090855
Caprì A, Frazzica A, Calabrese L. Recent Developments in Coating Technologies for Adsorption Heat Pumps: A Review. Coatings. 2020; 10(9):855. https://doi.org/10.3390/coatings10090855
Chicago/Turabian StyleCaprì, Angela, Andrea Frazzica, and Luigi Calabrese. 2020. "Recent Developments in Coating Technologies for Adsorption Heat Pumps: A Review" Coatings 10, no. 9: 855. https://doi.org/10.3390/coatings10090855
APA StyleCaprì, A., Frazzica, A., & Calabrese, L. (2020). Recent Developments in Coating Technologies for Adsorption Heat Pumps: A Review. Coatings, 10(9), 855. https://doi.org/10.3390/coatings10090855







