Spilanthes acmella Leaves Extract for Corrosion Inhibition in Acid Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
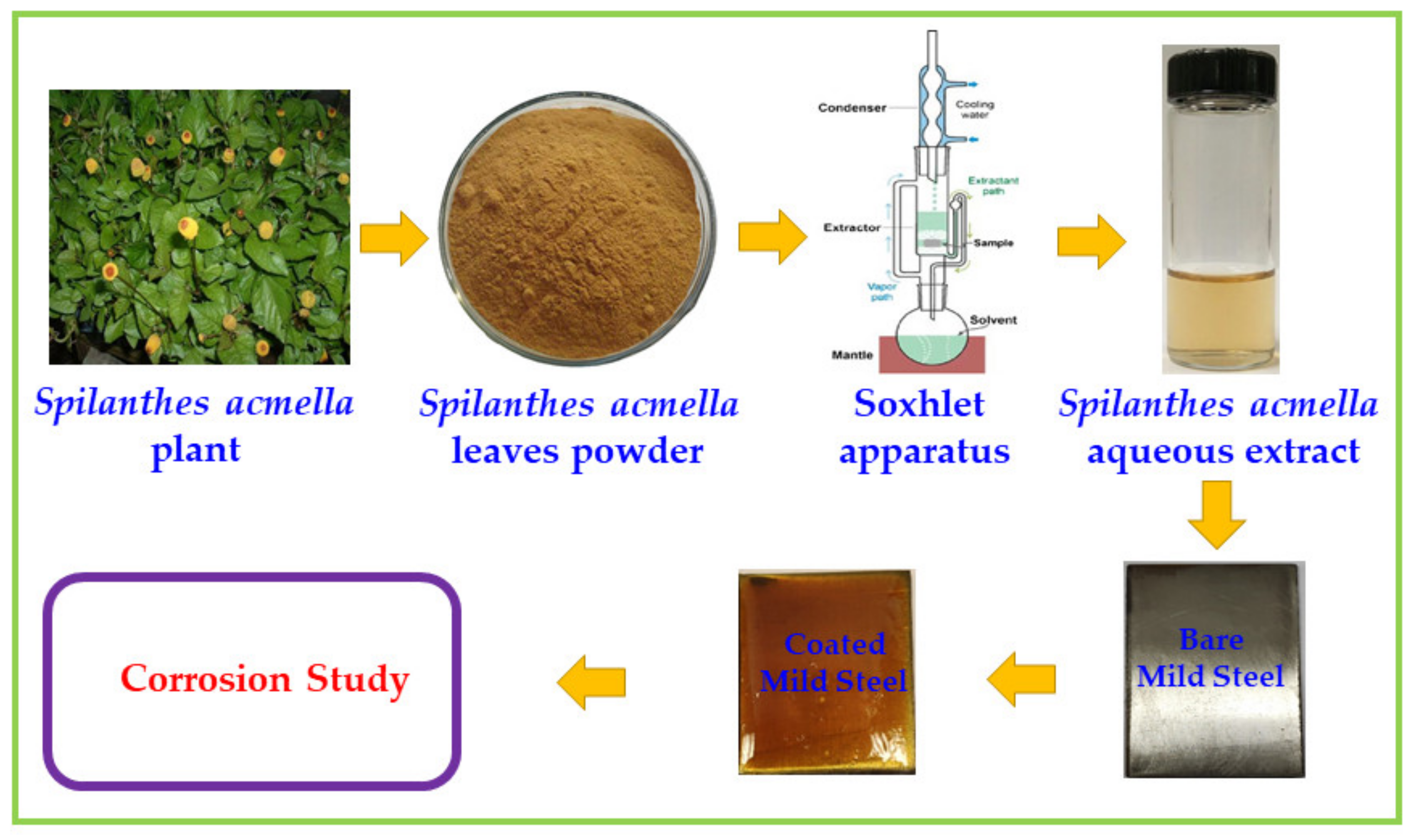
2.2.1. Preparation of Leaves Extract
2.2.2. Preparation of Mild Steel
2.2.3. Selection of Medium
2.3. Corrosion Inhibition Study
2.3.1. Mass Loss Method
2.3.2. Effect of Concentrations, Temperature, and Corrosion Rate
2.3.3. Adsorption Isotherm, Energy of Activation (Ea) and Free Energy of Adsorption (ΔGoads)
2.3.4. Electrochemical Methods
Potentiodynamic (Tafel) Polarization, Linear Polarization Resistance, Electrochemical Impedance, and Bode Studies
2.4. Surface Analysis
3. Results and Discussion
3.1. Characterization of Aqueous Leaves Extract of SA
3.1.1. Preliminary Phytochemical Screening of the Leaves Extract
3.1.2. FT-IR Spectroscopic Study of Aqueous Extract of the Leaves of SA
3.1.3. UV-Visible Absorption Spectral Studies
3.2. Weight Loss Method
3.2.1. Effect of Concentration of Leaves Extract on Corrosion Inhibition
3.2.2. Effect of Temperatures on the Anti-Corrosion Activity of Spilanthes Acmella Leaves SA-LE
3.3. Adsorption Isotherm
3.3.1. Langmuir Adsorption Isotherm
3.3.2. Temkin Adsorption Isotherm
3.4. Thermodynamic Adsorption Parameter
3.4.1. Free Energy Adsorption (ΔGoads)
3.4.2. Enthalpy and Entropy of Adsorption for Corrosion Process
3.5. Activation Parameter for Corrosion Inhibition Process
Energy of Activation (Ea)
3.6. Electrochemical Methods
Potentiodynamic Polarization Method
3.7. Surface Analytical Methods
3.7.1. Atomic Force Microscopy (AFM)
3.7.2. Scanning Electron Microscope Analysis (SEM)
3.7.3. Contact Angle Measurements
3.7.4. FT-IR Spectral Studies
FT-IR Results for SA-LE/Mild Steel/1.0 M HCl
UV-Visible Spectral Analysis
3.8. Phyto-Constituents of SA-LE
3.9. Corrosion Inhibition Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ukoba, O.; Oke, P.; Ibegbulam, M. Corrosion Behaviour of Ductile Iron in Different Environment. Int. J. Sci. Technol. 2012, 2, 618–621. [Google Scholar]
- Chen, Y.; Hong, T.; Gopal, M.; Jepson, W. EIS studies of a corrosion inhibitor behavior under multiphase flow conditions. Corros. Sci. 2000, 42, 979–990. [Google Scholar] [CrossRef]
- Riggs, O.L., Jr.; Hurd, R.M. Temperature coefficient of corrosion inhibition. Corrosion 1967, 23, 252–260. [Google Scholar] [CrossRef]
- Chigondo, M.; Chigondo, F. Recent natural corrosion inhibitors for mild steel: An overview. J. Chem. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Oyekunle, D.; Agboola, O.; Ayeni, A. Corrosion Inhibitors as Building Evidence for Mild Steel: A Review; Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; p. 032046. [Google Scholar]
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef] [Green Version]
- Zaferani, S.H.; Sharifi, M.; Zaarei, D.; Shishesaz, M.R. Application of eco-friendly products as corrosion inhibitors for metals in acid pickling processes-A review. J. Environ. Chem. Eng. 2013, 1, 652–657. [Google Scholar] [CrossRef]
- Raja, P.B.; Sethuraman, M.G. Natural products as corrosion inhibitor for metals in corrosive media-A review. Mater. Lett. 2008, 62, 113–116. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Bahadur, I.; Quraishi, M. An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. J. Mol. Liq. 2018, 266, 577–590. [Google Scholar] [CrossRef]
- Sanyal, B. Organic compounds as corrosion inhibitors in different environments-A review. Prog. Org. Coat. 1981, 9, 165–236. [Google Scholar] [CrossRef]
- Mo, S.; Luo, H.-Q.; Li, N.-B. Plant extracts as “green” corrosion inhibitors for steel in sulphuric acid. Chem. Pap. 2016, 70, 1131–1143. [Google Scholar] [CrossRef]
- Fang, Y.; Suganthan, B.; Ramasamy, R.P. Electrochemical characterization of aromatic corrosion inhibitors from plant extracts. J. Electroanal. Chem. 2019, 840, 74–83. [Google Scholar] [CrossRef]
- Miralrio, A.; Espinoza Vázquez, A. Plant Extracts as Green Corrosion Inhibitors for Different Metal Surfaces and Corrosive Media: A Review. Processes 2020, 8, 942. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Obot, I.B.; Suleiman, R.K. A critical review on the recent studies on plant biomaterials as corrosion inhibitors for industrial metals. J. Ind. Eng. Chem. 2019, 76, 91–115. [Google Scholar] [CrossRef]
- El Ibrahimi, B.; Jmiai, A.; Bazzi, L.; El Issami, S. Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arab. J. Chem. 2020, 13, 740–771. [Google Scholar] [CrossRef]
- Raja, P.B.; Fadaeinasab, M.; Qureshi, A.K.; Rahim, A.A.; Osman, H.; Litaudon, M.; Awang, K. Evaluation of green corrosion inhibition by alkaloid extracts of Ochrosia oppositifolia and isoreserpiline against mild steel in 1 M HCl medium. Ind. Eng. Chem. Res. 2013, 52, 10582–10593. [Google Scholar] [CrossRef]
- Morad, M.S.S.; Hermas, A.E.H.A.; Aal, M.S.A. Effect of amino acids containing sulfur on the corrosion of mild steel in phosphoric acid solutions polluted with Cl−, F− and Fe3+ ions–behaviour near and at the corrosion potential. J. Chem. Technol. Biotechnol Int. Res. Process Environ. Clean Technol. 2002, 77, 486–494. [Google Scholar] [CrossRef]
- Olivares-Xometl, O.; Likhanova, N.; Domínguez-Aguilar, M.; Arce, E.; Dorantes, H.; Arellanes-Lozada, P. Synthesis and corrosion inhibition of α-amino acids alkylamides for mild steel in acidic environment. Mater. Chem. Phys. 2008, 110, 344–351. [Google Scholar] [CrossRef]
- Dubey, S.; Maity, S.; Singh, M.; Saraf, S.A.; Saha, S. Phytochemistry, pharmacology and toxicology of Spilanthes acmella: A review. Adv. Pharmacol. Sci. 2013, 2013, 1–9. [Google Scholar]
- Dias, A.; Santos, P.; Seabra, I.; Júnior, R.; Braga, M.; De Sousa, H. Spilanthol from Spilanthes acmella flowers, leaves and stems obtained by selective supercritical carbon dioxide extraction. J. Supercrit. Fluids 2012, 61, 62–70. [Google Scholar] [CrossRef]
- Elufioye, T.O.; Habtemariam, S.; Adejare, A. Chemistry and Pharmacology of Alkylamides from Natural Origin. Rev. Bras. Farmacogn. 2020, 30, 1–19. [Google Scholar] [CrossRef]
- Durodola, S.S.; Adekunle, A.S.; Olasunkanmi, L.O.; Oyekunle, J.A. Inhibition of Mild Steel Corrosion in Acidic Medium by Extract of Spilanthes Uliginosa Leaves. Electroanalysis 2020, 32, 2693–2702. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance spectroscopy: Emphasizing solid materials and systems. ApOpt 1989, 28, 1083. [Google Scholar]
- Towfri, L.; Kadri, A.; Khelifa, A.; Aimeur, N.; Benlrahim, N. The inhibition and adsorption processes of L-cysteine against the corrosion of XC 18 carbon Steel in 2N H2SO4. J. Eng. Appl. Sci. 2008, 3, 688–696. [Google Scholar]
- Satapathy, A.; Gunasekaran, G.; Sahoo, S.; Amit, K.; Rodrigues, P. Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros. Sci. 2009, 51, 2848–2856. [Google Scholar] [CrossRef]
- Vankar, P.S.; Srivastava, J. Comparative study of total phenol, flavonoid contents and antioxidant activity in Canna indica and Hibiscus rosa sinensis: Prospective natural food dyes. Int. J. Food Eng. 2008, 4. [Google Scholar] [CrossRef]
- Acharya, J.; Sahu, J.; Sahoo, B.; Mohanty, C.; Meikap, B. Removal of chromium (VI) from wastewater by activated carbon developed from Tamarind wood activated with zinc chloride. Chem. Eng. J. 2009, 150, 25–39. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Fu, H. Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract. Corros. Sci. 2012, 62, 163–175. [Google Scholar] [CrossRef]
- Yadav, R. Phytochemical Screening of Spilanthes acmella plant parts. Int. J. Pharm. Erud. 2012, 1, 44–72. [Google Scholar]
- Harborne, A. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Berlin, Germany, 1998. [Google Scholar]
- Nabi, N.G.; Wani, T.A.; Shrivastava, M.; Rashid, A.; Shah, S. Spilanthes acmella an endangered medicinal plant-its Traditional, Phytochemical and Therapeutic properties-An overview. Int. J. Adv. Res. 2016, 4, 627–639. [Google Scholar]
- Cheng, Y.-B.; Liu, R.H.; Ho, M.-C.; Wu, T.-Y.; Chen, C.-Y.; Lo, I.-W.; Hou, M.-F.; Yuan, S.-S.; Wu, Y.-C.; Chang, F.-R. Alkylamides of Acmella oleracea. Molecules 2015, 20, 6970–6977. [Google Scholar] [CrossRef] [Green Version]
- Akalezi, C.O.; Enenebaku, C.K.; Oguzie, E.E. Inhibition of acid corrosion of mild steel by biomass extract from the Petersianthus macrocarpus plant. J. Mater. Environ. Sci. 2013, 4, 217–226. [Google Scholar]
- Solmaz, R.; Mert, M.; Kardaş, G.; Yazici, B.; Erbil, M. Adsorption and corrosion inhibition effect of 1, 1’-thiocarbonyldiimidazole on mild steel in H2SO4 solution and synergistic effect of iodide ion. Acta Phys. Chim. Sin. 2008, 24, 1185–1191. [Google Scholar] [CrossRef]
- Singh, A.K.; Quraishi, M. Effect of 2, 2’ benzothiazolyl disulfide on the corrosion of mild steel in acid media. Corros. Sci. 2009, 51, 2752–2760. [Google Scholar] [CrossRef]
- Noor, E.A.; Al-Moubaraki, A.H. Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-methyl-4 [4’(-X)-styryl pyridinium iodides/hydrochloric acid systems. Mater. Chem. Phys. 2008, 110, 145–154. [Google Scholar] [CrossRef]
- Shao, H.; Wang, J.; Zhang, Z.; Zhang, J.; Cao, C. The cooperative effect of calcium ions and tartrate ions on the corrosion inhibition of pure aluminum in an alkaline solution. Mater. Chem. Phys. 2003, 77, 305–309. [Google Scholar] [CrossRef]
- Sethi, T.; Chaturvedi, A.; Upadhyay, R.; Mathur, S. Corrosion inhibitory effects of some Schiff’s bases on mild steel in acid media. J. Chil. Chem. Soc. 2007, 52, 1206–1213. [Google Scholar] [CrossRef]
- Refaey, S.; Taha, F.; Abd El-Malak, A. Inhibition of stainless steel pitting corrosion in acidic medium by 2-mercaptobenzoxazole. Appl. Surf. Sci. 2004, 236, 175–185. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Obot, I.B.; Murulana, L. Quinoline and its derivatives as effective corrosion inhibitors for mild steel in acidic medium. Int. J. Electrochem. Sci 2010, 5, 1574–1586. [Google Scholar]
- Moretti, G.; Guidi, F.; Grion, G. Tryptamine as a green iron corrosion inhibitor in 0.5 M deaerated sulphuric acid. Corros. Sci. 2004, 46, 387–403. [Google Scholar] [CrossRef]
- Ahamad, I.; Prasad, R.; Quraishi, M. Adsorption and inhibitive properties of some new Mannich bases of Isatin derivatives on corrosion of mild steel in acidic media. Corros. Sci. 2010, 52, 1472–1481. [Google Scholar] [CrossRef]
- Shukla, S.K.; Ebenso, E.E. Corrosion inhibition, adsorption behavior and thermodynamic properties of streptomycin on mild steel in hydrochloric acid medium. Int. J. Electrochem. Sci. 2011, 6, 3277–3291. [Google Scholar]
- Gomma, G.K.; Wahdan, M.H. Effect of temperature on the acidic dissolution of copper in the presence of amino acids. Mater. Chem. Phys. 1994, 39, 142–148. [Google Scholar] [CrossRef]
- Obi-Egbedi, N.; Obot, I. Xanthione: A new and effective corrosion inhibitor for mild steel in sulphuric acid solution. Arab. J. Chem. 2013, 6, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Umoren, S.; Ebenso, E.; Okafor, P.; Ogbobe, O. Water-soluble polymers as corrosion inhibitors. Pigment Resin Technol. 2006, 35, 346–352. [Google Scholar] [CrossRef]
- Herrag, L.; Hammouti, B.; Elkadiri, S.; Aouniti, A.; Jama, C.; Vezin, H.; Bentiss, F. Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: Experimental and theoretical investigations. Corros. Sci. 2010, 52, 3042–3051. [Google Scholar] [CrossRef]
- Benali, O.; Benmehdi, H.; Hasnaoui, O.; Selles, C.; Salghi, R. Green corrosion inhibitor: Inhibitive action of tannin extract of Chamaerops humilis plant for the corrosion of mild steel in 0.5 M H2SO4. J. Mater. Environ. Sci. 2013, 4, 127–138. [Google Scholar]
- Badawy, W.; Al-Kharafi, F.; El-Azab, A. Electrochemical behaviour and corrosion inhibition of Al, Al-6061 and Al–Cu in neutral aqueous solutions. Corros. Sci. 1999, 41, 709–727. [Google Scholar] [CrossRef]
- Ghareba, S.; Omanovic, S. Corrosion inhibition of carbon steel in sulfuric acid by sodium caprylate. Balance 2016, 100, 74–84. [Google Scholar]
- Hassan, H.; Ismail, A.; Ahmad, S.; Soon, C. Super-Hydrophobic Green Corrosion Inhibitor on Carbon Steel; IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; pp. 1–5. [Google Scholar]
- Eddy, N.; Ebenso, E. Corrosion inhibition and adsorption properties of ethanol extract of Gongronema latifolium on mild steel in H2SO4. Pigment Resin Technol. 2010, 39, 77–83. [Google Scholar] [CrossRef]
- Abboud, Y.; Abourriche, A.; Saffaj, T.; Berrada, M.; Charrouf, M.; Bennamara, A.; Al Himidi, N.; Hannache, H. 2, 3-Quinoxalinedione as a novel corrosion inhibitor for mild steel in 1 M HCl. Mater. Chem. Phys. 2007, 105, 1–5. [Google Scholar] [CrossRef]
- Singh, A.; Ebenso, E.E.; Quraishi, M. Theoretical and electrochemical studies of Cuminum cyminum (Jeera) extract as green corrosion inhibitor for mild steel in hydrochloric acid solution. Int. J. Electrochem. Sci. 2012, 7, 8543–8559. [Google Scholar]
- Petchiammal, A.; Selvaraj, S. Investigation of Anti-Corrosive Effects of Lebbeck Seed Extract On Aluminum in Acid Environment. Pac. J. Sci. Technol. 2013, 14, 31–39. [Google Scholar]
- Bothi Raja, P.; Sethuraman, M. Strychnos nux-vomica an eco-friendly corrosion inhibitor for mild steel in 1 M sulfuric acid medium. Mater. Corros. 2009, 60, 22–28. [Google Scholar] [CrossRef]
- Arora, S.; Vijay, S.; Kumar, D. Phytochemical and antimicrobial studies on the leaves of Spilanthes acmella. J. Chem. Pharm. Res. 2011, 3, 145–150. [Google Scholar]
- Arif, M.; Juyal, D.; Joshi, A. A review on pharmacognostic and phytochemical study of a plant Spilanthes acmella Murr. Pharma. Innov. 2017, 6, 172. [Google Scholar]
- Obot, I.; Ebenso, E.; Gasem, Z.M. Eco-friendly corrosion inhibitors: Adsorption and inhibitive action of ethanol extracts of chlomolaena odorata l. For the corrosion of mild steel in H2So4 solutions. Int. J. Electrochem. Sci. 2012, 7, 1997–2008. [Google Scholar]
- Zakvi, S.; Mehta, G. Acid corrosion of mild steel and its inhibition by swertia aungustifolia-study by electrochemical techniques. Trans. SAEST 1988, 23, 407–410. [Google Scholar]
- Faiz, M.; Zahari, A.; Awang, K.; Hussin, H. Corrosion inhibition on mild steel in 1 M HCl solution by Cryptocarya nigra extracts and three of its constituents (alkaloids). RSC Adv. 2020, 10, 6547–6562. [Google Scholar] [CrossRef] [Green Version]
- Flick, E.W. Corrosion Inhibitors: An Industrial Guide; Noyes Publications: Park Ridge, NJ, USA, 1993. [Google Scholar]
- Soltani, N.; Tavakkoli, N.; Khayatkashani, M.; Jalali, M.R.; Mosavizade, A. Green approach to corrosion inhibition of 304 stainless steel in hydrochloric acid solution by the extract of Salvia officinalis leaves. Corros. Sci. 2012, 62, 122–135. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Lei, J.; He, J.; Zhang, S.; Pan, F. Adsorption and corrosion inhibition of Osmanthus fragran leaves extract on carbon steel. Corros. Sci. 2012, 63, 82–90. [Google Scholar] [CrossRef]
- Faustin, M.; Maciuk, A.; Salvin, P.; Roos, C.; Lebrini, M. Corrosion inhibition of C38 steel by alkaloids extract of Geissospermum laeve in 1 M hydrochloric acid: Electrochemical and phytochemical studies. Corros. Sci. 2015, 92, 287–300. [Google Scholar] [CrossRef]













| Phytochemical Constituents | Alkaloids | Carbohydrates | Flavonoids | Tannins | Amino Acids | Terpenoids |
|---|---|---|---|---|---|---|
| Aqueous extract of the leaves Spilanthes Acmella (SA-LE) | + | - | + | + | − | − |
| Phytochemical Constituents | Glycosides | Steroids | Anthraquinones | Saponins | Phenols | Proteins |
| Aqueous extract of the leaves Spilanthes Acmella (SA-LE) | + | + | − | − | − | − |
| Inhibitor System Leaf Extract | Absorption Bands (nm) | Transitions |
|---|---|---|
| SA | 231, 265 | η →π* π →π* η →σ* |
| Inhibitors | Concentration of Inhibitor (% v/v) | Corrosion Rate (mg/dm2 Day) | Inhibition Efficiency (%) |
|---|---|---|---|
| Blank | 615 | − | |
| SA | 2 | 219 | 64.34 |
| 4 | 176 | 71.30 | |
| 6 | 123 | 80.00 | |
| 8 | 096 | 84.34 | |
| 10 | 42 | 93.04 |
| Temperature (K) | Concentration of Inhibitor (% v/v) | Corrosion Rate (mg/dm2 Day) | Inhibition Efficiency (%) |
|---|---|---|---|
| 303 | Blank | 615 | − |
| 2 | 219 | 64.34 | |
| 4 | 176 | 71.30 | |
| 6 | 123 | 80.00 | |
| 8 | 96 | 84.34 | |
| 10 | 42 | 93.04 | |
| 313 | Blank | 979 | − |
| 2 | 379 | 61.20 | |
| 4 | 342 | 65.02 | |
| 6 | 310 | 68.30 | |
| 8 | 230 | 76.50 | |
| 10 | 187 | 80.87 | |
| 323 | Blank | 1181 | − |
| 2 | 535 | 52.15 | |
| 4 | 518 | 53.58 | |
| 6 | 470 | 57.89 | |
| 8 | 379 | 66.02 | |
| 10 | 294 | 73.68 | |
| 333 | Blank | 1251 | − |
| 2 | 690 | 44.87 | |
| 4 | 668 | 46.58 | |
| 6 | 577 | 53.84 | |
| 8 | 486 | 61.11 | |
| 10 | 428 | 65.81 |
| Inhibitor System | Temperature, K | R2 | Slope | Intercept | Kads | −ΔG0ads |
|---|---|---|---|---|---|---|
| SA | 303 | 0.9981 | 0.9582 | 0.1427 | 7.007 | 15.027 |
| 313 | 0.9968 | 1.1245 | 0.1953 | 5.120 | 14.706 | |
| 323 | 0.9635 | 1.2065 | 0.2310 | 4.329 | 14.725 | |
| 333 | 0.9676 | 1.298 | 1.0080 | 0.992 | 11.102 |
| Inhibitor System | Temperature (K) | R2 | Slope | Intercept | −a | Kads | −ΔGoads (KJ/mol) |
|---|---|---|---|---|---|---|---|
| SA | 303 | 0.9469 | 0.3942 | 0.5035 | 2.92 | 18.93 | 17.532 |
| 313 | 0.9859 | 0.4164 | 0.3833 | 2.76 | 8.32 | 15.972 | |
| 323 | 0.8519 | 0.3264 | 0.4085 | 2.81 | 6.295 | 15.731 | |
| 333 | 0.9378 | 0.6657 | 0.3153 | 2.97 | 2.97 | 14.143 |
| Inhibitor System | ΔGoads (kJ/mol−1) | |||
|---|---|---|---|---|
| 303 | 313 | 323 | 333 | |
| SA-LE | 15.027 | 14.706 | 14.725 | 11.102 |
| Inhibitor System | Mean Values | |
|---|---|---|
| −ΔHads0 KJ/mol | −ΔSads0 J/mol | |
| SA-LE | 15.932 | 126.3 |
| Inhibitor System | Ea (KJ/mol) |
|---|---|
| – | 24.30 |
| SA-LE | 67.45 |
| Concentration of the Leaves Extract (% v/v) | −Ecorr mV/SCE | Tafel Slope | Icorr mA/cm2 | Rp Ω.cm2 | Inhibition Efficiency (%) Calculated From: | ||
|---|---|---|---|---|---|---|---|
| ba mV/dec | bc mV/dec | Icorr mA/cm2 | Rp Ω.cm2 | ||||
| 0 | −434 | 140 | 232 | 6.488 × 10−3 | 5.9 | − | − |
| 2 | −429 | 10 | 225 | 1.527 × 10−3 | 20.4 | 76.4 | 71.0 |
| 10 | −473 | 108 | 096 | 1.024 × 10−4 | 216 | 98.4 | 97.2 |
| System | Surface Roughness | Line Roughness | ||||
|---|---|---|---|---|---|---|
| Average Roughness (Ra), nm | Root Mean Square Roughness (Rq), nm | Maximum Peak to Valley (P-V) Height, nm | Average Roughness (Ra), nm | Root Mean Square Roughness (Rq), nm | Maximum Peak to Valley (P-V) height, nm | |
| Polished mild steel | 117.5 | 162.7 | 171.5 | 64.1 | 76.9 | 359.0 |
| Mild steel + 1.0 M HCl+ 10% of aqueous leaf extract SA | 229.7 | 289.3 | 2524.1 | 185.9 | 221.3 | 1201.2 |
| Mild steel + 1.0 M HCl | 847.8 | 1086.9 | 8393.5 | 828.2 | 1084 | 4511.9 |
| System | Contact Angle (°) |
|---|---|
| Mild steel + 1.0 M HCl | 38.1 |
| Mild steel + 1.0 M HCl + 10% of SA-LE | 129.7 |
| Polished mild steel | 145.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begum, A.A.S.; Vahith, R.M.A.; Kotra, V.; Shaik, M.R.; Abdelgawad, A.; Awwad, E.M.; Khan, M. Spilanthes acmella Leaves Extract for Corrosion Inhibition in Acid Medium. Coatings 2021, 11, 106. https://doi.org/10.3390/coatings11010106
Begum AAS, Vahith RMA, Kotra V, Shaik MR, Abdelgawad A, Awwad EM, Khan M. Spilanthes acmella Leaves Extract for Corrosion Inhibition in Acid Medium. Coatings. 2021; 11(1):106. https://doi.org/10.3390/coatings11010106
Chicago/Turabian StyleBegum, Akbar Ali Samsath, Raja Mohamed Abdul Vahith, Vijay Kotra, Mohammed Rafi Shaik, Abdelatty Abdelgawad, Emad Mahrous Awwad, and Mujeeb Khan. 2021. "Spilanthes acmella Leaves Extract for Corrosion Inhibition in Acid Medium" Coatings 11, no. 1: 106. https://doi.org/10.3390/coatings11010106








