The Enhancement Effect of Salt Bath Chromizing for P20 Steel
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Experimental
2.3. Characterization of Modified Surface Layers
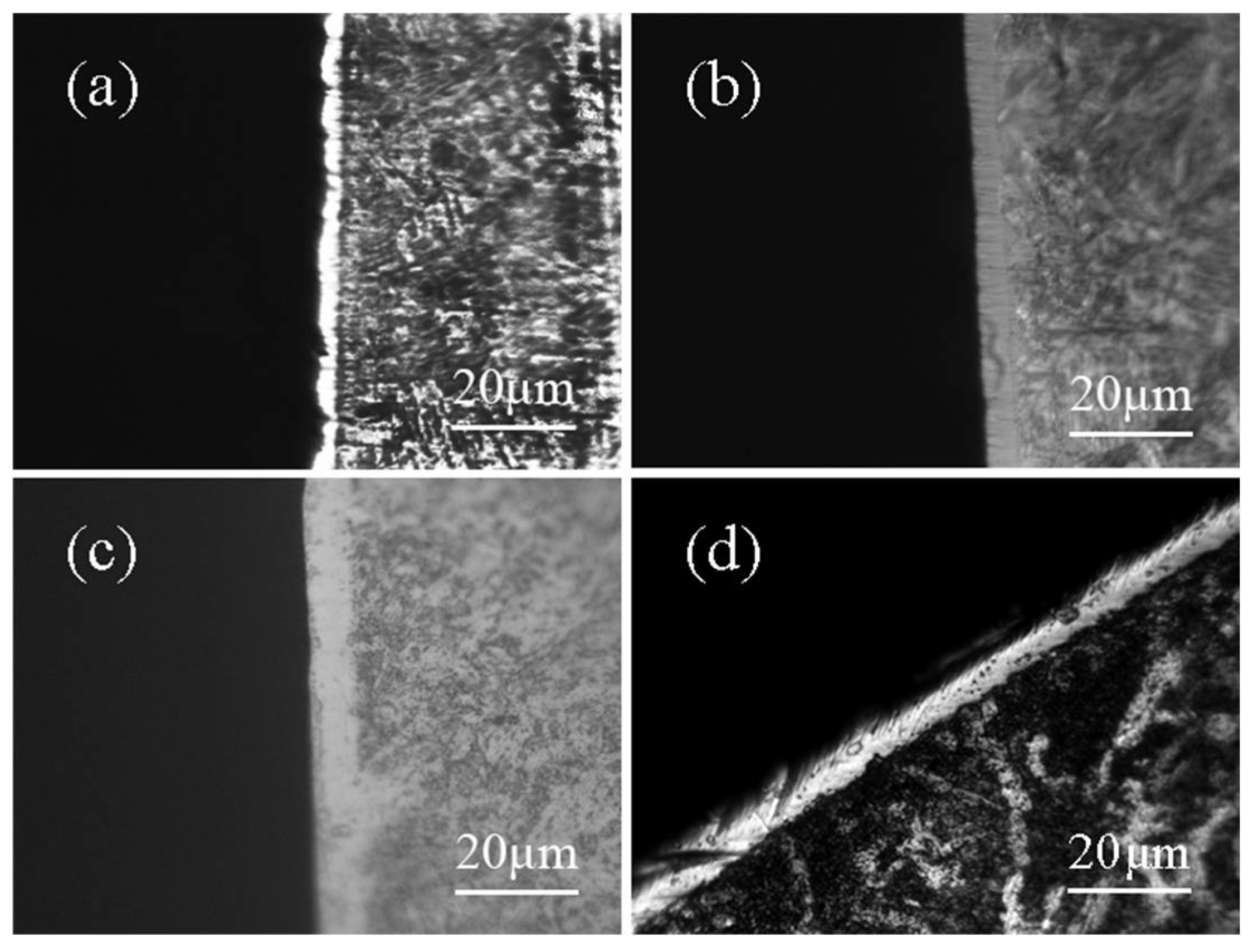
3. Results
3.1. Effect of Temperature on Chromizing in Salt Bath
3.2. Effect of Time on Chromizing in Salt Bath
3.3. Compositional Analysis
3.4. Micro-Hardness Profile
3.5. Corrosivity Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Campbell, I.E.; Barth, V.D. Salt-Bath Chromizing. J. Electrochem. Soc. 1949, 96, 262. [Google Scholar] [CrossRef]
- Su, X.; Zhao, S. Formation of chromium carbide coatings on HT250 steel by thermal diffusion processes in fluoride molten salt bath. Vacuum 2018, 155, 219–223. [Google Scholar] [CrossRef]
- Khalaj, G.; Nazari, A. Chromium carbonitride coating produced on DIN 1.2210 steel by thermo-reactive deposition technique: Thermodynamics, kinetics and modeling. Surf. Coat. Technol. 2013, 255, 1–10. [Google Scholar] [CrossRef]
- Saduman, S. Influence of chromium carbide coating on tribological performance of steel. Mater. Des. 2006, 25527, 85–91. [Google Scholar]
- Aliakbar, G.; Hassan, S. Diffusion mechanism in molten salt baths during the production of carbide coatings via thermal reactive diffusion. Int. J. Min. Met. Mater. 2017, 24, 1448–1458. [Google Scholar]
- Yuan, Z.; Wang, Z. Preparation and property of chromium carbide thermal diffusion coating on cold working die materials. J. Wuhan Univ. Technol. 2010, 25, 596–599. [Google Scholar] [CrossRef]
- Sen, S. A study on kinetics of CrxC-coated high-chromium steel by thermo-reactive diffusion technique. Vacuum 2005, 79, 63–70. [Google Scholar] [CrossRef]
- Wu, C.; Yuan, M. Microstructure of the composite chromized layer on 0.2 wt% steel after 500 °C salt-bath chromizing. J. Hunan Univ. (Nat. Sci.) 2010, 37, 60–64. [Google Scholar]
- Liu, X.; Wang, H. Study on kinetics of carbide coating growth by thermal diffusion process. Surf. Coat. Technol. 2006, 201, 2414–2418. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H. Effects of rare earths in borax salt bath immersion vanadium carbide coating process on steel matrix. Surf. Coat. Technol. 2008, 202, 4788–4792. [Google Scholar] [CrossRef]
- Platten, J.K. The soret effect: A review of recent experimental results. J. Appl. Mech. 2006, 73, 5–15. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J. Segregation of molten salt on chromizing in thermal diffusion process. J. Wuhan Univ. Technol. 2011, 26, 1189–1192. [Google Scholar] [CrossRef]
- Lu, X.G.; Selleby, M. Calculations of thermophysical properties of cubic carbides and nitrides using the Debye–Grüneisen model. Acta Mater. 2007, 55, 1215–1226. [Google Scholar] [CrossRef] [Green Version]
- Ganji, O.; Sajjadi, S.A. On the formation and properties of chromium carbide and vanadium carbide coatings produced on W1 tool steel through thermal reactive diffusion (TRD). Ceram. Int. 2020, 46, 25320–25329. [Google Scholar] [CrossRef]
- Biesuz, M.; Sglavo, V.M. Chromium and vanadium carbide and nitride coatings obtained by TRD techniques on UNI 42CrMoS4 (AISI 4140) steel. Surf. Coat. Technol. 2016, 286, 319–326. [Google Scholar] [CrossRef]
- Wu, C.; Hong, Y. A double strengthened surface layer fabricated by nitro-chromizing on carbon steel. Surf. Coat. Technol. 2016, 298, 83–92. [Google Scholar] [CrossRef]
- Liang, S.X.; Yin, L.X. Kinetics of thermodiffusion of TZ20 titanium alloy gas-nitride within temperature of 500–650 °C. J. Alloy. Compd. 2018, 734, 172–178. [Google Scholar] [CrossRef]
- Ye, J.L.; Ye, F. Effects of strong carbide-forming elements on low temperature salt-bath chromizing. Adv. Mater. Res. 2011, 214, 646–650. [Google Scholar] [CrossRef]
- Mahdavi, A.; Medvedovski, E. Corrosion resistance of boronized, aluminized, and chromized thermal diffusion-coated steels in simulated high-temperature recovery boiler conditions. Coatings 2018, 8, 257. [Google Scholar] [CrossRef] [Green Version]





| Experiment Group | Time/h | Temperature/°C |
|---|---|---|
| Group 1 | 5 | 930, 960, 980 |
| Group 2 | 3, 4.5, 6 | 960 |
| Spectrum | C | O | Na | Si | Cr | Mn | Fe |
|---|---|---|---|---|---|---|---|
| Spectrum 1 | – | – | – | – | 81.81 | – | 18.19 |
| Spectrum 2 | – | – | – | – | 52.83 | 1.25 | 45.92 |
| Spectrum 3 | – | – | – | 0.39 | 7.84 | 1.29 | 90.47 |
| Spectrum 4 | – | – | 1.81 | – | 2.04 | 1.43 | 74.70 |
| Spectrum | C | O | Na | Si | Cr | Mn | Fe |
|---|---|---|---|---|---|---|---|
| Spectrum 1 | – | – | – | – | 100.00 | – | – |
| Spectrum 2 | – | – | – | – | 99.39 | – | 0.61 |
| Spectrum 3 | – | – | – | – | 3.04 | 1.56 | 95.40 |
| Spectrum 4 | – | 1.24 | 2.01 | – | 2.14 | 1.42 | 93.89 |
| Hardness (HV2.94) | Chromium Carbide Coating | Chromium Plating Coating | Matrix | ||
|---|---|---|---|---|---|
| 3 h | 4.5 h | 6 h | |||
| Coatings | 1561.8 ± 0.5 | 1595.8 ± 0.5 | 1640.5 ± 0.5 | 690.8 ± 0.5 | 297.5 ± 0.5 |
| Matrix | 556.3 ± 0.5 | 562.4 ± 0.5 | 584.1 ± 0.5 | 320.4 ± 0.5 | – |
| Hardness (HV2.94) | Chromium Carbide Coating | Chromium Plating Coating | Matrix | ||
|---|---|---|---|---|---|
| 930 °C | 960 °C | 980 °C | |||
| Coatings | 1552.6 ± 0.5 | 1619.2 ± 0.5 | 1640.5 ± 0.5 | 690.8 ± 0.5 | 297.5 ± 0.5 |
| Matrix | 552.7 ± 0.5 | 573.8 ± 0.5 | 590.2 ± 0.5 | 320.4 ± 0.5 | – |
| Corrosion Time (h) | Corrosion Amount Per Unit Area of Three Groups of Specimens (mg/cm2) | Corrosion Rate of Three Groups of Specimens (g(m2·h)−1) | ||||
|---|---|---|---|---|---|---|
| Untreated | Chromium Plating | Chromium Carbide | Untreated | Chromium Plating | Chromium Carbide | |
| 6 | 0.0732 | 0.0138 | 0.0112 | 0.1220 | 0.0023 | 0.0019 |
| 12 | 0.2137 | 0.0362 | 0.0312 | 0.1781 | 0.0031 | 0.0026 |
| 24 | 0.9874 | 0.0618 | 0.0557 | 0.4114 | 0.0258 | 0.0232 |
| 36 | 2.1674 | 0.1583 | 0.1149 | 0.4515 | 0.0329 | 0.0239 |
| 48 | 2.7538 | 0.3867 | 0.2513 | 0.3671 | 0.0537 | 0.0349 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Zhu, C.; Zhou, L.; Wang, L. The Enhancement Effect of Salt Bath Chromizing for P20 Steel. Coatings 2021, 11, 27. https://doi.org/10.3390/coatings11010027
Wei Z, Zhu C, Zhou L, Wang L. The Enhancement Effect of Salt Bath Chromizing for P20 Steel. Coatings. 2021; 11(1):27. https://doi.org/10.3390/coatings11010027
Chicago/Turabian StyleWei, Zihao, Chundong Zhu, Lianpu Zhou, and Liming Wang. 2021. "The Enhancement Effect of Salt Bath Chromizing for P20 Steel" Coatings 11, no. 1: 27. https://doi.org/10.3390/coatings11010027
APA StyleWei, Z., Zhu, C., Zhou, L., & Wang, L. (2021). The Enhancement Effect of Salt Bath Chromizing for P20 Steel. Coatings, 11(1), 27. https://doi.org/10.3390/coatings11010027




