Engineering Copper Adhesion on Poly-Epoxy Surfaces Allows One-Pot Metallization of Polymer Composite Telecommunication Waveguides
Abstract
:1. Introduction
2. Materials and Methods
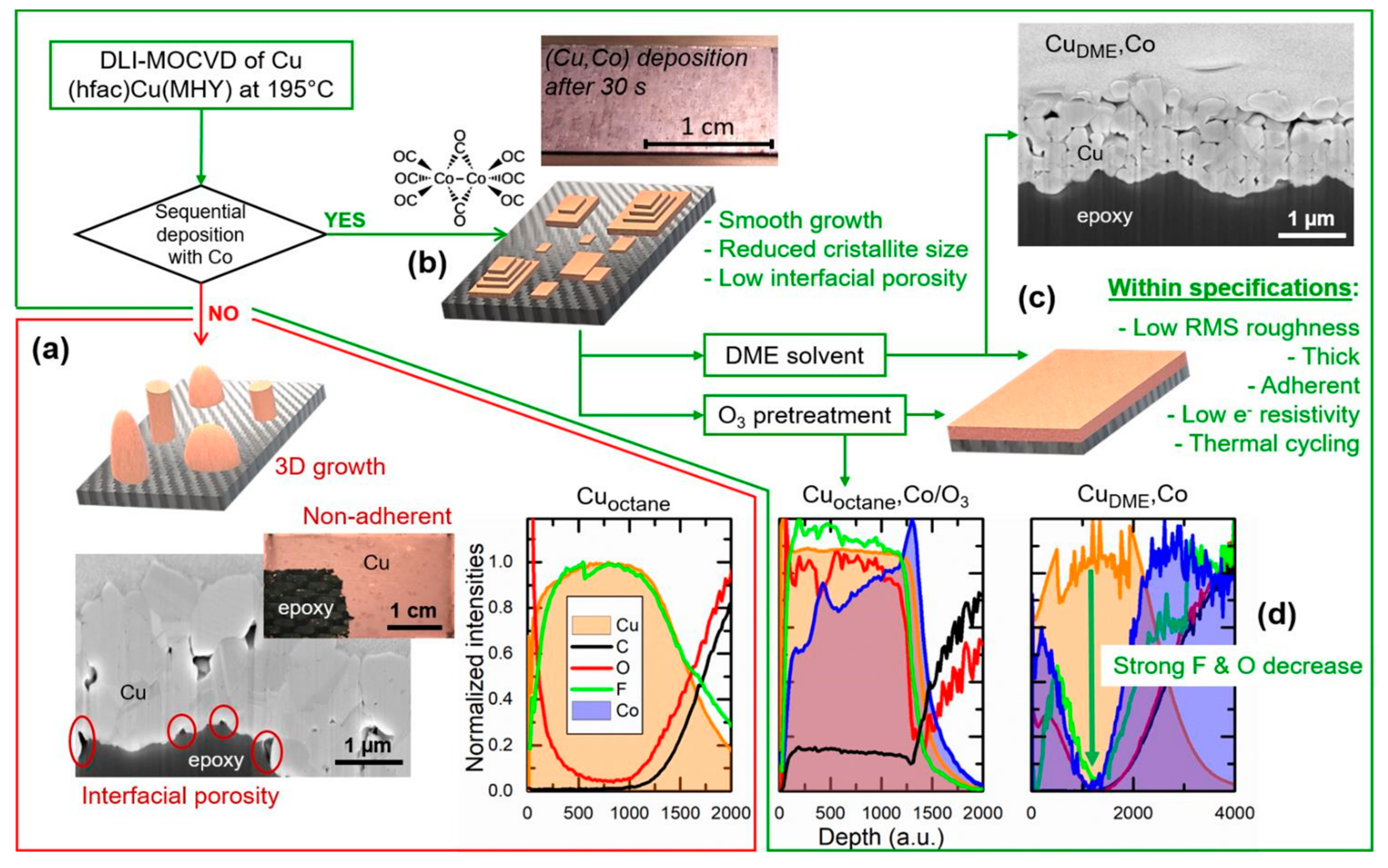
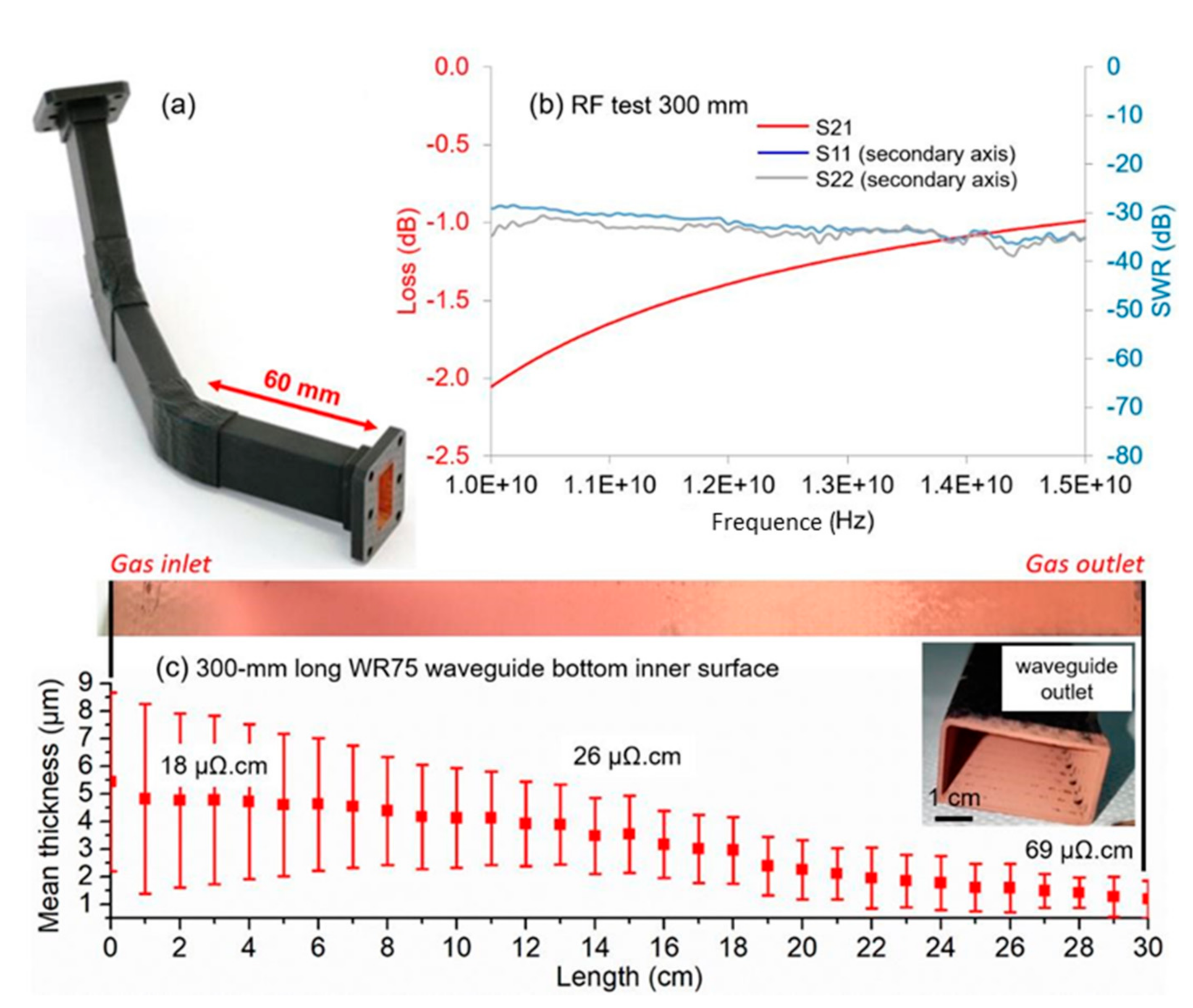
3. Results and Discussion
3.1. Relative Surface Energy
3.2. Microstructure and Composition
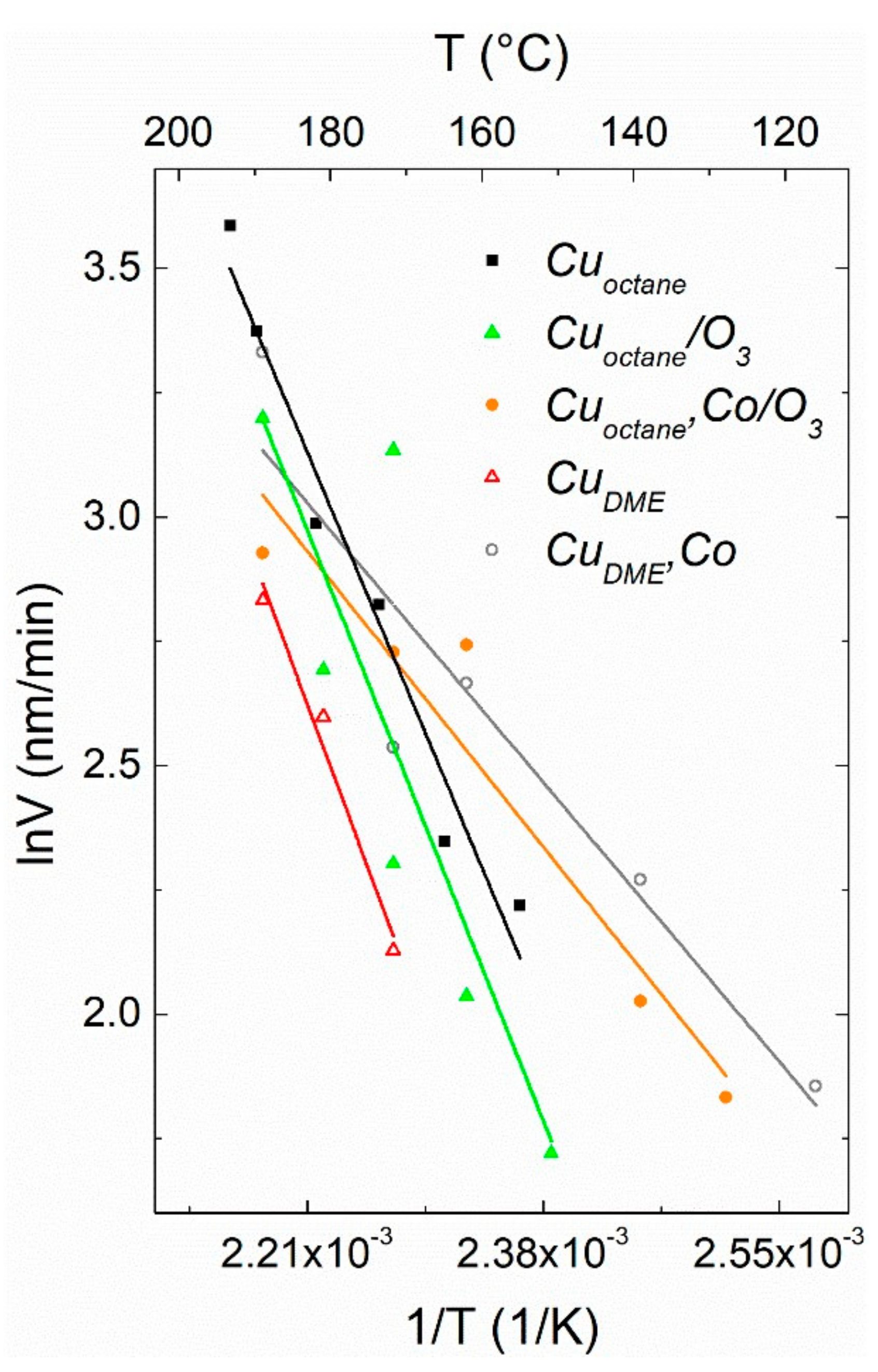
3.3. Chemical Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beck, M. Composites: The Final Frontier. Compos. Manuf. 2014, 30, 14–17. [Google Scholar]
- Reinhart, T.J. Engineered Materials Handbook: Composites; ASM International: Materials Park, OH, USA, 1987. [Google Scholar]
- Anguita, J.V.; Smith, C.T.G.; Stute, T.; Funke, M.; Delkowski, M.; Silva, S.R.P. Dimensionally and environmentally ultra-stable polymer composites reinforced with carbon fibres. Nat. Mater. 2020, 19, 317–322. [Google Scholar] [CrossRef]
- Alonso, F.; Fagoaga, I.; Oregui, P. Erosion protection of carbon epoxy composites by plasma-sprayed coatings. Surf. Coat. Technol. 1991, 49, 482–488. [Google Scholar] [CrossRef]
- Lopera-Valle, A.; McDonald, A. Application of flame-sprayed coatings as heating elements for polymer-based composite structures. J. Therm. Spray Technol. 2015, 24, 1289–1301. [Google Scholar] [CrossRef]
- Fallah, P.; Rajagopalan, S.; McDonald, A.; Yue, S. Development of hybrid metallic coatings on carbon fiber-reinforced polymers (CFRPs) by cold spray deposition of copper-assisted copper electroplating process. Surf. Coat. Technol. 2020, 400, 10. [Google Scholar] [CrossRef]
- Granado, L.; Kempa, S.; Gregoriades, L.J.; Bruning, F.; Bernhard, T.; Flaud, V.; Anglaret, E.; Frety, N. Improvements of the Epoxy-Copper Adhesion for Microelectronic Applications. ACS Appl. Electron. Mater. 2019, 1, 1498–1505. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhou, T.; Wen, L. Selective Metallization Induced by Laser Activation: Fabricating Metallized Patterns on Polymer via Metal Oxide Composite. ACS Appl. Mater. Interfaces 2017, 9, 8996–9005. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, P.; Zhang, H.Z.; Li, X.H.; Lei, L.; Chen, L.N.; Zheng, Z.J.; Yu, Y. Universal Nature-Inspired and Amine-Promoted Metallization for Flexible Electronics and Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 28963–28970. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.J.; Shi, X.Y.; Chai, M.Y.; Ji, J.; Xu, Z.K.; Wan, L.S. Surface Metallization of Porous Polymer Materials for Multifunctional Applications. Langmuir 2020, 36, 1454–1461. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, C.; Zheng, Z.J. Polymer-Assisted Metal Deposition (PAMD): A Full-Solution Strategy for Flexible, Stretchable, Compressible, and Wearable Metal Conductors. Adv. Mater. 2014, 26, 5508–5516. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.S.; He, L.C.; Wang, L.; Man, Y.C.; Huang, L.Y.; Xu, Z.; Ge, D.; Li, J.M.; Liu, C.; Wang, L.D. Significant Enhancement of the Adhesion between Metal Films and Polymer Substrates by UV-Ozone Surface Modification in Nanoscale. ACS Appl. Mater. Interfaces 2016, 8, 30576–30582. [Google Scholar] [CrossRef]
- Addou, F.; Duguet, T.; Bosso, P.; Zhang, A.N.; Amin-Chalhoub, E.; Fanelli, F.; Vahlas, C. Metallization of carbon fiber reinforced polymers: Chemical kinetics, adhesion, and properties. Surf. Coat. Technol. 2016, 308, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Boisselier, G.; Maury, F.; Schuster, F. SiC coatings grown by liquid injection chemical vapor deposition using single source metal-organic precursors. Surf. Coat. Technol. 2013, 215, 152–160. [Google Scholar] [CrossRef] [Green Version]
- Czok, G.S.; Werther, J. Liquid spray vs. gaseous precursor injection—Its influence on the performance of particle coating by CVD in the fluidized bed. Powder Technol. 2006, 162, 100–110. [Google Scholar] [CrossRef]
- Joulaud, M.; Angekort, C.; Doppelt, P.; Mourier, T.; Mayer, D. Evaluation of (hfac)Cu(MHY) for CuCVD. Microelec. Eng. 2002, 64, 107–115. [Google Scholar] [CrossRef]
- Gooch, J.W. Encyclopedic Dictionary of Polymers; Springer: New York, NY, USA, 2011. [Google Scholar]
- Duguet, T.; Senocq, F.; Laffont, L.; Vahlas, C. Metallization of polymer composites by metalorganic chemical vapor deposition of Cu: Surface functionalization driven films characteristics. Surf. Coat. Technol. 2013, 230, 254–259. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.; Turunen, M.P.K.; Kivilahti, J.K. Surface modification and characterization of photodefinable epoxy/copper systems. Thin Solid Film. 2003, 440, 198–207. [Google Scholar] [CrossRef]
- Prysiazhnyi, V.; Stupavska, M.; Rahel, J.; Kleber, C.; Cernak, M.; Rafailovic, L.D. A comparison of chemical and atmospheric plasma assisted copper plating on carbon fiber reinforced epoxy polymer surfaces. Surf. Coat. Technol. 2014, 258, 1082–1089. [Google Scholar] [CrossRef]
- Schaubroeck, D.; De Baets, J.; Desmet, T.; Van Vlierberghe, S.; Schacht, E.; Van Calster, A. Introduction of amino groups on the surface of thin photo definable epoxy resin layers via chemical modification. Appl. Surf. Sci. 2009, 255, 8780–8787. [Google Scholar] [CrossRef]
- Yu, Z.J.; Kang, E.T.; Neoh, K.G. Electroless plating of copper on polyimide films modified by surface grafting of tertiary and quaternary amines polymers. Polymer 2002, 43, 4137–4146. [Google Scholar] [CrossRef]
- Ge, J.; Tuominen, R.; Kivilahti, J.K. Adhesion of electrolessly-deposited copper to photosensitive epoxy. J. Adhes. Sci. Technol. 2001, 15, 1133–1143. [Google Scholar] [CrossRef]
- Siau, S.; Vervaet, A.; Schacht, E.; Van Calster, A. Influence of Chemical Pretreatment of Epoxy Polymers on the Adhesion Strength of Electrochemically Deposited Cu for Use in Electronic Interconnections. J. Electroch. Soc. 2004, 151, C133–C141. [Google Scholar] [CrossRef]
- Weiss, C.; Muenstedt, H. Surface modification of polyether ether ketone (peek) films for flexible printed circuit boards. J. Adhes. 2002, 78, 507–519. [Google Scholar] [CrossRef]
- Addou, F. Metallization of waveguides in epoxy matrix composite material by a DLI-MOCVD process. Ph.D. Thesis, Institut National Polytechnique de Toulouse, Toulouse, France, 2017. [Google Scholar]
- Allen, N.S.; Edge, M.; Mourelatou, D.; Wilkinson, A.; Liauw, C.M.; Parellada, M.D.; Barrio, J.A.; Quiteria, V.R.S. Influence of ozone on styrene-ethylene-butylene-styrene (SEBS) copolymer. Polym. Degrad. Stab. 2003, 79, 297–307. [Google Scholar] [CrossRef]
- Gatenholm, P.; Ashida, T.; Hoffman, A.S. Hybrid biomaterials prepared by ozone-induced polymerization. 1. Ozonation of microporous polypropylene. J. Polym. Sci. Part A: Polym. Chem. 1997, 35, 1461–1467. [Google Scholar] [CrossRef]
- Siau, S.; Vervaet, A.; Nalines, S.; Schacht, E.; Van Calster, A. Kinetic study of wet chemical treatments on the surface roughness of epoxy polymer layers for buildup layers—I. Sweller influence. J. Electroch. Soc. 2004, 151, C816–C830. [Google Scholar] [CrossRef]
- Siau, S.; Vervaet, A.; Nalines, S.; Schacht, E.; Van Calster, A. Kinetic study of wet chemical treatments on the surface roughness of epoxy polymer layers for buildup layers—II. Oxidative treatment of the surface. J. Electroch. Soc. 2004, 151, C831–C849. [Google Scholar] [CrossRef]
- Siau, S.; Vervaet, A.; Van Vaeck, L.; Schacht, E.; Demeter, U.; Van Calster, A. Adhesion strength of the epoxy polymer/copper interface for use in microelectronics. J. Electroch. Soc. 2005, 152, C442–C455. [Google Scholar] [CrossRef]
- Amin-Chalhoub, E.; Duguet, T.; Samélor, D.; Debieu, O.; Ungureanu, E.; Vahlas, C. Chemical vapor deposition of low reflective cobalt (II) oxide films. Appl. Surf. Sci. 2016, 360, 540–546. [Google Scholar] [CrossRef] [Green Version]
- Duguet, T.; Amin-Chalhoub, E.; Samelor, D.; Pugliara, A.; Vahlas, C. Black Co oxides coatings for thermosensitive polymer surfaces by low-temperature DLI-MOCVD. Surf. Coat. Technol. 2018, 349, 941–948. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.W.; Gordon, R.G.; Farmer, D.B.; Lin, Y.B.; Vlassak, J. Nucleation and adhesion of ALD copper on cobalt adhesion layers and tungsten nitride diffusion barriers. Electrochem. Sol. State Lett. 2005, 8, G182–G185. [Google Scholar] [CrossRef]
- Su, S.K.; Akiba, M.; Takahashi, T.; Saburi, M.; Hidai, M.; Uchida, Y. Chemical deposition in liquid-phase (CDL)—A convenient method for cobalt-coating or nickel-coating of carbon fibers using zerovalent organometallic compounds. Chem. Lett. 1987, 16, 337–340. [Google Scholar] [CrossRef]
- Shimizu, H.; Sakoda, K.; Shimogaki, Y. CVD of cobalt-tungsten alloy film as a novel copper diffusion barrier. Microelec. Eng. 2013, 106, 91–95. [Google Scholar] [CrossRef]
- Ye, D.X.; Pimanpang, S.; Jezewski, C.; Tang, F.; Senkevich, J.J.; Wang, G.C.; Lu, T.M. Low temperature chemical vapor deposition of Co thin films from Co2(CO)8. Thin Solid Film. 2005, 485, 95–100. [Google Scholar] [CrossRef]
- Eustathopoulos, N.; Nicholas, M.G.; Drevet, B. Wettability at High Temperatures; Pergamon Press: Oxford, UK, 1999; Volume 13, pp. 1–420. [Google Scholar]
- Owens, D.K.; Wendt, R. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Michau, A.; Gazal, Y.; Addou, F.; Maury, F.; Duguet, T.; Boichot, R.; Pons, M.; Monsifrot, E.; Maskrot, H.; Schuster, F. Scale up of a DLI-MOCVD process for the internal treatment of a batch of 16 nuclear fuel cladding segments with a CrCx protective coating. Surf. Coat. Technol. 2019, 375, 894–902. [Google Scholar] [CrossRef] [Green Version]

| Sample | γL (mJ/m2) ±20% | Dispersive | Polar | r2 | O/C Ratio |
|---|---|---|---|---|---|
| Untreated 1 | 35 | 33 | 2 | 0.95 | 0.18 |
| O3 | 43 | 30 | 13 | 0.85 | 0.78 |
| Untreated 2 | 53 | 28 | 25 | 0.85 | 0.18 |
| DME-exposed (195 °C, 45 min) | 62 | 4 | 58 | 0.96 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addou, F.; Duguet, T.; Ledru, Y.; Mesnier, D.; Vahlas, C. Engineering Copper Adhesion on Poly-Epoxy Surfaces Allows One-Pot Metallization of Polymer Composite Telecommunication Waveguides. Coatings 2021, 11, 50. https://doi.org/10.3390/coatings11010050
Addou F, Duguet T, Ledru Y, Mesnier D, Vahlas C. Engineering Copper Adhesion on Poly-Epoxy Surfaces Allows One-Pot Metallization of Polymer Composite Telecommunication Waveguides. Coatings. 2021; 11(1):50. https://doi.org/10.3390/coatings11010050
Chicago/Turabian StyleAddou, Fouzi, Thomas Duguet, Yohann Ledru, Didier Mesnier, and Constantin Vahlas. 2021. "Engineering Copper Adhesion on Poly-Epoxy Surfaces Allows One-Pot Metallization of Polymer Composite Telecommunication Waveguides" Coatings 11, no. 1: 50. https://doi.org/10.3390/coatings11010050
APA StyleAddou, F., Duguet, T., Ledru, Y., Mesnier, D., & Vahlas, C. (2021). Engineering Copper Adhesion on Poly-Epoxy Surfaces Allows One-Pot Metallization of Polymer Composite Telecommunication Waveguides. Coatings, 11(1), 50. https://doi.org/10.3390/coatings11010050






