Effect of Microstructure on Layered Double Hydroxides Film Growth on Mg-2Zn-xMn Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of LDHs Film on Mg Alloy
2.2. Characterization
3. Results
3.1. Alloy Microstructure
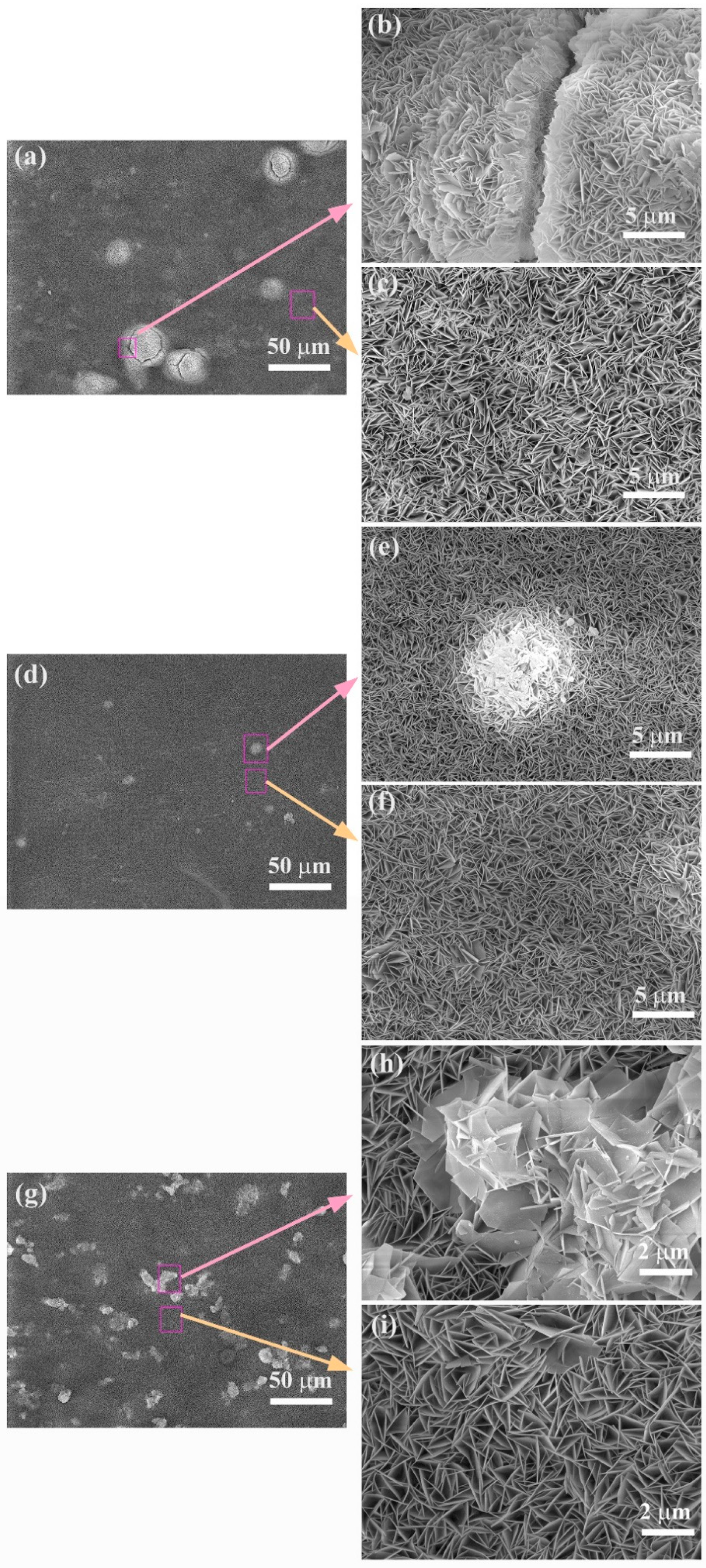
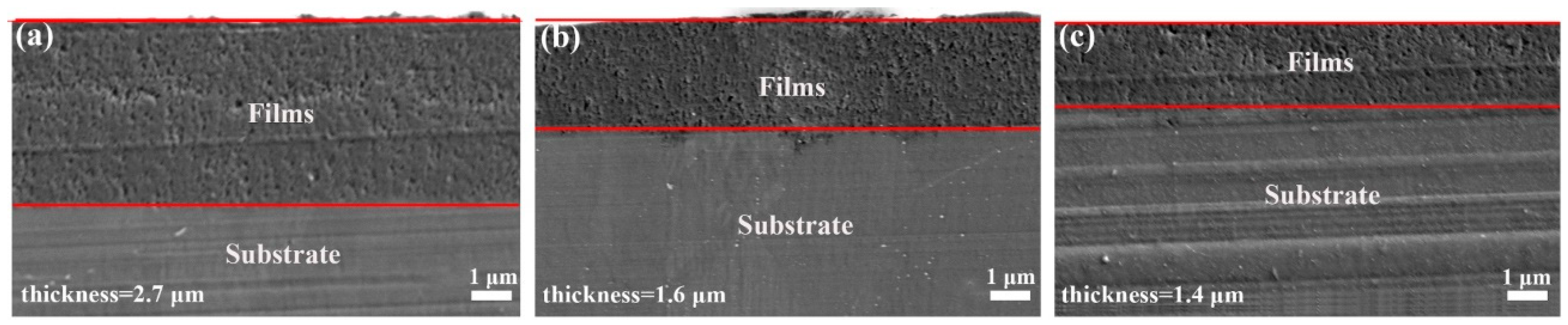
3.2. Surface Morphology of the LDHs Film on Mg Alloy
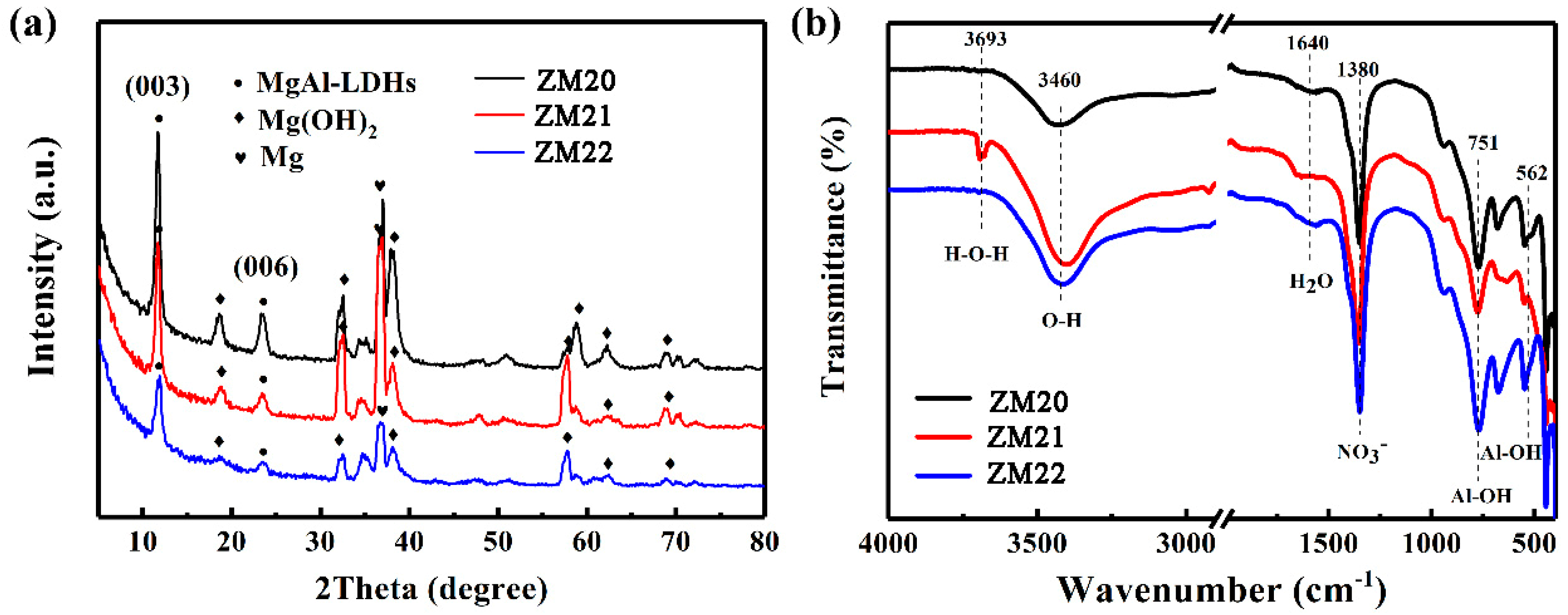
3.3. Surface Chemistry of the LDHs Film on Mg Alloy
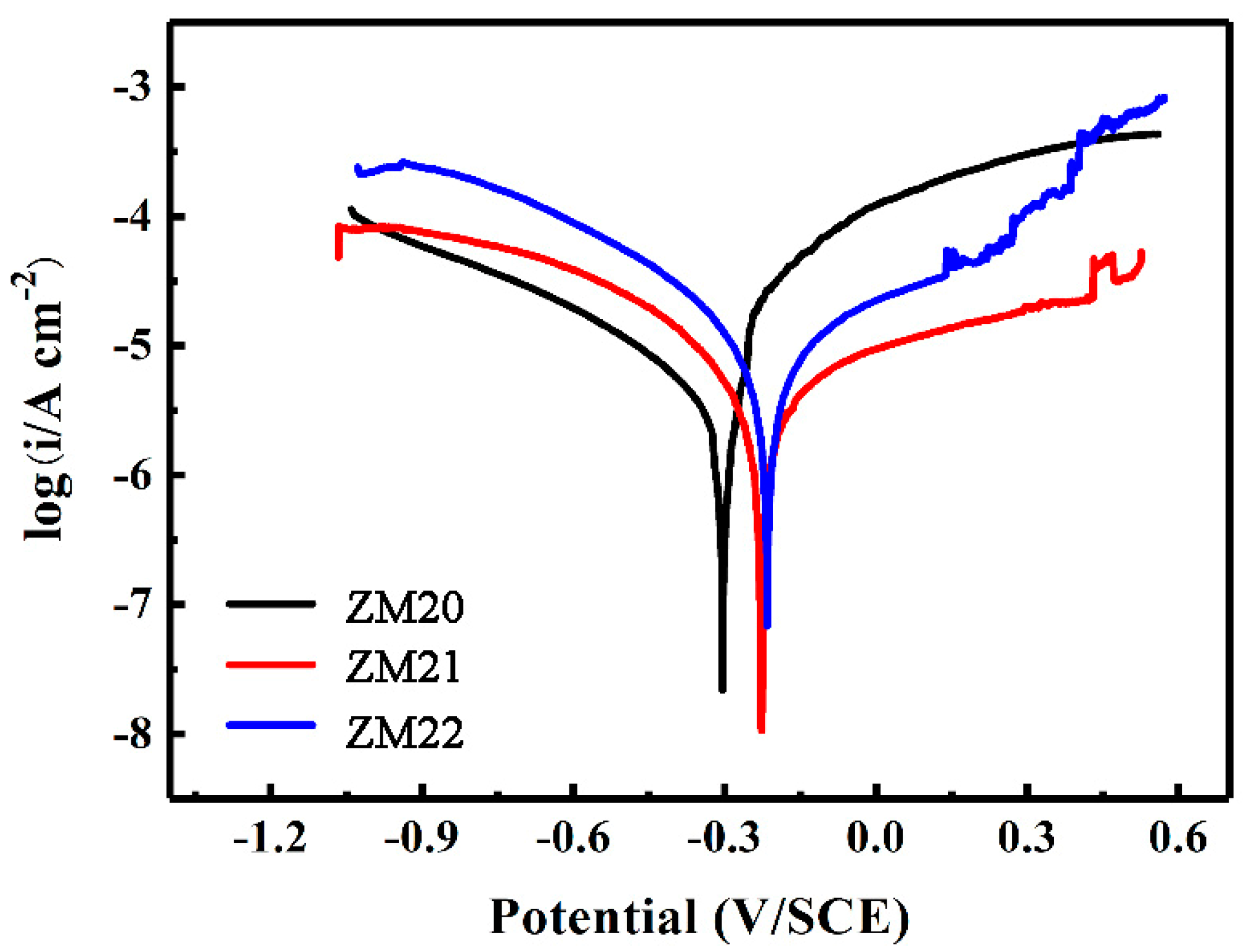
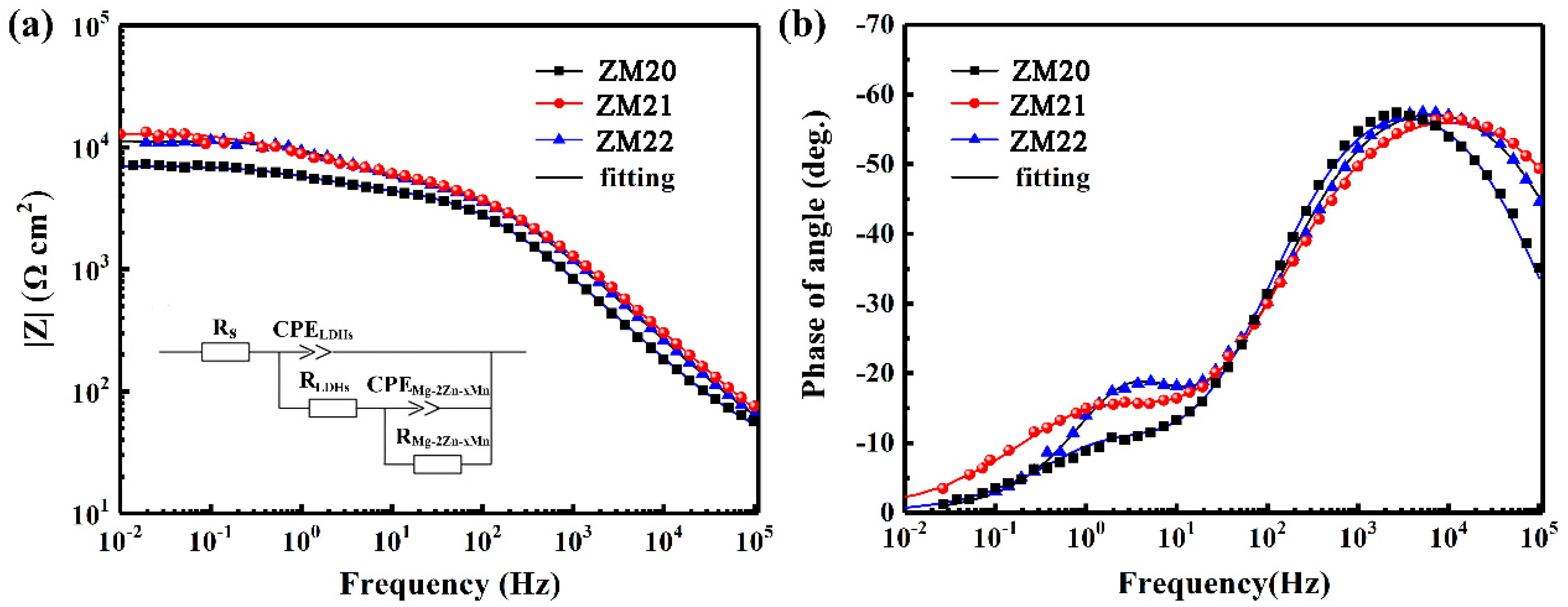
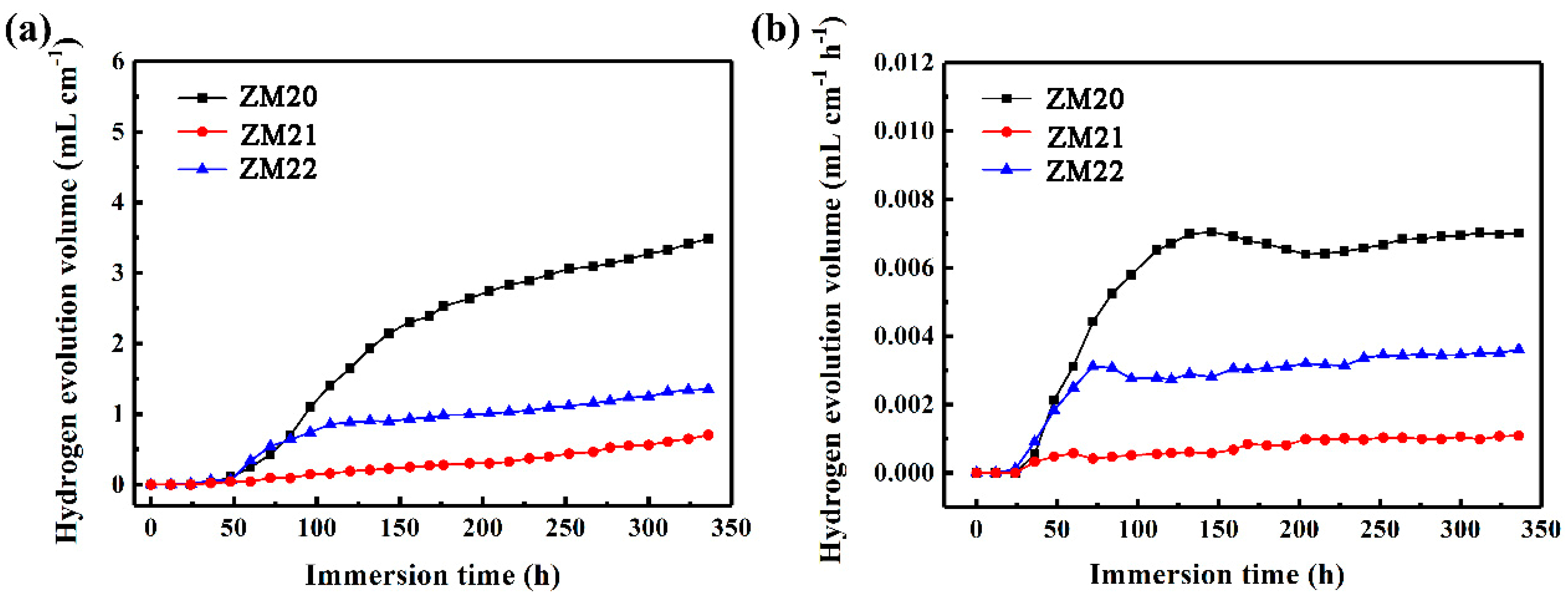
3.4. Corrosion Protection of the LDHs Film on Mg Alloy
3.5. Formation Mechanism of the LDHs Film on Mg Alloy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; Ahmad, R.; Yin, B.; Sandlöbes, S.; Curtin, W. Mechanistic origin and prediction of enhanced ductility in magnesium alloys. Science 2018, 359, 447–451. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, Y.; Zhang, K.; Li, X.; Ma, M.; Shi, G.; Yuan, J.; Wang, K. Microstructure, hot deformation behavior, and textured evolution of Mg-3 wt%Zn-1 wt%Ca-0.5 wt% Sr alloy. J. Mater. Sci. 2020, 55, 12434–12447. [Google Scholar] [CrossRef]
- Wu, J.; Chen, J.; Yan, H.; Xia, W.; Su, B.; Yu, L.; Liu, G.; Song, M. Enhancing the mechanical properties of high strain rate rolled Mg-6Zn-1Mn alloy by pre-rolling. J. Mater. Sci. 2017, 52, 10557–10566. [Google Scholar] [CrossRef]
- Hu, K.; Liao, Q.; Li, C.; Le, Q.; Zhou, W.; Cheng, C.; Ning, S.; Chen, X.; Yu, F. High ductility induced by un-DRXed grains in a Mg–Zn–Mn–La–Ce alloy. J. Mater. Sci. 2020, 54, 10902–10917. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, C.; Shen, L.; Bao, N. Ion-exchange modification of potassium magnesium titanate for high-performance wear-corrosion-resistant composite coatings. J. Mater. Sci. 2020, 55, 13836–13851. [Google Scholar] [CrossRef]
- Nezamdoust, S.; Seifzadeh, D.; Rajabalizadeh, Z. Application of novel sol-gel composites on magnesium alloy. J. Magnes. Alloy. 2019, 7, 419–432. [Google Scholar] [CrossRef]
- Yao, W.; Wu, L.; Huang, G.; Jiang, B.; Atrens, A.; Pan, F. Superhydrophobic coatings for corrosion protection of magnesium alloys. J. Mater. Sci. Technol. 2020, 52, 100–118. [Google Scholar] [CrossRef]
- Shulha, T.; Serdechnova, M.; Lamaka, S.; Wieland, D.; Lapko, K.; Zheludkevich, M. Chelating agent-assisted in situ LDH growth on the surface of magensium alloy. Sci. Rep. 2018, 8, 16409. [Google Scholar] [CrossRef]
- Zeng, R.; Liu, Z.; Zhang, F.; Li, S.; He, Q.; Cui, H.; Han, E. Corrosion resistance of in-situ Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy by one-step formation. Trans. Nonferrous Met. Soc. China 2015, 25, 1917–1925. [Google Scholar] [CrossRef]
- Seifzadeh, D.; Farhoudi, L. Electroless Co–P plating on magnesium alloy and its anti-corrosion properties. Surf. Eng. 2016, 32, 348–355. [Google Scholar] [CrossRef]
- Niu, Y.; Hou, J.; Ning, F.; Chen, X.; Jia, Y.; Le, Q. Hot deformation behavior and processing map of Mg-2Zn-1Al-0.2RE alloy. J. Rare Earth 2020, 38, 665–675. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Liu, Y.; Chen, L. Microstructure characteristics, film layer rupture mechanism and corrosion behavior of hot-rolled Mg-2Zn-0.2Mn-xNd. Mater. Charact. 2020, 165, 110368. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Le, Q.; Jia, Y.; Zhou, X.; Yu, F.; Atrens, A. The role of long-period stacking ordered phase on the discharge and electrochemical behaviors of magnesium anode Mg–Zn-Y for the primary Mg-air battery. Int. J. Energy Res. 2020, 44, 8865–8876. [Google Scholar] [CrossRef]
- Petrova, E.; Serdechnova, M.; Shulha, T.; Lamaka, S.; Wieland, D.; Karlova, P.; Blawert, C.; Starykevich, M.; Zheludkevich, M. Use of synergistic mixture of chelating agents for in situ LDH growth on the surface of PEO-treated AZ91. Sci. Rep. 2020, 10, 8645. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, D.; Li, M.; Liu, X.; Zhang, Y.; Qian, S.; Peng, F. Osteogenesis, angiogenesis and immune response of Mg–Al layered double hydroxide coating on pure Mg. Bioact. Mater. 2021, 6, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Mu, P.; Wang, Q.; Li, J. Superhydrophobic ZIF-8-based dual-layer coating for enhanced corrosion protection of Mg alloy. ACS Appl. Mater. Interfaces 2020, 12, 35453–35463. [Google Scholar] [CrossRef]
- Lin, J.; Jeng, K.; Uan, J. Crystallization of a chemical conversion layer that forms on AZ91D magnesium alloy in carbonic acid. Corros. Sci. 2011, 53, 3832–3839. [Google Scholar] [CrossRef]
- Iqbal, M.; Fedel, M. Effect of synthesis conditions on the controlled growth of MgAl-LDH corrosion resistance film: Structure and corrosion resistance properties. Coatings 2019, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Pan, F.; Liu, Y.; Zhang, G.; Tang, A.; Atrens, A. Influence of pH on the growth behaviour of Mg–Al LDH films. Surf. Eng. 2018, 34, 674–681. [Google Scholar] [CrossRef]
- Tedim, J.; Zheludkevich, M.; Salak, A.; Lisenkov, A.; Ferreira, M. Nanostructured LDH-container layer with active protection functionality. J. Mater. Chem. 2011, 21, 15464. [Google Scholar] [CrossRef]
- Campestrini, P.; Westing, E.; Rooijen, H.; Wit, J. Relation between microstructural aspects of AA2024 and its corrosion behaviour investigated using AFM scanning potential technique. Corros. Sci. 2000, 42, 1853–1861. [Google Scholar] [CrossRef]
- Komatsu, S.; Ikeda, M.; Mori, U. Solidus composition in Mg side phase diagram of Mg–Zn binary system and its estimation method. J. Jpn. Inst. Light Met. 2004, 54, 25–30. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Ma, Y.; Song, G.; Zheng, D.; Jiang, B.; Atrens, A.; Pan, F. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31. Corros. Sci. 2018, 32, 370–382. [Google Scholar] [CrossRef] [Green Version]



| Samples | Name | Zn (wt.%) | Mn (wt.%) | Mg (wt.%) |
|---|---|---|---|---|
| Mg-2Zn | ZM20 | 1.95 | 0 | Bal. |
| 1.6 | 0 | 98.4 | ||
| Mg-2Zn-Mn | ZM21 | 1.97 | 0.84 | Bal. |
| 1.8 | 0.9 | 97.3 | ||
| Mg-2Zn-2Mn | ZM22 | 1.94 | 1.87 | Bal. |
| 1.6 | 1.9 | 96.5 |
| Samples | O (at.%) | Mg (at.%) | Al (at.%) |
|---|---|---|---|
| ZM20-LDHs | 63.9 | 22.4 | 13.7 |
| ZM21-LDHs | 64.1 | 23.6 | 12.3 |
| ZM22-LDHs | 62.6 | 23.2 | 14.3 |
| Samples | Ecorr (VSCE) | icorr (μA·cm−2) |
|---|---|---|
| ZM20-LDHs | −0.35 | 1.8 |
| ZM21-LDHs | −0.22 | 0.4 |
| ZM22-LDHs | −0.23 | 1.1 |
| Samples | Rsol (Ω·cm2) | CPEout (μF·cm−2) | nout | Rout (Ω·cm2) | CPEinn (μF cm−2) | ninn | Rinn (Ω·cm2) | X2 ×10−3 |
|---|---|---|---|---|---|---|---|---|
| ZM20-LDHs | 30.44 | 2.05 × 10−6 | 0.72 | 2.46 × 103 | 10.4 × 10−5 | 0.71 | 4.58 × 103 | 3.1 |
| ZM21-LDHs | 15.75 | 1.87 × 10−6 | 0.68 | 5.94 × 103 | 5.57× 10−5 | 0.61 | 8.01 × 103 | 8.3 |
| ZM22-LDHs | 21.62 | 1.66 × 10−6 | 0.70 | 5.51 × 103 | 2.25 ×10−5 | 0.83 | 5.72 × 103 | 9.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yao, W.; Wu, L.; Chen, J.; Pan, F. Effect of Microstructure on Layered Double Hydroxides Film Growth on Mg-2Zn-xMn Alloy. Coatings 2021, 11, 59. https://doi.org/10.3390/coatings11010059
Chen Y, Yao W, Wu L, Chen J, Pan F. Effect of Microstructure on Layered Double Hydroxides Film Growth on Mg-2Zn-xMn Alloy. Coatings. 2021; 11(1):59. https://doi.org/10.3390/coatings11010059
Chicago/Turabian StyleChen, Yonghua, Wenhui Yao, Liang Wu, Jing Chen, and Fusheng Pan. 2021. "Effect of Microstructure on Layered Double Hydroxides Film Growth on Mg-2Zn-xMn Alloy" Coatings 11, no. 1: 59. https://doi.org/10.3390/coatings11010059





