Effect of Chitosan Coating on PLGA Nanoparticles for Oral Delivery of Thymoquinone: In Vitro, Ex Vivo, and Cancer Cell Line Assessments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines
2.3. Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC) Technique
2.4. Experimental Design
2.5. Formulation of TQ-CS-PLGA-NPs
2.6. Characterization
2.6.1. Particle Characterization
2.6.2. Entrapment Efficiency
2.7. In Vitro Gastrointestinal Stability Study
2.8. Colloidal Stability
2.9. TQ Release Study
2.10. Mucoadhesion Study
2.11. Antioxidant Activity
2.12. Cell Viability Assay
2.13. Intestinal Permeation Study
2.14. Statistical Analysis
3. Results and Discussion
3.1. Optimization
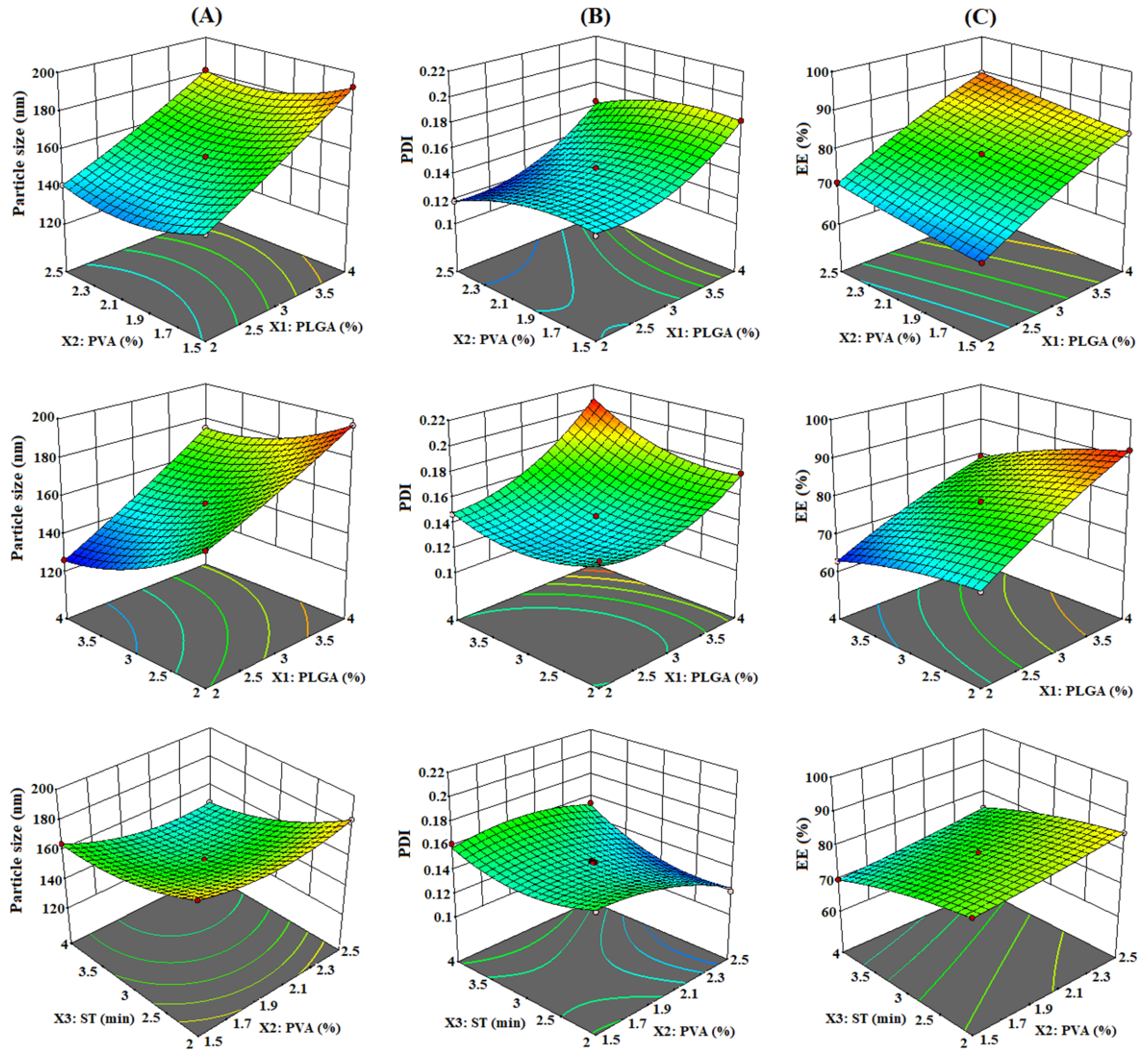
3.1.1. Effect of PLGA (X1), PVA (X2), and ST (X3) on PS (Y1)
3.1.2. Effect of PLGA (X1), PVA (X2), and ST (X3) on PDI (Y2)
3.1.3. Effect of PLGA (X1), PVA (X2), and ST (X3) on EE (Y3)
3.1.4. Optimization by Point Prediction
3.2. Characterization
3.2.1. Particle Characterization
3.2.2. Entrapment Efficiency (%EE)
3.3. In Vitro Gastrointestinal Stability Study
3.4. Colloidal Stability
3.5. TQ Release Study
3.6. Mucoadhesion Study
3.7. Antioxidant Activity
3.8. Cell Viability Assay
3.9. Ex Vivo Intestinal Permeation Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francies, F.Z.; Hull, R.; Khanyile, R.; Dlamini, Z. Breast cancer in low-middle income countries: Abnormality in splicing and lack of targeted treatment options. Am. J. Cancer Res. 2020, 10, 1568–1591. [Google Scholar] [PubMed]
- Rizwanullah, M.; Alam, M.; Harshita; Mir, S.R.; Rizvi, M.M.A.; Amin, S. Polymer-lipid hybrid nanoparticles: A next-generation nanocarrier for targeted treatment of solid tumors. Curr. Pharm. Des. 2020, 26, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, M.; Samy, A.; Raslan, M.A.; Salama, A.; Said, R.A.; Abdelaziz, A.E.; El-Eraky, W.; El Awdan, S.; Viitala, T. Enhancement of bioavailability and pharmacodynamic effects of thymoquinone via nanostructured lipid carrier (NLC) formulation. AAPS PharmSciTech. 2016, 17, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Tania, M.; Fu, S.; Fu, J. Thymoquinone, as an anticancer molecule: From basic research to clinical investigation. Oncotarget 2017, 8, 51907–51919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballout, F.; Habli, Z.; Rahal, O.N.; Fatfat, M.; Gali-Muhtasib, H. Thymoquinone-based nanotechnology for cancer therapy: Promises and challenges. Drug Discov. Today 2018, 23, 1089–1098. [Google Scholar] [CrossRef]
- Khan, F.A.; Rizwanullah, M.; Perwez, A.; Ahmad, M.Z.; Ahmad, J. Polymeric Nanomedicines in Treatment of Breast Cancer: Review of Contemporary Research. Front. Anti-Cancer Drug Discov. 2020, 11, 36–66. [Google Scholar]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Shafiq, N.; Malhotra, S. Drug-loaded PLGA nanoparticles for oral administration: Fundamental issues and challenges ahead. Crit. Rev. Ther. Drug Carrier Syst. 2012, 29, 149–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xie, L.Q.; Qin, J.; Jia, Y.; Cai, X.; Nan, W.; Yang, W.; Lv, F.; Zhang, Q.Q. Surface modification of PLGA nanoparticles with biotinylated chitosan for the sustained in vitro release and the enhanced cytotoxicity of epirubicin. Colloids Surf. B Biointerfaces 2016, 138, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, M.; Zheng, A.; Cao, D.; Bi, Y.; Sun, J. Preparation and characterization of insulin loaded bioadhesive PLGA nanoparticles for oral administration. Eur. J. Pharm. Sci. 2012, 45, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Kong, L. Chitosan modified PLGA nanoparticles with versatile surface for improved drug delivery. AAPS PharmSciTech. 2013, 14, 585–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqahtani, S.; Simon, L.; Astete, C.E.; Alayoubi, A.; Sylvester, P.W.; Nazzal, S.; Shen, Y.; Xu, Z.; Kaddoumi, A.; Sabliov, C.M. Cellular uptake, antioxidant and antiproliferative activity of entrapped α-tocopherol and γ-tocotrienol in poly(lactic-co-glycolic) acid (PLGA) and chitosan covered PLGA nanoparticles(PLGA-Chi). J. Colloid. Interface Sci. 2015, 445, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.A.; Khalil, N.M.; Tominaga, T.T.; Lechanteur, A.; Sarmento, B.; Mainardes, R.M. Mucoadhesive chitosan-coated PLGA nanoparticles for oral delivery of ferulic acid. Artif. Cells Nanomed. Biotechnol. 2018, 46, 993–1002. [Google Scholar] [CrossRef] [Green Version]
- Chuah, L.H.; Billa, N.; Roberts, C.J.; Burley, J.C.; Manickam, S. Curcumincontaining chitosan nanoparticles as a potential mucoadhesive delivery system to the colon. Pharm. Dev. Technol. 2013, 18, 591–599. [Google Scholar] [CrossRef]
- Gilani, S.J.; Imam, S.S.; Ahmed, A.; Chauhan, S.; Mirza, M.A.; Taleuzzaman, M. Formulation and evaluation of thymoquinone niosomes: Application of developed and validated RP-HPLC method in delivery system. Drug Dev. Ind. Pharm. 2019, 4, 1799–1806. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Amin, S.; Ahmad, J. Improved pharmacokinetics and antihyperlipidemic efficacy of rosuvastatin-loaded nanostructured lipid carriers. J. Drug Target. 2017, 25, 58–74. [Google Scholar] [CrossRef]
- Khatoon, K.; Rizwanullah, M.; Amin, S.; Mir, S.R.; Akhter, S. Cilnidipine loaded transfersomes for transdermal application: Formulation optimization, in-vitro and in-vivo study. J. Drug Deliv. Sci. Tech. 2019, 54, 101303. [Google Scholar] [CrossRef]
- Abd El Hady, W.E.; Mohamed, E.A.; Soliman, O.A.; El-Sabbagh, H.M. In vitro–in vivo evaluation of chitosan-PLGA nanoparticles for potentiated gastric retention and anti-ulcer activity of diosmin. Int. J. Nanomed. 2019, 14, 7191–7213. [Google Scholar] [CrossRef] [Green Version]
- Shahab, M.S.; Rizwanullah, M.; Alshehri, S.; Imam, S.S. Optimization to development of chitosan decorated polycaprolactone nanoparticles for improved ocular delivery of dorzolamide: In vitro, ex vivo and toxicity assessments. Int. J. Biol. Macromol. 2020, 163, 2392–2404. [Google Scholar] [CrossRef]
- Saifi, Z.; Rizwanullah, M.; Mir, S.R.; Amin, S. Bilosomes nanocarriers for improved oral bioavailability of acyclovir: A complete characterization through in vitro, ex-vivo and in vivo assessment. J. Drug Deliv. Sci. Tech. 2020, 57, 101634. [Google Scholar] [CrossRef]
- Kaithwas, V.; Dora, C.P.; Kushwah, V.; Jain, S. Nanostructured lipid carriers of olmesartan medoxomil with enhanced oral bioavailability. Colloids Surf. B Biointerfaces 2017, 154, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Dalmoro, A.; Bochicchio, S.; Nasibullin, S.F.; Bertoncin, P.; Lamberti, G.; Barba, A.A.; Moustafine, R.I. Polymer-lipid hybrid nanoparticles as enhanced indomethacin delivery systems. Eur. J. Pharm. Sci. 2018, 121, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.; Rizwanullah, M.; Kohli, K. Development and optimization of sulforaphane-loaded nanostructured lipid carriers by the Box-Behnken design for improved oral efficacy against cancer: In vitro, ex vivo and in vivo assessments. Artif. Cells Nanomed. Biotechnol. 2018, 46, 15–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipović-Grčić, J.; Škalko-Basnet, N.; Jalšienjak, I. Mucoadhesive chitosan-coated liposomes: Characteristics and stability. J. Microencapsul. 2001, 18, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Fakhria, A.; Gilani, S.J.; Imam, S.S. Formulation of thymoquinone loaded chitosan nano vesicles: In-vitro evaluation and in-vivo anti-hyperlipidemic assessment. J. Drug Deliv. Sci. Technol. 2019, 50, 339–346. [Google Scholar] [CrossRef]
- Imam, S.S.; Alshehri, S.; Alzahrani, T.A.; Hussain, A.; Altamimi, M.A. Formulation and Evaluation of Supramolecular Food-Grade Piperine HP β CD and TPGS Complex: Dissolution, Physicochemical Characterization, Molecular Docking, In Vitro Antioxidant Activity, and Antimicrobial Assessment. Molecules 2020, 25, 4716. [Google Scholar] [CrossRef]
- Khan, M.A.; Zafaryab, M.; Mehdi, S.H.; Quadri, J.; Rizvi, M.M. Characterization and carboplatin loaded chitosan nanoparticles for the chemotherapy against breast cancer in vitro studies. Int. J. Biol. Macromol. 2017, 97, 115–122. [Google Scholar] [CrossRef]
- Al-Joudi, F.S.; Alias, I.Z.; Samsudin, A.R. The effects of chemotherapeutic drugs on viabilty, apoptosis, and survivin expression in MCF-7 cells. Acta Histochem. Cytochem. 2005, 38, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Jahangir, M.A.; Khan, R.; Imam, S.S. Formulation of sitagliptin-loaded oral polymeric nano scaffold: Process parameters evaluation and enhanced anti-diabetic performance. Artif. Cells Nanomed. Biotechnol. 2018, 46, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Barkat, M.A.; Rizwanullah, M.; Beg, S.; Pottoo, F.H.; Siddiqui, S.; Ahmad, F.J. Paclitaxel-loaded nanolipidic carriers with improved oral bioavailability and anticancer activity against human liver carcinoma. AAPS PharmSciTech. 2019, 20, 87. [Google Scholar]
- Lepeltier, E.; Bourgaux, C.; Couvreur, P. Nanoprecipitation and the “Ouzo effect”: Application to drug delivery devices. Adv. Drug Deliv. Rev. 2014, 71, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.R.; de Menezes, L.R.; Dutra Filho, J.C.; Cabral, L.M.; Tavares, M.I. Surface-coated polycaprolactone nanoparticles with pharmaceutical application: Structural and molecular mobility evaluation by TD-NMR. Polym. Test. 2017, 60, 39–48. [Google Scholar] [CrossRef]
- Zafar, A.; Khan, N.; Alruwaili, N.K.; Bukhari, S.N.; Alsuwayt, B.; Afzal, M.; Akhtar, S.; Yasir, M.; Elmowafy, M.; Shalaby, K.; et al. Improvement of Ocular Efficacy of Levofloxacin by Bioadhesive Chitosan Coated PLGA Nanoparticles: Box-behnken Design, In-vitro Characterization, Antibacterial Evaluation and Scintigraphy Study. Iran. J. Pharm. Res. 2020, 19, 292–311. [Google Scholar]
- Mishra, A.; Imam, S.S.; Aqil, M.; Ahad, A.; Sultana, Y.; Ameeduzzafar; Ali, A. Carvedilolnano lipid carriers: Formulation, characterization and in-vivo evaluation. Drug Deliv. 2016, 23, 1486–1494. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Khanna, K.; Bhatnagar, A.; Ahmad, F.J.; Ali, A. Chitosan coated PLGA nanoparticles amplify the ocular hypotensive effect of forskolin: Statistical design, characterization and in vivo studies. Int. J. Biol. Macromol. 2018, 116, 648–663. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Madan, P.; Lin, S. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A cosurfactant study. Asian J. Pharm. Sci. 2016, 11, 404–416. [Google Scholar] [CrossRef] [Green Version]
- Sathyamoorthy, N.; Magharla, D.; Chintamaneni, P.; Vankayalu, S. Optimization of paclitaxel loaded poly (ε-caprolactone) nanoparticles using Box Behnken design. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 362–373. [Google Scholar] [CrossRef]
- Shah, M.; Pathak, K. Development and Statistical Optimization of Solid Lipid Nanoparticles of Simvastatin by Using 2(3) Full-Factorial Design. AAPS PharmSciTech. 2010, 11, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Pan, H.; He, F.; Wang, X.; Li, J.; Yang, X.; Pan, W. Effect of particle size on oral absorption of carvedilol nanosuspensions: In vitro and in vivo evaluation. Int. J. Nanomedicine 2015, 10, 6425–6434. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Lv, X.; Le, Y. Chitosan-modified PLGA nanoparticles for control-released drug delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoes, S.; Figueiras, A.; Veiga, F.; Concheiro, A.; Alvarez-Lorenzo, C. Polymeric micelles for oral drug administration enabling locoregional and systemic treatments. Expert Opin. Drug Deliv. 2014, 12, 297–318. [Google Scholar] [CrossRef] [PubMed]
- Anwer, M.K.; Mohammad, M.; Ezzeldin, E.; Fatima, F.; Alalaiwe, A.; Iqbal, M. Preparation of sustained release apremilast-loaded PLGAnanoparticles: In vitro characterization and in vivo pharmacokinetic study in rats. Int. J. Nanomed. 2019, 14, 1587–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.S.; Kim, C.S.; Lee, K.M. The intracellular uptake ability of chitosan-coated poly (d,l-lactideco-glycolide) nanoparticles. Arch. Pharm. Res. 2008, 31, 1050–1054. [Google Scholar] [CrossRef]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is chitosan mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef]
- Zarai, Z.; Boujelbene, E.; Ben Salem, N.; Gargouri, Y.; Sayari, A. Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT Food Sci. Technol. 2013, 50, 634–641. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Massimi, M.; Giardi, M.F.; Cametti, C.; Devirgiliis, L.C.; Dentini, M.; Palocci, C. Chitosan coated PLGA nanoparticles: A sustained drug release strategy for cell cultures. Colloids Surf. B Biointerfaces 2013, 103, 310–317. [Google Scholar] [CrossRef]
- Ng, W.K.; Yazan, L.S.; Yap, L.H.; Wan Nor Hafiza, W.A.G.; How, C.W.; Abdullah, R. Thymoquinone-loaded nanostructured lipid carrier exhibited cytotoxicity towards breast cancer cell lines (MDA-MB-231 and MCF-7) and cervical cancer cell lines (HeLa and SiHa). Biomed. Res. Int. 2015, 2015, 263131. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, J.; Mir, S.R.; Kohli, K.; Chuttani, K.; Mishra, A.K.; Panda, A.K.; Amin, S. Solid-nanoemulsion preconcentrate for oral delivery of paclitaxel: Formulation design, biodistribution, and γ-scintigraphy imaging. Biomed. Res. Int. 2014, 2014, 984756. [Google Scholar] [CrossRef] [Green Version]








| Independent Variables | Levels Used | Dependent Variables | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| X1 = PLGA concentration (%) | 2 | 3 | 4 | Y1 = Particle size (nm) |
| X2 = PVA concentration (%) | 1.5 | 2 | 2.5 | Y2 = Polydispersity index |
| X3 = Sonication time (min) | 2 | 3 | 4 | Y3 = Entrapment efficiency (%) |
| Runs | Independent Variables | Dependent Variables | |||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | Y3 | ||||
| Actual | Predicted | Actual | Predicted | Actual | Predicted | ||||
| F1 | 4 | 2 | 2 | 196.71 | 196.91 | 0.178 | 0.176 | 92.17 | 92.03 |
| F2 | 3 | 2 | 3 | 156.94 | 156.48 | 0.139 | 0.145 | 78.23 | 78.64 |
| F3 | 4 | 2 | 4 | 174.84 | 175.1 | 0.205 | 0.208 | 79.34 | 79.05 |
| F4 | 4 | 1.5 | 3 | 192.59 | 192.4 | 0.182 | 0.181 | 84.21 | 84.54 |
| F5 | 3 | 2.5 | 2 | 184.65 | 184.72 | 0.12 | 0.123 | 85.32 | 85.36 |
| F6 | 3 | 2 | 3 | 153.46 | 156.48 | 0.136 | 0.145 | 78.84 | 78.64 |
| F7 | 4 | 2.5 | 3 | 181.42 | 181.15 | 0.164 | 0.162 | 89.69 | 89.79 |
| F8 | 3 | 1.5 | 2 | 184.42 | 184.41 | 0.157 | 0.158 | 80.68 | 80.49 |
| F9 | 2 | 2 | 4 | 126.03 | 125.83 | 0.147 | 0.148 | 62.75 | 62.89 |
| F10 | 3 | 1.5 | 4 | 164.74 | 164.67 | 0.162 | 0.158 | 69.85 | 69.81 |
| F11 | 3 | 2.5 | 4 | 147.98 | 147.99 | 0.154 | 0.152 | 74.86 | 75.05 |
| F12 | 2 | 2.5 | 3 | 141.19 | 141.38 | 0.118 | 0.118 | 71.27 | 70.94 |
| F13 | 2 | 1.5 | 3 | 146.21 | 146.48 | 0.138 | 0.139 | 66.19 | 66.09 |
| F14 | 3 | 2 | 3 | 157.42 | 156.48 | 0.148 | 0.145 | 78.84 | 78.64 |
| F15 | 2 | 2 | 2 | 160.74 | 160.48 | 0.154 | 0.15 | 70.62 | 70.91 |
| Model | R2 | Adjusted R2 | Predicted R2 | SD | Desirability |
|---|---|---|---|---|---|
| Response (Y1) | 0.987 | ||||
| Linear | 0.9215 | 0.9001 | 0.8666 | 6.47 | |
| 2F1 | 0.9425 | 0.8993 | 0.8393 | 6.49 | |
| Quadratic | 0.9999 | 0.9998 | 0.9988 | 0.2965 | |
| Response (Y2) | |||||
| Linear | 0.7002 | 0.6184 | 0.3938 | 0.0139 | 0.932 |
| 2F1 | 0.7703 | 0.5980 | 0.4724 | 0.0143 | |
| Quadratic | 0.9917 | 0.9767 | 0.8671 | 0.0034 | |
| Response (Y3) | 0.973 | ||||
| Linear | 0.9817 | 0.9767 | 0.9649 | 1.28 | |
| 2F1 | 0.9881 | 0.9791 | 0.9532 | 1.21 | |
| Quadratic | 0.9992 | 0.9978 | 0.9912 | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshehri, S.; Imam, S.S.; Rizwanullah, M.; Fakhri, K.U.; Rizvi, M.M.A.; Mahdi, W.; Kazi, M. Effect of Chitosan Coating on PLGA Nanoparticles for Oral Delivery of Thymoquinone: In Vitro, Ex Vivo, and Cancer Cell Line Assessments. Coatings 2021, 11, 6. https://doi.org/10.3390/coatings11010006
Alshehri S, Imam SS, Rizwanullah M, Fakhri KU, Rizvi MMA, Mahdi W, Kazi M. Effect of Chitosan Coating on PLGA Nanoparticles for Oral Delivery of Thymoquinone: In Vitro, Ex Vivo, and Cancer Cell Line Assessments. Coatings. 2021; 11(1):6. https://doi.org/10.3390/coatings11010006
Chicago/Turabian StyleAlshehri, Sultan, Syed Sarim Imam, Md Rizwanullah, Khalid Umar Fakhri, Mohd Moshahid Alam Rizvi, Wael Mahdi, and Mohsin Kazi. 2021. "Effect of Chitosan Coating on PLGA Nanoparticles for Oral Delivery of Thymoquinone: In Vitro, Ex Vivo, and Cancer Cell Line Assessments" Coatings 11, no. 1: 6. https://doi.org/10.3390/coatings11010006







