Effect of Cross-Linking Modification on Structural and Film-Forming Characteristics of Pearl Millet (Pennisetum glaucum L.) Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Starch Isolation and Method for Preparation of CL Starch
2.2.2. Degree of Cross-Linking (DC)
2.2.3. Physicochemical Properties
2.2.4. Pasting Properties
2.2.5. Rheological Properties
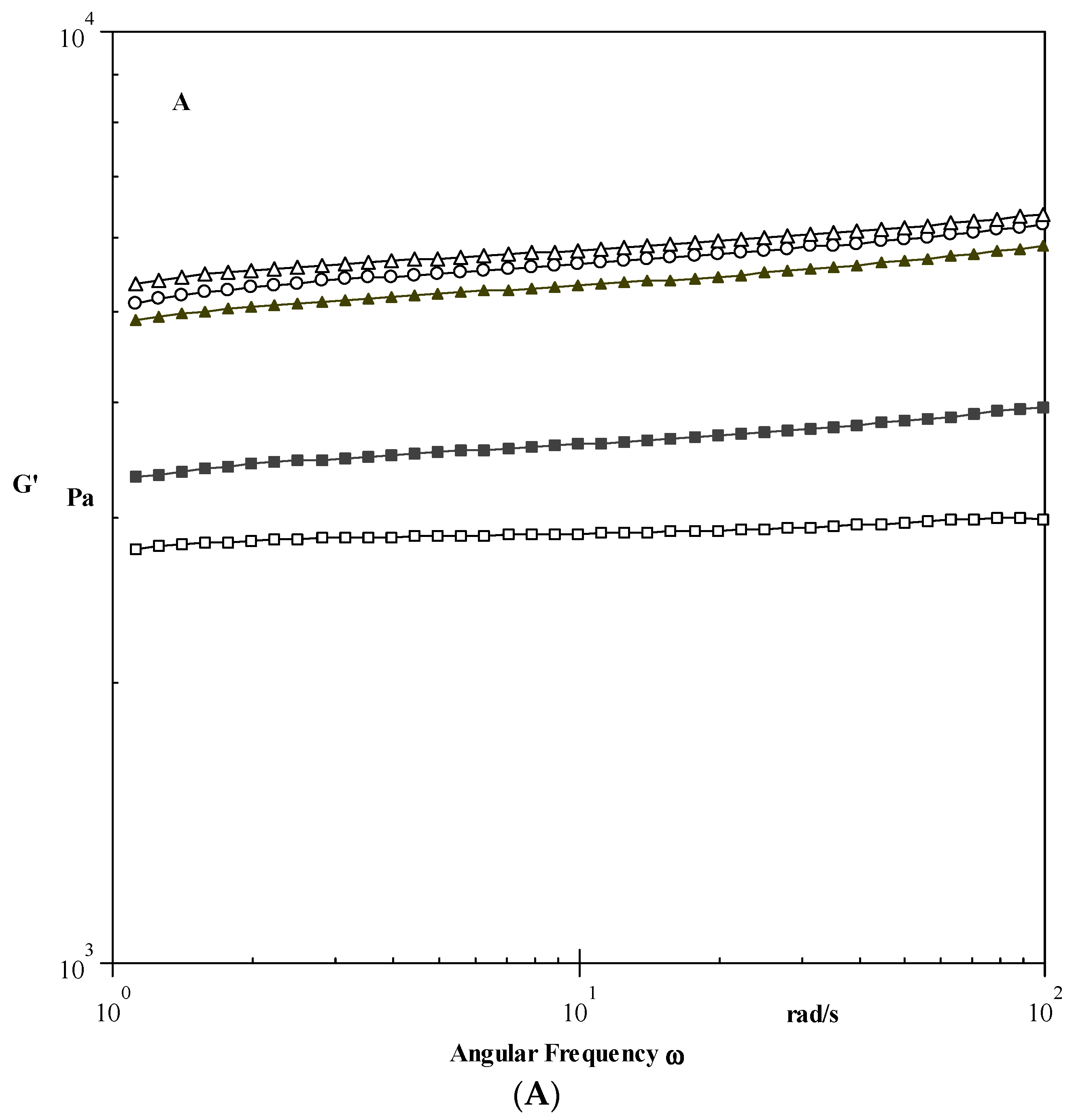
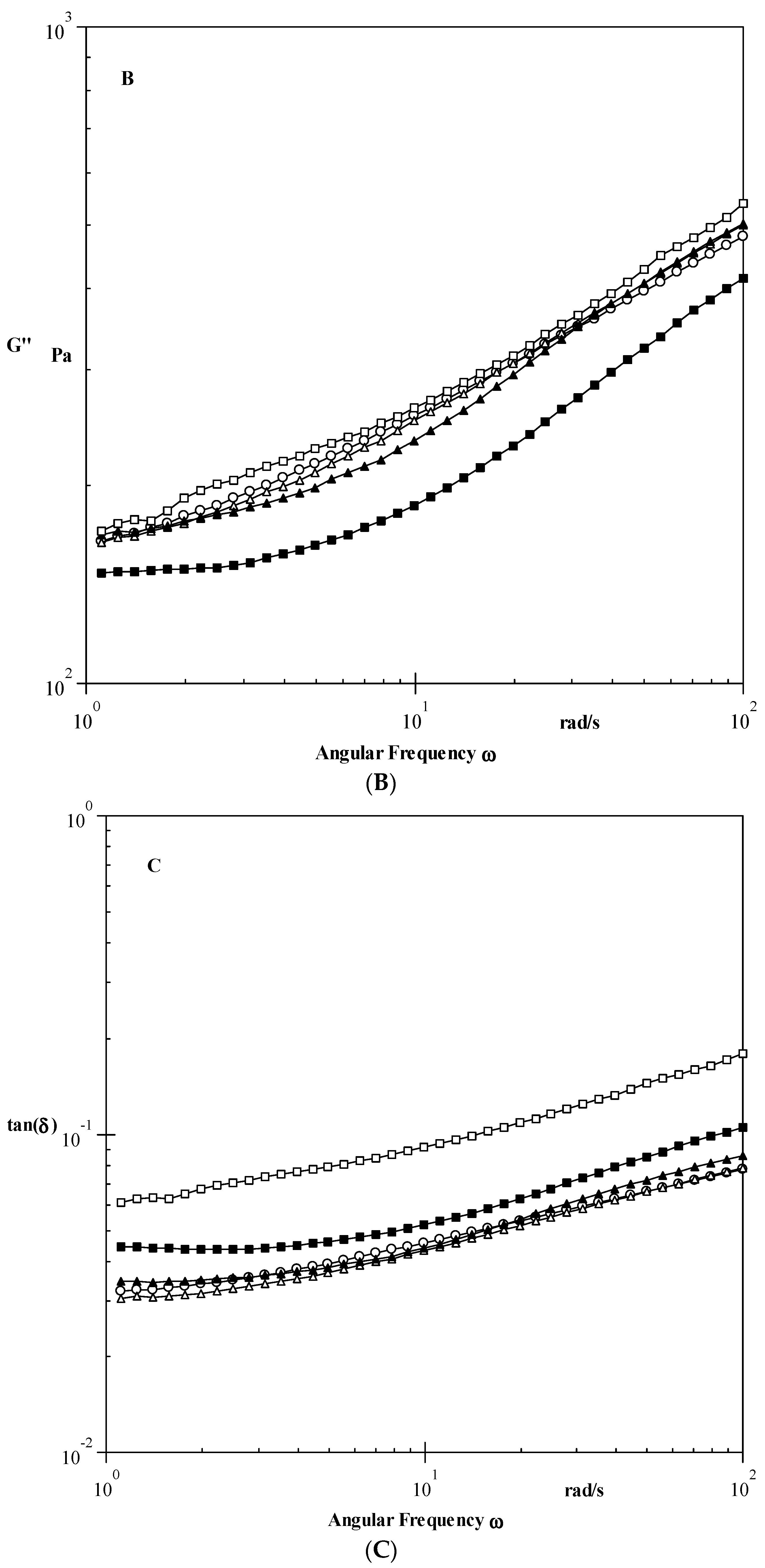
Dynamic Properties
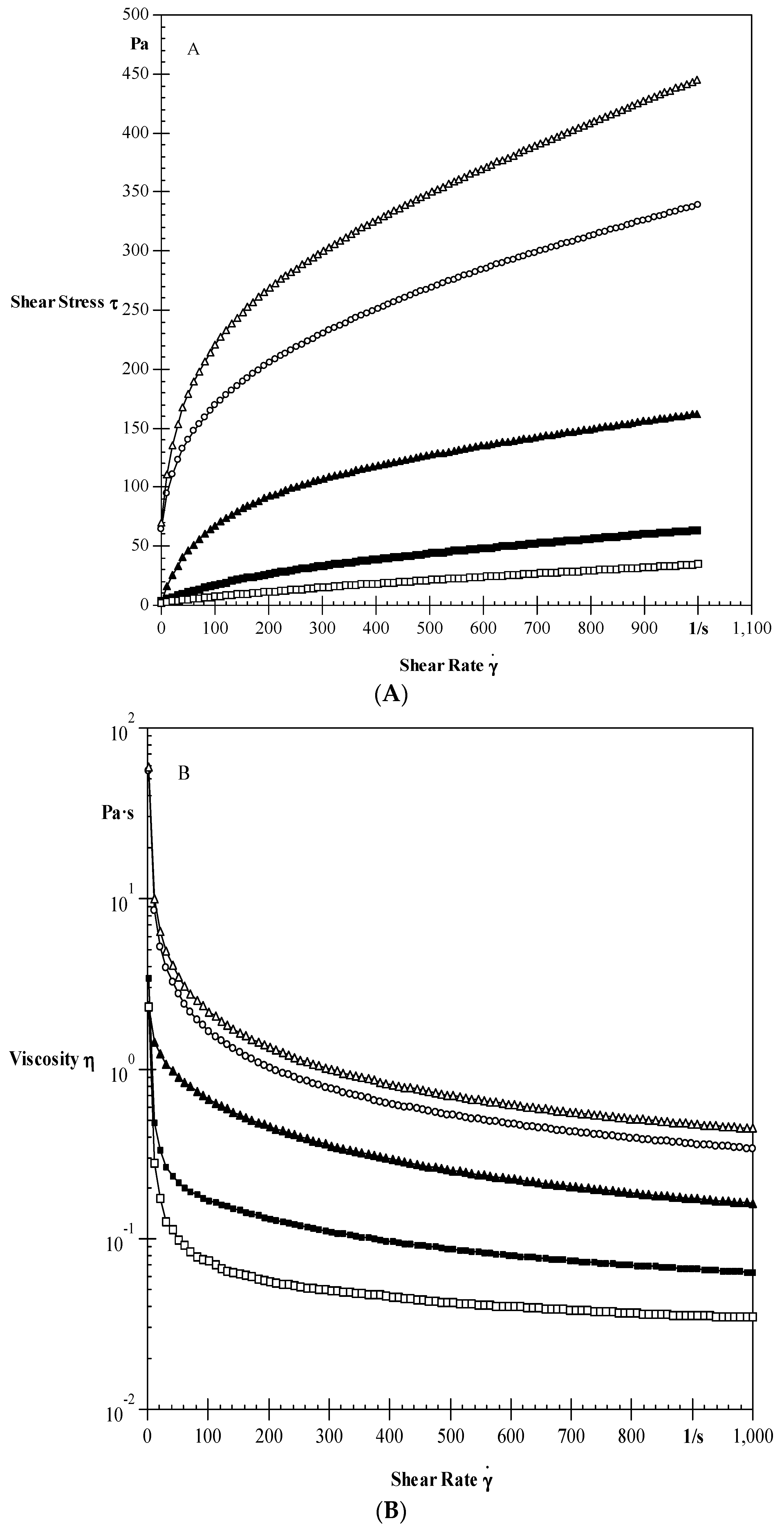
Steady Shear Properties
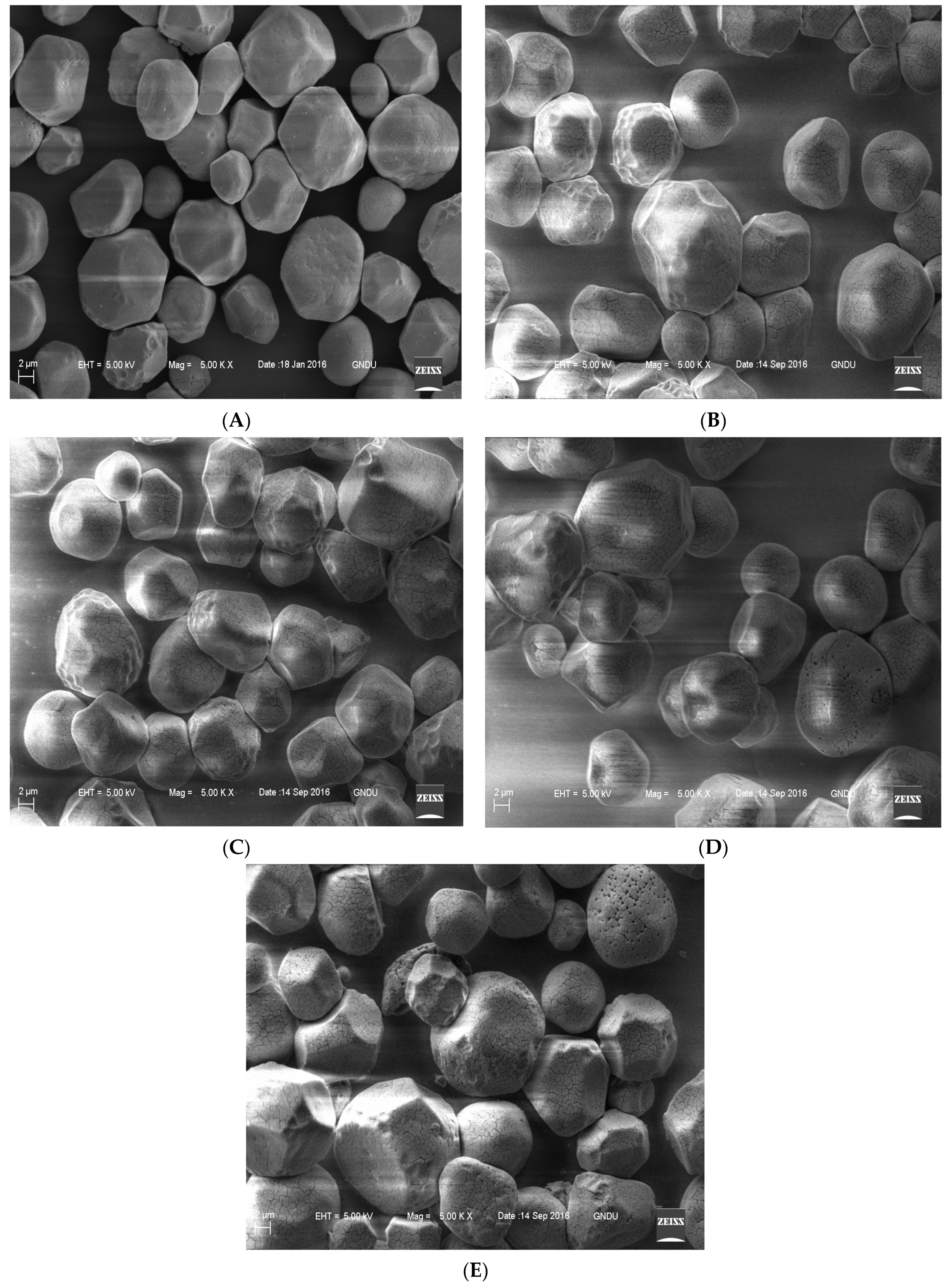
2.2.6. Morphological Characteristics
2.2.7. In Vitro Starch Digestibility
2.2.8. Film Formation
Properties of Films
Tensile Strength (TS) and Elongation at Break Point (EAB)
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. DC and Physicochemical Characteristics
3.2. Pasting Characteristics
3.3. Rheological Properties
3.3.1. Dynamic Shear Properties
3.3.2. Steady Shear Characteristics
3.4. In Vitro Digestibility
3.5. Morphological Properties
3.6. Properties of Starch Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Food and Agricultural Organization of the United Nations). The Statistical Division. 2019. Available online: http://faostat.fao.org/beta/en/#data/QC (accessed on 7 April 2021).
- Xiao, H.; Lin, Q.; Liu, G.Q.; Wu, Y.; Tian, W.; Wu, W.; Fu, X. Physicochemical properties of chemically modified starches from different botanical origin. Sci. Res. Essays 2011, 6, 4517–4525. [Google Scholar]
- Jyothi, A.N.; Moorthy, S.N.; Rajasekharan, K.N. Effect of cross-linking with epichlorohydrin on the properties of cassava (Manihot esculenta Crantz) starch. Starch/Stärke 2006, 58, 292–299. [Google Scholar] [CrossRef]
- Mehboob, S.; Ali, T.M.; Sheikh, M.; Hasnain, A. Effects of cross linking and/or acetylation on sorghum starch and film characteristics. Int. J. Biol. Macromol. 2020, 155, 786–794. [Google Scholar] [CrossRef] [PubMed]
- El-Tahlawy, K.; Venditti, R.A.; Pawlak, J.J. Aspects of the preparation of starch microcellular foam particles crosslinked with glutaraldehyde using a solvent exchange technique. Carbohydr. Polym. 2007, 67, 319–331. [Google Scholar] [CrossRef]
- Chinma, C.E.; Ariahu, C.C.; Alakali, J.S. Effect of temperature and relative humidity on the water vapour permeability and mechanical properties of cassava starch and soy protein concentrate based edible films. J. Food Sci. Technol. 2015, 52, 2380–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effects of carbohydrate/protein ratio on the microstructure and the barrier and sorption properties of wheat starch–whey protein blend edible films. J. Sci. Food Agric. 2017, 97, 858–867. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, J.; Cheng, F. Effects of starches from different botanical sources and modification methods on physicochemical properties of starch-based edible films. Int. J. Biol. Macromol. 2019, 132, 897–905. [Google Scholar] [CrossRef]
- Farahnaky, A.; Saberi, B.; Majzoobi, M. Effect of glycerol on physical and mechanical properties of wheat starch edible films. J. Texture Stud. 2013, 44, 176–186. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Gonçalves, J.R.; El Halal, S.L.M.; Pinto, V.Z.; Dias, A.R.G.; Jacques, A.C.; da Rosa Zavareze, E. Oxidation of potato starch with different sodium hypochlorite concentrations and its effect on biodegradable films. LWT-Food Sci. Technol. 2015, 60, 714–720. [Google Scholar] [CrossRef] [Green Version]
- da Rosa Zavareze, E.; Pinto, V.Z.; Klein, B.; El Halal, S.L.M.; Elias, M.C.; Prentice-Hernández, C.; Dias, A.R.G. Development of oxidised and heat–moisture treated potato starch film. Food Chem. 2012, 132, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Woggum, T.; Sirivongpaisal, P.; Wittaya, T. Properties and characteristics of dual-modified rice starch based biodegradable films. Int. J. Biol. Macromol. 2014, 67, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.S.; Singh, N. Relationships between selected properties of starches from different corn lines. Int. J. Food Prop. 2005, 8, 481–491. [Google Scholar] [CrossRef]
- Wurtzburg, O.B. Preparation Of Starch Derivatives. U.S. Patent No. 2,935,510, 3 May 1960. [Google Scholar]
- Chatakanonda, P.; Varavinit, S.; Chinachoti, P. Relationship of gelatinization and recrystallization of cross-linked rice to glass transition temperature. Cereal Chem. 2000, 77, 315–319. [Google Scholar] [CrossRef]
- Williams, P.C.; Kuzina, F.D.; Hlynka, L. A rapid calorimetric procedure for estimating the amylose content of starches and flour. Cereal Chem. 1970, 47, 411–421. [Google Scholar]
- Leach, H.W.; McCowen, L.D.; Schoch, T.J. Structure of the starch granule I. swelling and solubility patterns of various starches. Cereal Chem. 1959, 36, 534–544. [Google Scholar]
- Park, S.; Chung, M.G.; Yoo, B. Effect of octenyl succinylated on rheological properties of corn starch pastes. Starch/Stärke 2004, 56, 399–406. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar]
- Chung, H.J.; Lim, H.S.; Lim, S.T. Effect of partial gelatinization and retrogradation on the enzymatic digestion of waxy rice starch. J. Cereal Sci. 2006, 43, 353–359. [Google Scholar] [CrossRef]
- Galus, S.; Mathieu, H.; Lenart, A.; Debeaufort, F. Effect of modified starch or maltodextrin incorporation on the barrier and mechanical properties, moisture sensitivity and appearance of soy protein isolate-based edible films. Innov. Food Sci. Emerg. Technol. 2012, 16, 148–154. [Google Scholar] [CrossRef]
- Fan, H.; Ji, N.; Zhao, M.; Xiong, L.; Sun, Q. Characterization of starch films impregnated with starch nanoparticles prepared by 2, 2, 6, 6-tetramethylpiperidine-1-oxyl (TEMPO)-mediated oxidation. Food Chem. 2016, 192, 865–872. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S.; CUQ, J.L. Edible wheat gluten films: Influence of the main process variables on film properties using response surface methodology. J. Food Sci. 1992, 57, 190–195. [Google Scholar] [CrossRef]
- Han, J.H.; Floros, J.D. Casting antimicrobial packaging films and measuring their physical properties and antimicrobial activity. J. Plast. Film Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Siroha, A.K.; Punia, S.; Sangwan, L.; Nehra, M.; Purewal, S.S. Effect of degree of cross linking on physicochemical, rheological and morphological properties of sorghum starch. Carbohydr. Polym. Techno. Appl. 2021, 1, 100073. [Google Scholar]
- Siroha, A.K.; Punía, S.; Sandhu, K.S.; Karwasra, B.L. Physicochemical, pasting, and rheological properties of pearl millet starches from different cultivars and their relations. Acta Aliment. 2020, 49, 49–59. [Google Scholar] [CrossRef]
- Singh, A.V.; Nath, L.K. Synthesis and evaluation of physicochemical properties of cross-linked sago starch. Int. J. Biol. Macromol. 2012, 50, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Singh, J.; Singh, N. Effect of cross-linking on some properties of potato (Solanum tuberosum L.) starches. J. Sci. Food Agric. 2006, 86, 1945–1954. [Google Scholar] [CrossRef]
- Luo, F.X.; Huang, Q.; Fu, X.; Zhang, L.X.; Yu, S.J. Preparation and characterisation of cross-linked waxy potato starch. Food Chem. 2009, 115, 563–568. [Google Scholar] [CrossRef]
- Ackar, D.; Babic, J.; Subaric, D.; Kopjar, M.; Milicevic, B. Isolation of starch from two wheat varieties and their modification with epichlorohydrin. Carbohydr. Polym. 2010, 81, 76–82. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Siroha, A.K. Relationships between physicochemical, thermal, rheological and in vitro digestibility properties of starches from pearl millet cultivars. LWT-Food Sci. Technol. 2017, 83, 213–224. [Google Scholar] [CrossRef]
- Chan, H.T.; Leh, C.P.; Bhat, R.; Senan, C.; Williams, P.A.; Karim, A.A. Molecular structure, rheological and thermal characteristics of ozone-oxidized starch. Food Chem. 2011, 126, 1019–1024. [Google Scholar] [CrossRef]
- Kim, B.Y.; Yoo, B. Effects of cross-linking on the rheological and thermal properties of sweet potato starch. Starch/Stärke 2010, 62, 577–583. [Google Scholar] [CrossRef]
- Shukri, R.; Shi, Y.C. Physiochemical properties of highly cross-linked maize starches and their enzymatic digestibilities by three analytical methods. J. Cereal Sci. 2015, 63, 72–80. [Google Scholar] [CrossRef]
- Huber, K.C.; BeMiller, J.N. Channels of maize and sorghum starch granules. Carbohydr. Polym. 2000, 41, 269–276. [Google Scholar] [CrossRef]
- Annor, G.A.; Marcone, M.; Bertoft, E.; Seetharaman, K. Physical and molecular characterization of millet starches. Cereal Chem. 2014, 91, 286–292. [Google Scholar] [CrossRef]
- Siroha, A.K.; Sandhu, K.S. Physicochemical, rheological, morphological, and in vitro digestibility properties of cross-linked starch from pearl millet cultivars. Int. J. Food Prop. 2018, 21, 1371–1385. [Google Scholar] [CrossRef] [Green Version]
- Mirmoghtadaie, L.; Kadivar, M.; Shahedi, M. Effects of cross-linking and acetylation on oat starch properties. Food Chem. 2009, 116, 709–713. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Role of powder particle size on the encapsulation efficiency of oils during spray drying. Drying Technol. 2007, 25, 1081–1089. [Google Scholar] [CrossRef]
- Hosseini, S.N.; Pirsa, S.; Farzi, J. Biodegradable nano composite film based on modified starch-albumin/MgO; antibacterial, antioxidant and structural properties. Polym. Test. 2021, 97, 107182. [Google Scholar] [CrossRef]
- Wang, B.; Yan, S.; Gao, W.; Kang, X.; Yu, B.; Liu, P.; Guo, L.; Cui, B.; Abd El-Aty, A.M. Antibacterial activity, optical, and functional properties of corn starch-based films impregnated with bamboo leaf volatile oil. Food Chem. 2021, 357, 129743. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Nehra, M.; Siroha, A.K.; Petrů, M.; Ilyas, R.A.; Devi, U.; Devi, P. Development and characterization of physical modified pearl millet starch-based films. Foods 2021, 10, 1609. [Google Scholar] [CrossRef]
- Bangar, S.P.; Siroha, A.K.; Nehra, M.; Trif, M.; Ganwal, V.; Kumar, S. Structural and Film-Forming Properties of Millet Starches: A Comparative Study. Coatings 2021, 11, 954. [Google Scholar] [CrossRef]
- Bruni, G.P.; de Oliveira, J.P.; El Halal, S.L.M.; Flores, W.H.; Gundel, A.; de Miranda, M.Z.; Dias, A.R.G.; da Rosa Zavareze, E. Phosphorylated and cross-linked wheat starches in the presence of polyethylene oxide and their application in biocomposite films. Starch/Stärke 2018, 70, 1700192. [Google Scholar] [CrossRef] [Green Version]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef] [Green Version]
- González, K.; Martin, L.; González, A.; Retegi, A.; Eceiza, A.; Gabilondo, N. D-isosorbide and 1, 3-propanediol as plasticizers for starch-based films: Characterization and aging study. J. Appl. Polym. Sci. 2017, 134, 44793. [Google Scholar] [CrossRef]
- Mitrea, L.; Călinoiu, L.-F.; Martău, G.-A.; Szabo, K.; Teleky, B.-E.; Mureșan, V.; Rusu, A.V.; Socol, C.T.; Vodnar, D.-C. Poly (vinyl alcohol)-based biofilms plasticized with polyols and colored with pigments extracted from tomato by-products. Polymers 2020, 12, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Samples | DC (%) | Amylose Content (%) | Swelling Power (g/g) | Solubility (%) |
|---|---|---|---|---|
| Native | ND | 10.0 ± 0.5 d | 14.2 ± 0.3 c | 14.1 ± 0.1 c |
| 0.1 EPI | ND | 9.8 ± 0.3 c | 18.1 ± 0.2 e | 16.5 ± 0.2 e |
| 0.3 EPI | ND | 9.5 ± 0.3 c | 15.2 ± 0.2 d | 14.9 ± 0.1 d |
| 0.5 EPI | 40.8 ± 0.3 a | 9.0 ± 0.1 b | 8.5 ± 0.1 b | 7.5 ± 0.2 b |
| 0.8 EPI | 74.6 ± 0.5 b | 6.5 ± 0.4 a | 7.8 ± 0.2 a | 7.1 ± 0.2 a |
| Samples | PV (cP) | TV (cP) | BV (cP) | SV (cP) | FV (cP) | P T (°C) |
|---|---|---|---|---|---|---|
| Native | 1860 ± 22 c | 839 ± 11 c | 1021 ± 12 e | 591 ± 14 e | 1430 ± 15 c | 88.5 ± 0.1 a |
| 0.1 EPI | 2247 ± 25 d | 1873 ± 15 d | 374 ± 9 c | 524 ± 12 d | 2397 ± 18 e | 88.3 ± 0.1 a |
| 0.3 EPI | 2588 ± 19 a | 1970 ± 21 a | 618 ± 8 d | 210 ± 9 c | 2180 ± 21 d | 89.1 ± 0.2 b |
| 0.5 EPI | 1101 ± 15 b | 803 ± 14 b | 298 ± 6 b | 103 ± 8 b | 906 ± 16 b | 89.6 ± 0.1 b |
| 0.8 EPI | 471 ± 09 a | 388 ± 09 a | 83 ± 4 a | 64 ± 4 a | 452 ± 11 a | 90.2 ± 0.2 c |
| Samples | G′ (Pa) | G″ (Pa) | tan δ |
|---|---|---|---|
| Native | 5541 ± 25 d | 228 ± 9 d | 0.04 |
| 0.1 EPI | 5744 ± 23 e | 221 ± 8 c | 0.03 |
| 0.3 EPI | 5263 ± 21 c | 209 ± 6 b | 0.03 |
| 0.5 EPI | 3558 ± 18 b | 168 ± 8 a | 0.04 |
| 0.8 EPI | 2877 ± 19 a | 237 ± 9 e | 0.08 |
| Samples | σο (Pa) | K (Pa·s) | n | R2 |
|---|---|---|---|---|
| Native | 25.59 ± 2 e | 31.38 ± 2 d | 0.32 ± 0.007 a | 0.999 |
| 0.1% EPI | 4.64 ± 0.9 d | 49.29 ± 3 e | 0.32 ± 0.005 a | 0.999 |
| 0.3% EPI | 4.33 ± 0.7 c | 1.46 ± 0.3 c | 0.77 ± 0.003 b | 0.985 |
| 0.5% EPI | 3.22 ± 0.5 b | 0.27 ± 0.02 b | 0.85 ± 0.005 c | 0.999 |
| 0.8% EPI | 2.3 ± 0.5 a | 0.08 ± 0.01 a | 0.87 ± 0.009 c | 0.995 |
| Samples | RDS (%) | SDS (%) | RS (%) |
|---|---|---|---|
| Native | 49.9 ± 0.9 d | 38.1 ± 0.6 d | 12.0 ± 0.5 a |
| 0.1% EPI | 49.6 ± 0.6 c | 37.8 ± 0.5 c | 12.6 ± 0.3 b |
| 0.3% EPI | 49.9 ± 0.8 d | 36.0 ± 0.8 b | 14.1 ± 0.8 c |
| 0.5% EPI | 48.3 ± 0.9 b | 36.4 ± 0.7 b | 15.3 ± 0.7 d |
| 0.8% EPI | 45.9 ± 0.6 a | 35.1 ± 0.7 a | 19.0 ± 0.8 e |
| Samples | Moisture Content (%) | Thickness (mm) | Water Solubility (%) | Opacity (%) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|---|
| Native | 26.08 ± 1.9 d | 0.103 ± 0.004 | 38.34 ± 2.5 d | 1.679 ± 00 b | 6.80 ± 0.25 a | 62.8 ± 1.9 e |
| 0.1 EPI | 28.11 ± 2.1 e | 0.105 ± 0.001 | 39.10 ± 2.1 e | 1.695 ± 00 a | 7.35 ± 0.30 b | 58.7 ± 1.7 d |
| 0.3 EPI | 25.01 ± 2.2 c | 0.102 ± 0.002 | 36.14 ± 2.4 c | 2.656 ± 00 c | 7.91 ± 0.11 c | 56.9 ± 1.1 c |
| 0.5 EPI | 22.88 ± 1.4 b | 0.099 ± 0.001 | 33.11 ± 1.9 b | 2.711 ± 00 d | 8.11 ± 0.22 d | 52.1 ± 1.5 b |
| 0.8 EPI | 20.09 ± 1.7 a | 0.094 ± 0.005 | 28.36 ± 2.5 a | 2.730 ± 00 e | 9.60 ± 0.14 e | 47.5 ± 2.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siroha, A.K.; Bangar, S.P.; Sandhu, K.S.; Trif, M.; Kumar, M.; Guleria, P. Effect of Cross-Linking Modification on Structural and Film-Forming Characteristics of Pearl Millet (Pennisetum glaucum L.) Starch. Coatings 2021, 11, 1163. https://doi.org/10.3390/coatings11101163
Siroha AK, Bangar SP, Sandhu KS, Trif M, Kumar M, Guleria P. Effect of Cross-Linking Modification on Structural and Film-Forming Characteristics of Pearl Millet (Pennisetum glaucum L.) Starch. Coatings. 2021; 11(10):1163. https://doi.org/10.3390/coatings11101163
Chicago/Turabian StyleSiroha, Anil Kumar, Sneh Punia Bangar, Kawaljit Singh Sandhu, Monica Trif, Manoj Kumar, and Prixit Guleria. 2021. "Effect of Cross-Linking Modification on Structural and Film-Forming Characteristics of Pearl Millet (Pennisetum glaucum L.) Starch" Coatings 11, no. 10: 1163. https://doi.org/10.3390/coatings11101163









