Polyethylene Films Coated with Antibacterial and Antiviral Layers Based on CO2 Extracts of Raspberry Seeds, of Pomegranate Seeds and of Rosemary
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Preparation
- (1)
- An amount of 12 g of MHPC was introduced into the baker containing 288 mL of distilled water. The mixture was stirred for 1 h using a magnetic stirrer (Ika, Warsaw, Poland) at 1500 rpm.
- (2)
- An amount of 2 g of CO2 extract of raspberry seeds (I), pomegranate seeds (II), and rosemary (III) were introduced into beakers separately. Then, 1 g of Decyl Glucoside and 1 g of Caprylyl/Capryl glucoside were introduced into each beaker and mixed for 10 min using a magnetic stirrer at 500 rpm (Ika, Warsaw, Poland). As a next step, 36 g of 4% MHPC was introduced into each mixture. Mixtures I, II, and III were mixed for 15 min (separately) using a magnetic stirrer (Ika, Warsaw, Poland) at 1000 rpm.
- (3)
- An amount of 1 g of CO2 extract of raspberry seeds and 1 g of CO2 extract of pomegranate seeds (IV) were introduced into the first beaker; 1 g of CO2 extract of raspberry seeds and 1 g of CO2 extract of rosemary (V) were introduced into the second beaker; 1 g of CO2 extract of pomegranate seeds, and 1 g of rosemary (VI) were introduced into the third beaker. These were mixed for 5 min using a magnetic stirrer at 500 rpm (Ika, Warsaw, Poland). Then, 1 g of Decyl Glucoside and 1 g of Caprylyl/Capryl glucoside were introduced into each beaker and mixed for 10 min using a magnetic stirrer at 500 rpm (Ika, Warsaw, Poland). As a next step, 36 g of 4% MHPC was introduced into each mixture. The mixtures IV, V, and VI were also mixed for 15 min (separately, at 1000 rpm).
- (4)
- An amount of 0.7 g of CO2 extract of raspberry seeds, 0.7 g of rosemary and 0.7 g of CO2 extract of pomegranate seeds (VII) were introduced into the beaker; this was mixed for 5 min using a magnetic stirrer at 500 rpm (Ika, Warsaw, Poland). Then, 1 g of Decyl Glucoside and 1 g of Caprylyl/Capryl glucoside were introduced into the beaker and mixed for 10 min using a magnetic stirrer at 500 rpm (Ika, Warsaw, Poland). Next, 36 g of 4% MHPC was introduced into the mixture. Mixture VII was also mixed for 15 min at 1000 rpm.
2.3. Antibacterial Properties Analysis
2.4. Antiviral Properties Analysis
2.5. SEM
2.6. Statistical Analysis
3. Results
3.1. Antibacterial Properties Analysis
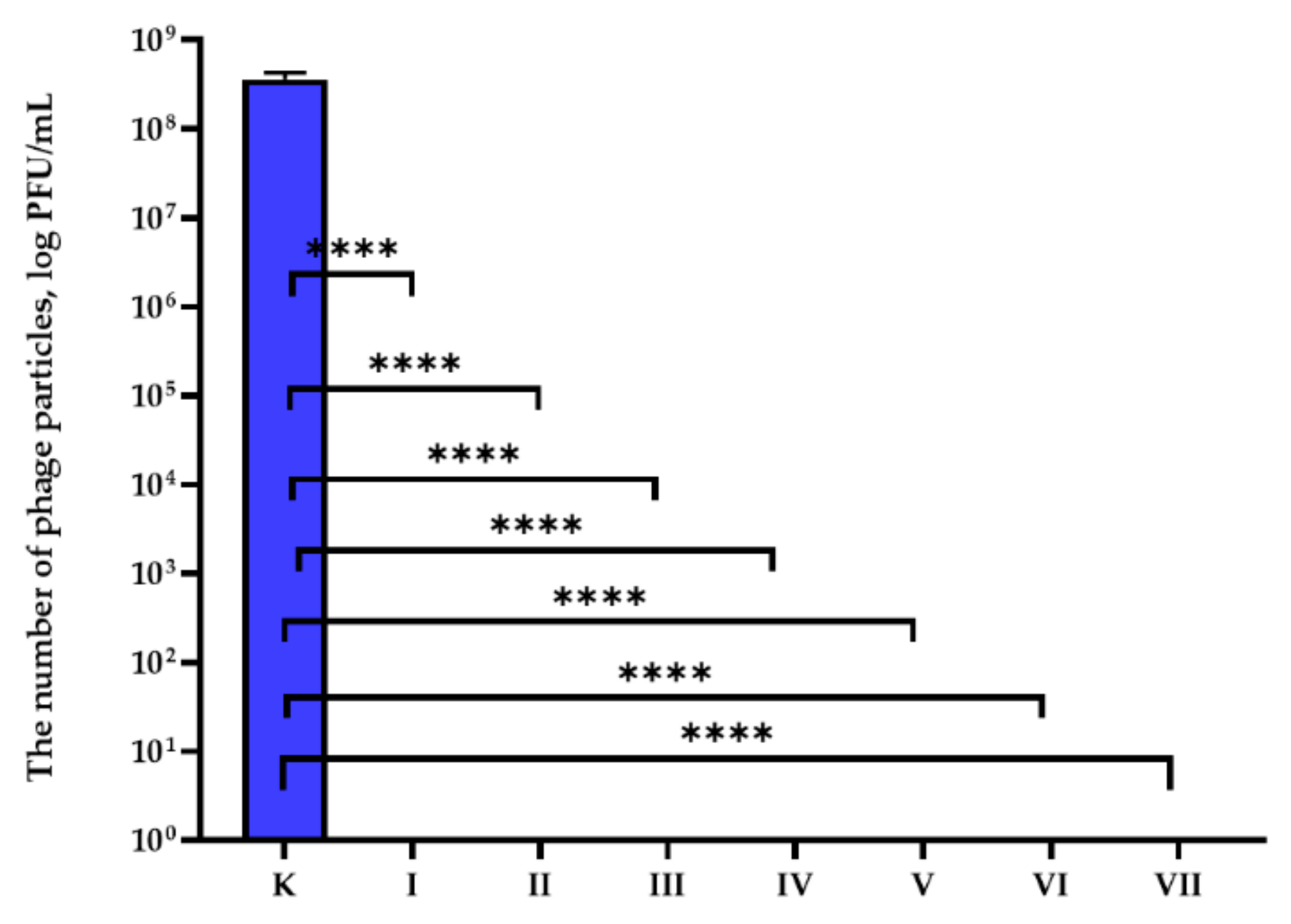
3.2. Antiviral Properties Analysis
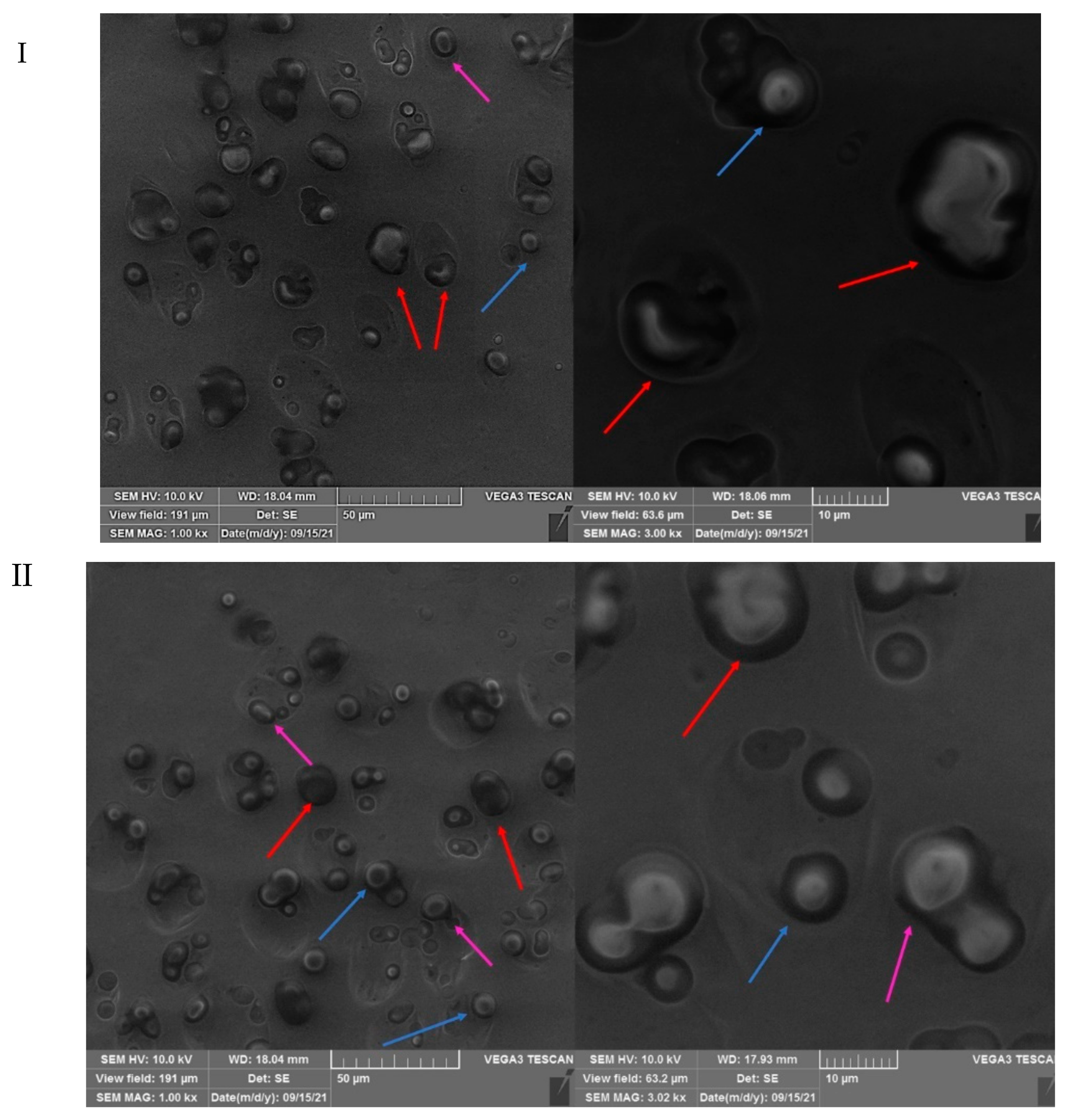
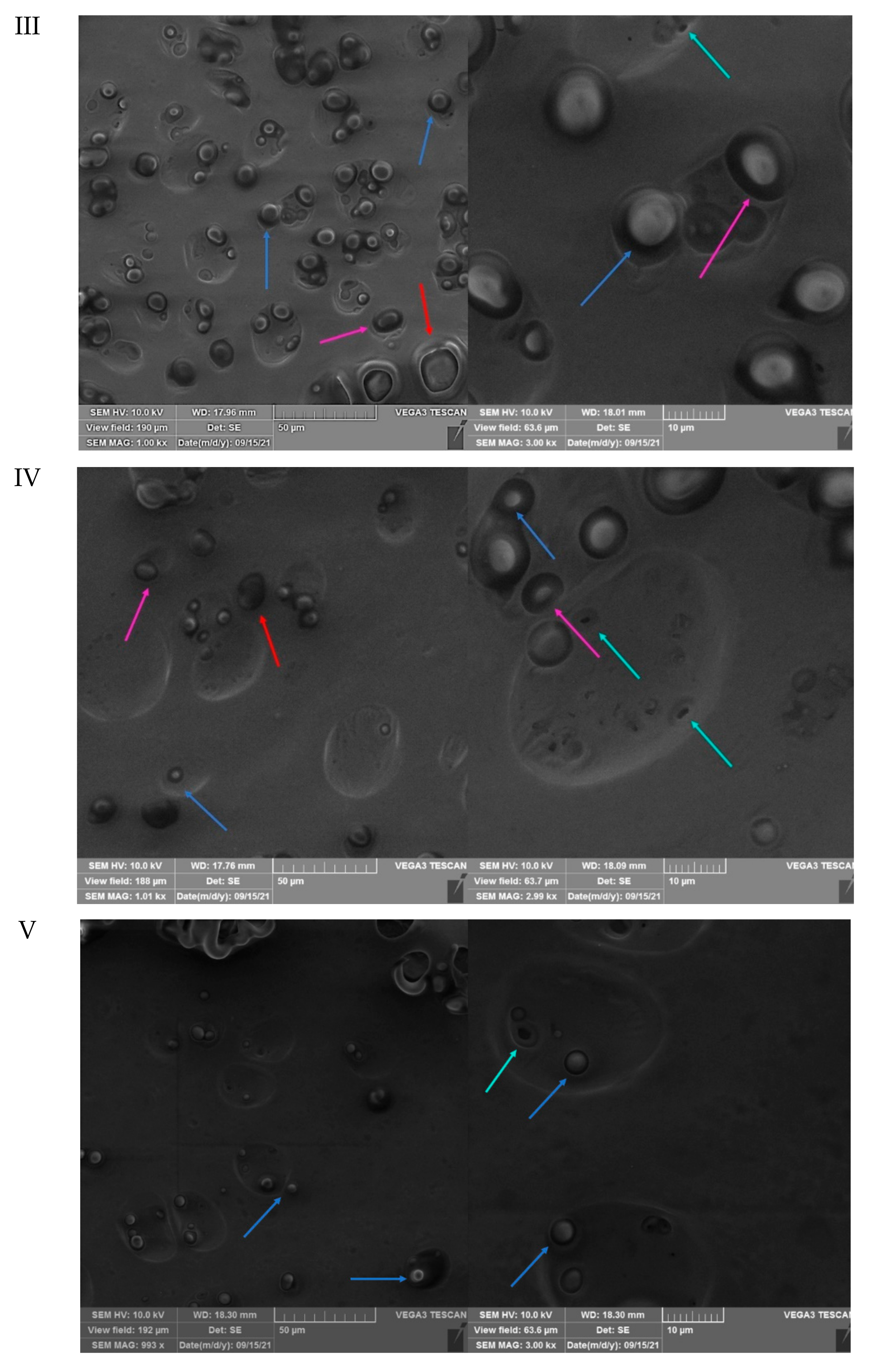
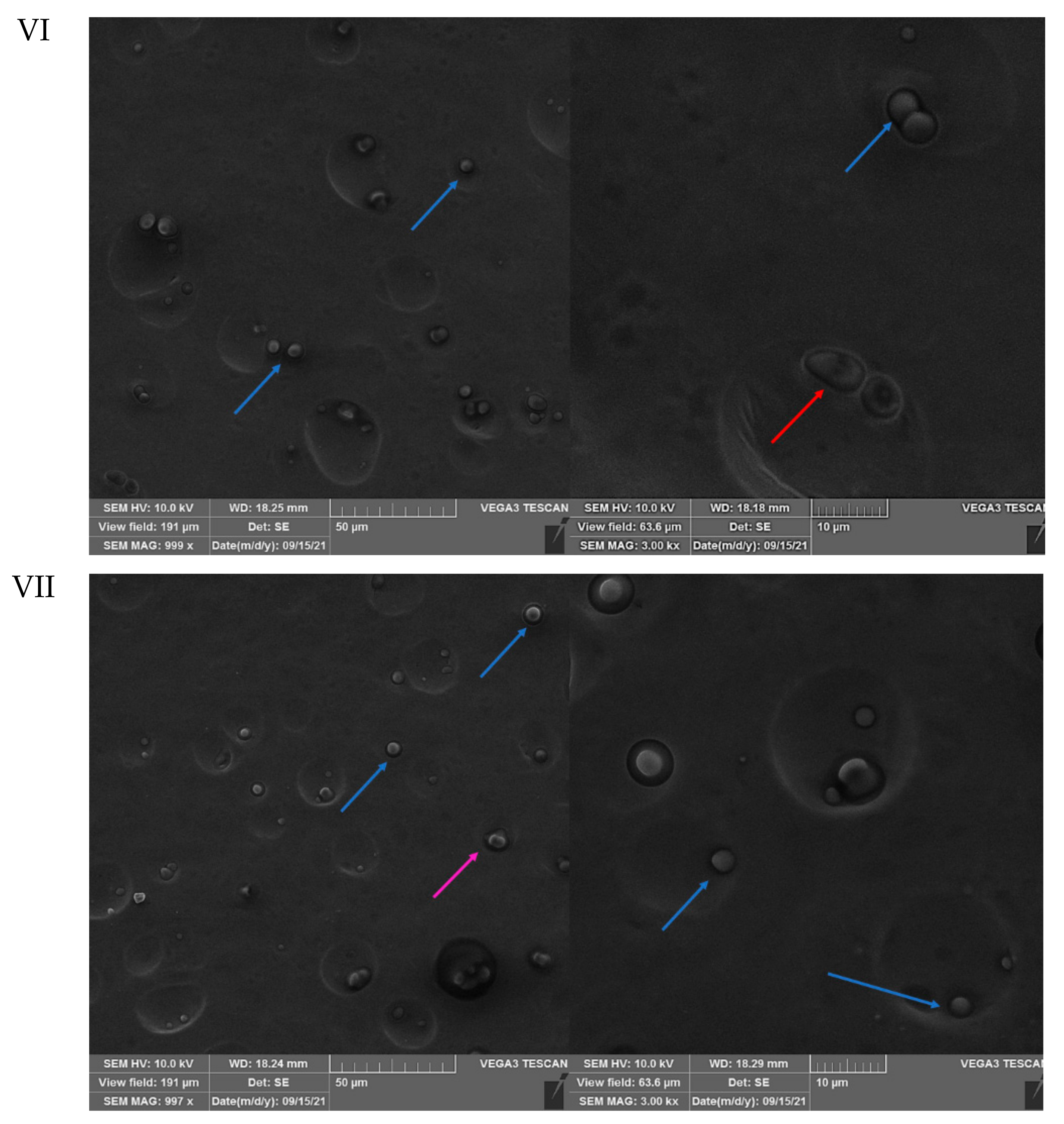
3.3. SEM Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mizielińska, M.; Kowalska, U.; Jarosz, M.; Sumińska Landercy, N.; Duquesne, E. The Effect of UV Aging on Antimicrobial and Mechanical Properties of PLA Films with Incorporated Zinc Oxide Nanoparticles. Int. J. Environ. Res. Public Health 2018, 15, 794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Jiang, H.; Rhim, J.W.; Cao, J.; Jiang, W. Effective strategies of sustained release and retention enhancement of essential oils in active food packaging films/coatings. Food Chem. 2022, 367, 130671. [Google Scholar] [CrossRef] [PubMed]
- Mizielińska, M.; Kowalska, U.; Jarosz, M.; Sumińska, P. A comparison of the effects of packaging containing nano ZnO or polylysine on the microbial purity and texture of cod (Gadus morhua) fillets. Nanomaterials 2018, 8, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizielińska, M.; Nawrotek, P.; Stachurska, X.; Ordon, M.; Bartkowiak, P. Packaging covered with antiviral and antibacterial coatings based on ZnO nanoparticles, geraniol and carvacrol. Int. J. Mol. Sci. 2021, 22, 1717. [Google Scholar] [CrossRef] [PubMed]
- Mizielińska, M.; Salachna, P.; Ordon, M.; Łopusiewicz, Ł. Antimicrobial activity of water and acetone extracts of some Eucomis taxa. Asian Pac. J. Trop. Med. 2017, 10, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Harscoat-Schiavo, C.; Khoualdia, B.; Savoire, R.; Hobloss, S.; Buré, C.; Samiab, B.A.; Subra-Paternault, P. Extraction of phenolics from pomegranate residues: Selectivity induced by the methods. J. Supercrit. Fluid. 2021, 176, 105300. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I.R.N.A. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep.-UK 2021, 11, 1–14. [Google Scholar]
- Hanafy, S.M.; El-Shafea, Y.M.A.; Saleh, W.D.; Fathy, H.M. Chemical profiling, in vitro antimicrobial and antioxidant activities of pomegranate, orange and banana peel-extracts against pathogenic microorganisms. J. Gen. Eng. Biotechnol. 2020, 19, 1–10. [Google Scholar]
- De Silva, L.O.; Garrett, R.; Monteiro, M.L.G.; Conte-Junior, C.A.; Torres, A.G. Pomegranate (Punica granatum) peel fractions obtained by supercritical CO2 increase oxidative and colour stability of bluefish (Pomatomus saltatrix) patties treated by UV-C irradiation. Food Chem. 2021, 362, 130159. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Al-Ansi, W.; Ali Mahdi, A.; Al-Gheethi, A.A.S.; Mushtaq, B.S.; Al-Adeeb, A.; Wei, M.; Yao, W. Supercritical fluid extraction of four aromatic herbs and assessment of the volatile compositions, bioactive compounds, antibacterial, and anti-biofilm activity. Environ. Sci. Pollut. Res. 2021, 28, 25479–25492. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Ali Mahdi, A.; Al-Ansi, W.; Mohammed, J.K.; Wei, M.; Yao, W. Evaluation of bioactive compounds and antibacterial activity of Pulicaria jaubertii extract obtained by supercritical and conventional methods. J. Food Meas. Charact. 2021, 15, 449–456. [Google Scholar] [CrossRef]
- Jung, H.; Kim, I.; Jung, S.; Lee, J. Oxidative stability of chia seed oil and fax seed oil and impact of rosemary (Rosmarinus ofcinalis L.) and garlic (Allium cepa L.) extracts on the prevention of lipid oxidation. Appl. Biol. Chem. 2021, 64, 1–16. [Google Scholar] [CrossRef]
- Alavi, M.S.; Fanoudi, S.; Rahbardar, M.G.; Mehri, S.; Hosseinzadeh, H. An updated review of protective effects of rosemary and its active constituents against natural and chemical toxicities. Phytother. Res. 2021, 35, 1313–1328. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Ezzat, S.M.; El Bishbishy, M.H.; Mnayer, D.; Sharopov, F.; Kılıç, C.S.; Neagu, M.; Constantin, C.; Sharifi-Rad, M.; Atanassova, M.; et al. Rosmarinus plants: Key farm concepts towards food applications. Phytother. Res. 2020, 34, 1474–1518. [Google Scholar] [CrossRef]
- Peñaranda, I.; Auqui, S.M.; Egea, M.; Linares, M.B.; Garrido, M.D. Effects of Dietary Rosemary Extract Supplementation on Pork Quality of Chato Murciano Breed during Storage. Animals 2021, 11, 2295. [Google Scholar] [CrossRef]
- Golovinskaia, O.; Wang, C.K. Review of Functional and Pharmacological Activities of Berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef]
- Junior, A.G.; Tolouei, S.E.L.; Dos Reis Lívero, F.A.; Gasparotto, F.; Boeing, T.; de Souza, P. Natural Agents Modulating ACE-2: A Review of Compounds with Potential against SARS-CoV-2 Infections. Curr. Pharm. Des. 2021, 27, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Mònica Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and their potential role to fight viral diseases: An overview. Sci. Total Environ. 2021, 801, 149719. [Google Scholar] [CrossRef]
- Mesomo Bombardelli, M.C.; Schineider Machado, C.; Kotovicz, V.; Letícia Kruger, R.; Dalla Santa, O.R.; Reyes Torres, Y.; Corazza, M.L.; Silva, E.A. Extracts from red Araçá (Psidium cattleianum) fruits: Extraction process, modelling and assessment of the bioactivity potentialities. J. Supercrit. Fluid. 2021, 176, 105278. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Chandu, A.G.S.; Devi, S.S.; Quirin, K.W.; Pasha, A.; Vijayendra, S.V.N. In-vitro evaluation of antimicrobial and insect repellent potential of supercritical-carbon dioxide (SCF-CO2) extracts of selected botanicals against stored product pests and foodborne pathogens. J. Food Sci. Technol. 2020, 57, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Prussin, A.J., II; Schwake, D.O.; Lin, K.; Gallagher, D.L.; Buttling, L.; Marr, L.C. Survival of the Enveloped Virus Phi6 in Droplets as a Function of Relative Humidity, Absolute Humidity and Temperature. Appl. Environ. Microbiol. 2018, 84, e00551-18. [Google Scholar] [CrossRef] [Green Version]
- ASTM. Standard Test Method for Determining the Activity of Incorporated Antimicrobial Agent(s) in Polymeric or Hydrophobic Materials; ASTM E 2180-01; ASTM: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Bhetwal, A.; Maharjan, A.; Shakya, S.; Satyal, D.; Ghimire, S.; Khanal, P.R.; Parajuli, N.P. Isolation of Potential Phages against Multidrug-Resistant Bacterial Isolates: Promising Agents in the Rivers of Kathmandu, Nepal. Hindawi BioMed Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, N.; Rojas, M.J.; Cruz, G.N.F.; Hung, S.H.; Rohwer, F.; Barr, J.J. Phage on tap–a quick and efficient protocol for the preparation of bacteriophage laboratory stocks. PeerJ 2016, 4, 2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 22196-2011: Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. Available online: https://www.iso.org/standard/54431.html (accessed on 20 August 2021).
- Mizielińska, M.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. The influence of accelerated UV-A and Q-SUN irradiation on the antimicrobial properties of coatings containing ZnO nanoparticles. Molecules 2017, 22, 1556. [Google Scholar] [CrossRef] [Green Version]
- Patrício Silva, A.L.; Prata, J.C.; Walker, T.R.; Duarte, A.C.; Ouyangd, W.; Barcelòe, D.; Rocha-Santos, T. Increased plastic pollution due to COVID-19 pandemic: Challenges and recommendations. Chem. Eng. J. 2021, 405, 126683. [Google Scholar] [CrossRef]
- Alvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Sharma, D.; Sharma, S.; Kaitha, B.S.; Rajputa, J.; Kaur, M. Synthesis of ZnO nanoparticles using surfactant free in-air and microwave method. Appl. Surf. Sci. 2011, 257, 9661–9672. [Google Scholar] [CrossRef]
- Zhu, F. Polysaccharide based films and coatings for food packaging: Effect of added polyphenols. Food Chem. 2021, 359, 129871. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, A.; Grinberg, M.; Orevi, T.; Kashtan, N. Survival of the enveloped bacteriophage Phi6 (a surrogate for SARS-CoV-2) in evaporated saliva microdroplets deposited on glass surfaces. Sci. Rep.-UK 2020, 10, 22419. [Google Scholar] [CrossRef]
- Wink, M. Potential of DNA Intercalating Alkaloids and Other Plant Secondary Metabolites against SARS-CoV-2 Causing COVID-19. Diversity 2020, 12, 175. [Google Scholar] [CrossRef]
- Pan, M.; Carol, L.; Lednicky, J.A.; Eiguren-Fernandez, A.; Hering, S.; Fan, Z.H.; Wu, C.Y. Determination of the distribution of infectious viruses in aerosol particles using water-based condensational growth technology and a bacteriophage MS2 model. Aerosol Sci. Technol. 2019, 53, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Surucic, R.; Travar, M.; Petkovic, M.; Tubic, B.; Stojiljkovic, M.P.; Grabez, M.; Savikin, K.; Zdunic, G.; Skrbic, R. Pomegranate peel extract polyphenols attenuate the SARS-CoV-2 S-glycoprotein binding ability to ACE2 Receptor: In silico and in vitro studies. Bioorg. Chem. 2021, 114, 105145. [Google Scholar] [CrossRef] [PubMed]
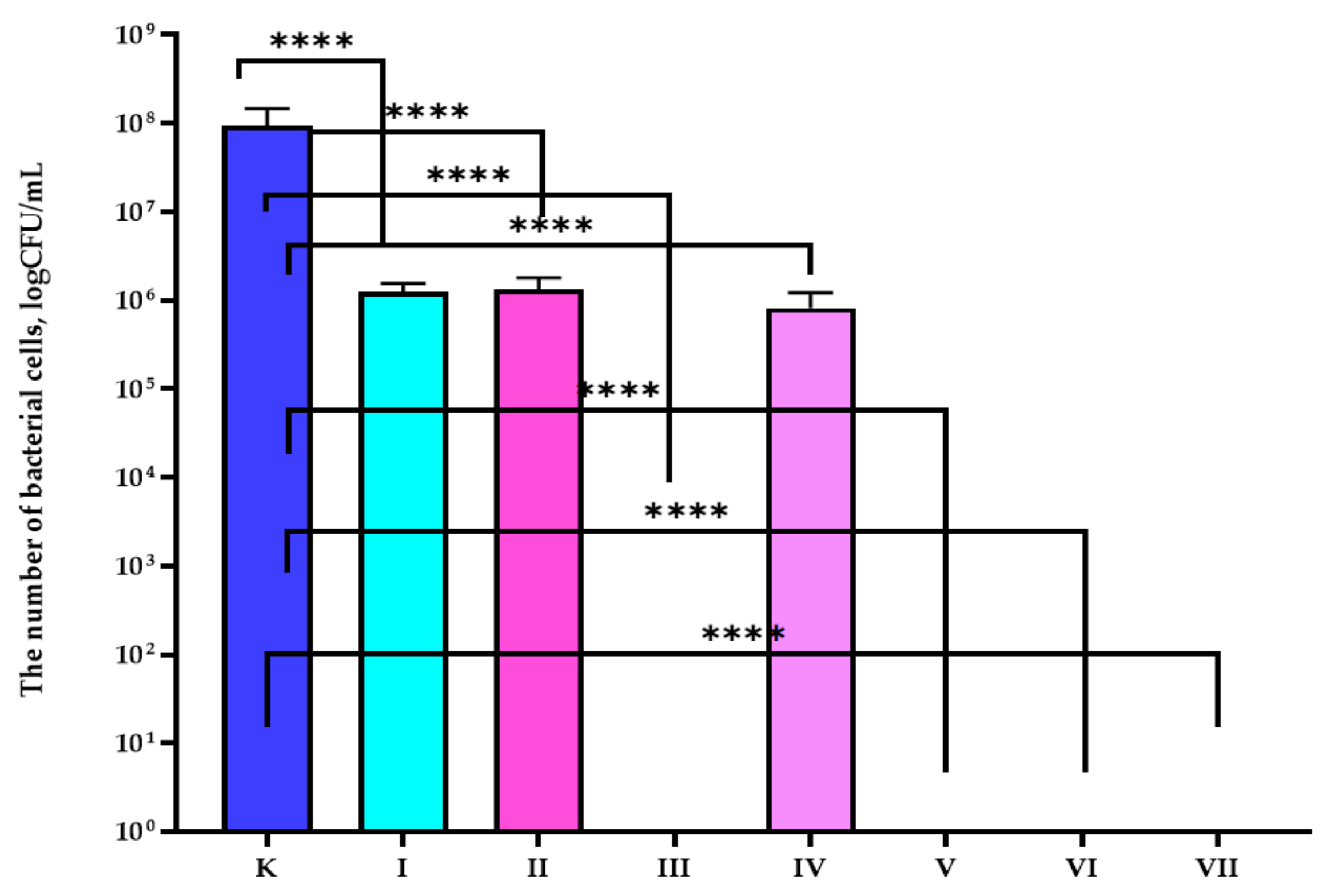
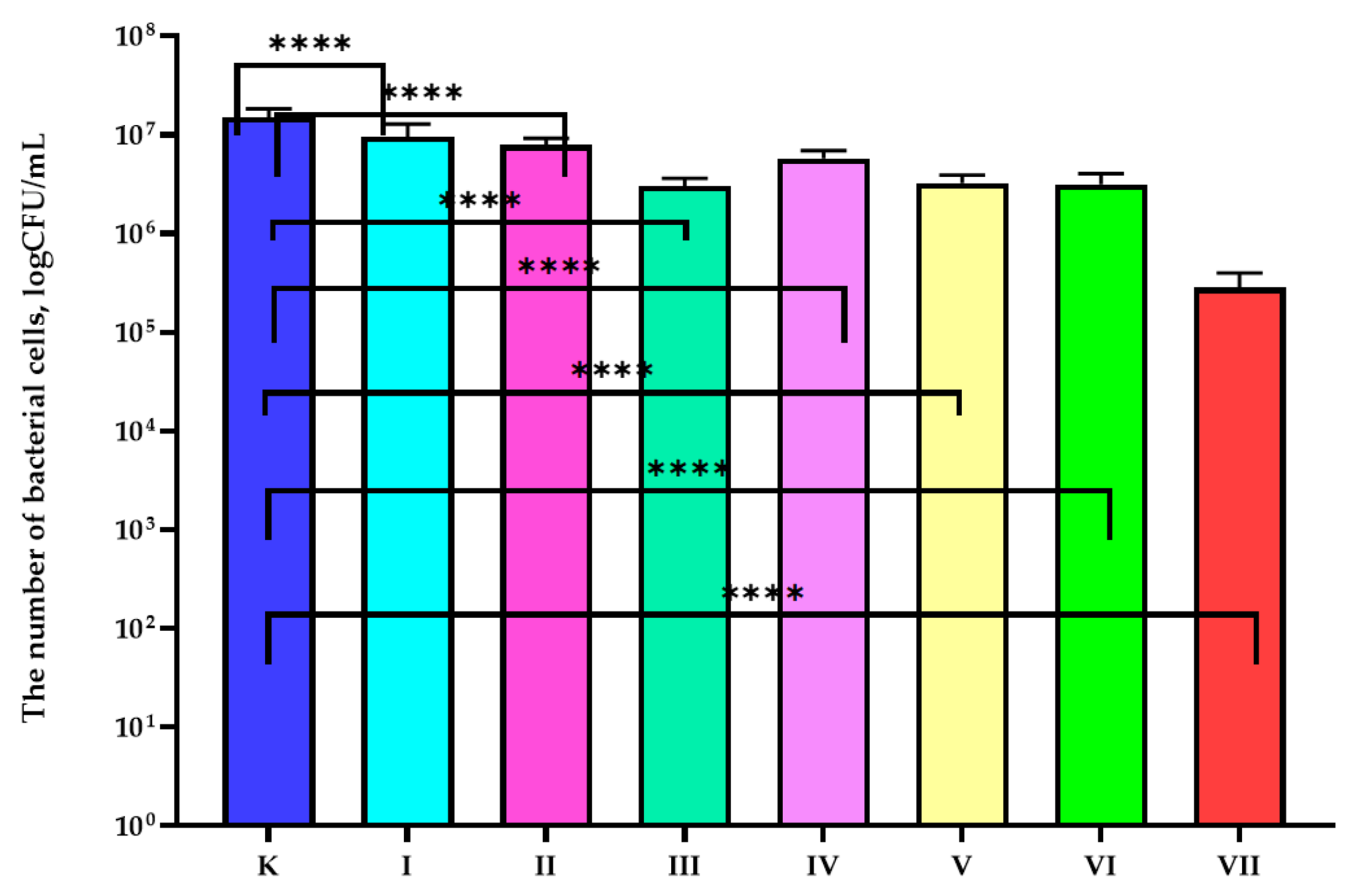
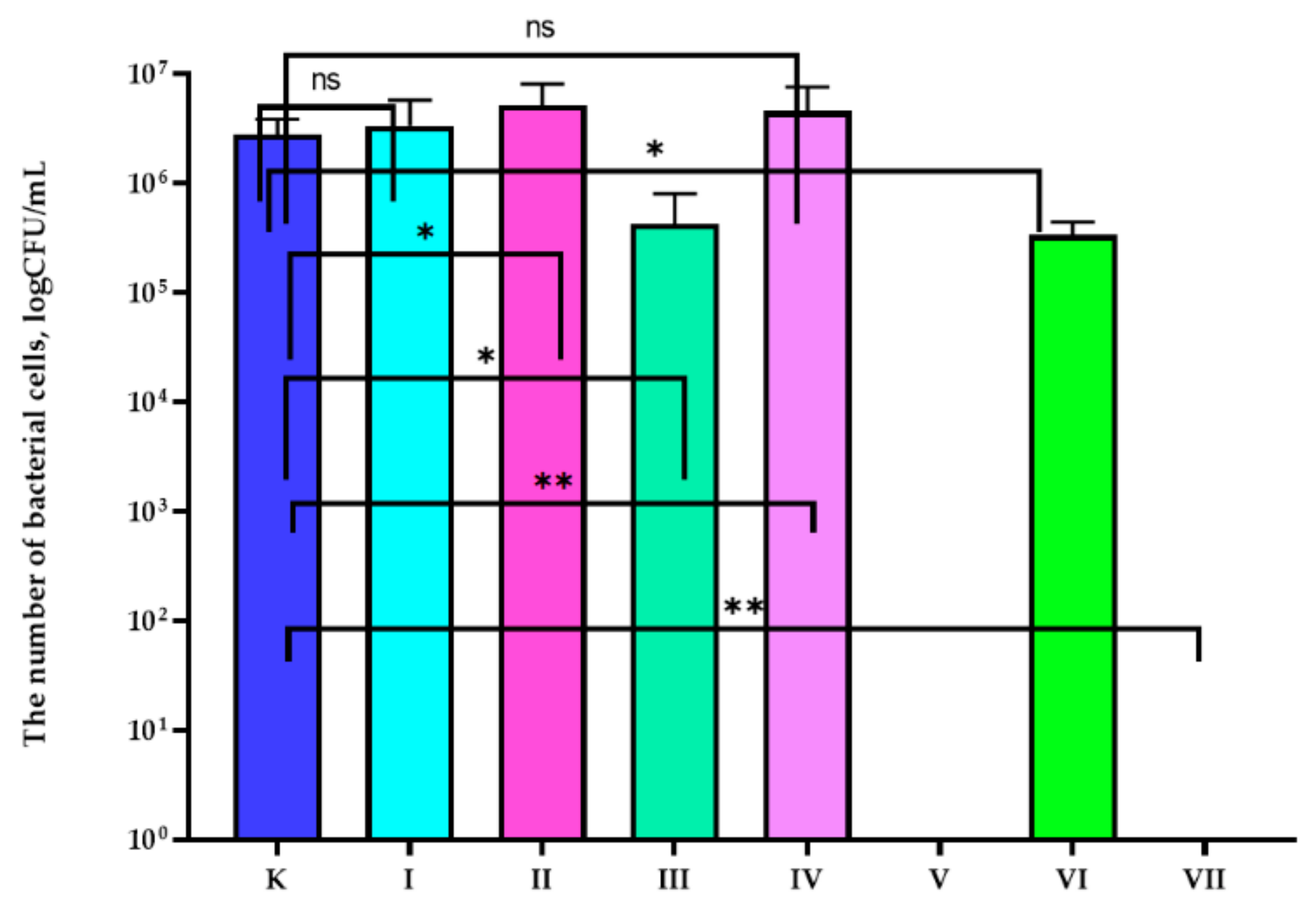
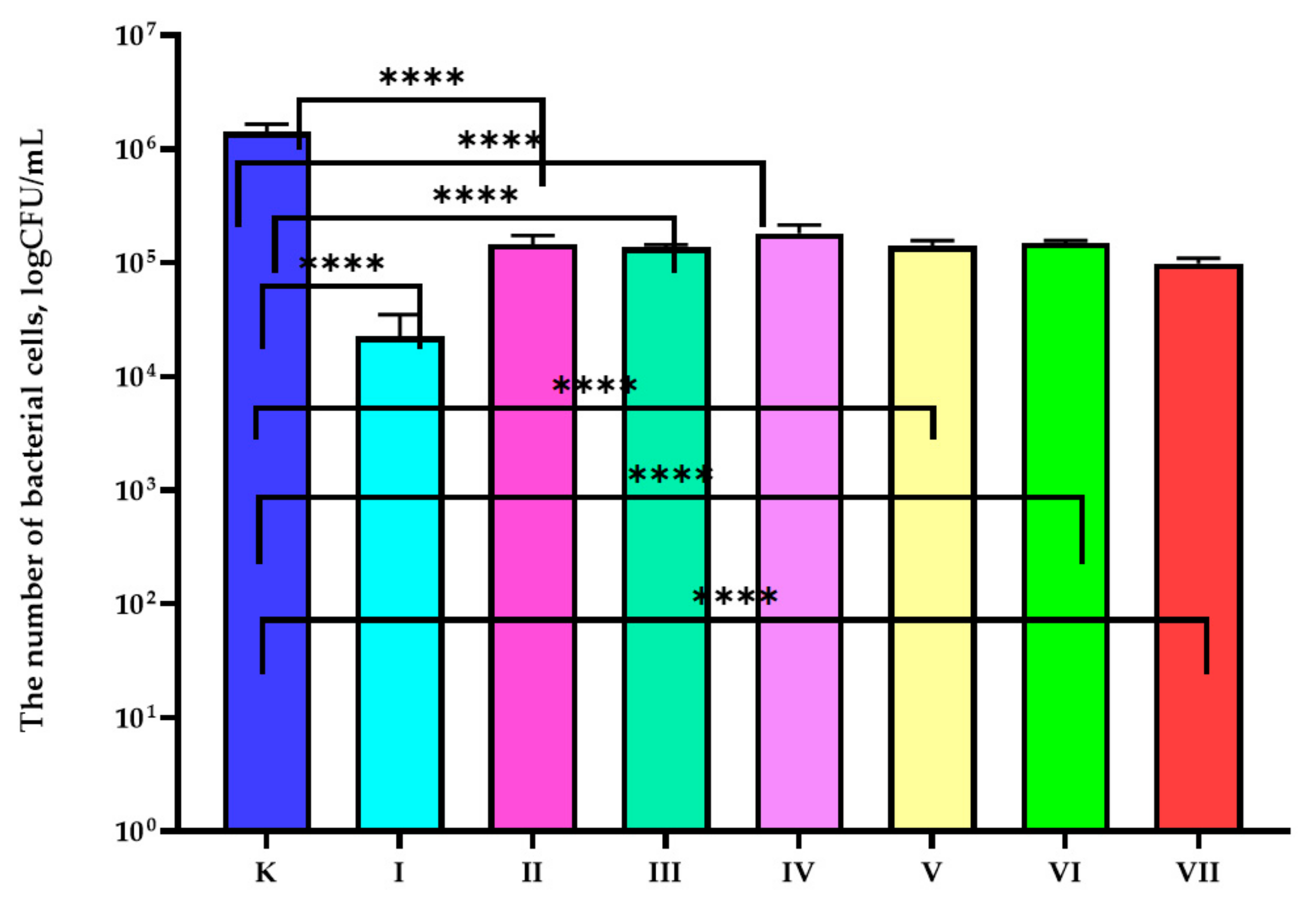

| Sample | Composition of the Active Coatings | |
|---|---|---|
| K | PE film | |
| I | PE film covered with the MHPC coating containing: | CO2 raspberry seed extract as an active compound |
| II | CO2 pomegranate seed extract as an active compound | |
| III | CO2 rosemary extract as an active compound | |
| IV | CO2 raspberry and pomegranate seed extracts | |
| V | CO2 raspberry seed and rosemary extracts | |
| VI | CO2 pomegranate seed and rosemary extracts | |
| VII | CO2 extracts of raspberry seeds, pomegranate seeds and rosemary | |
| The Number of phi6 Phage Particles (PFU/mL) | |||||||
|---|---|---|---|---|---|---|---|
| K | I | II | III | IV | V | VI | VII |
| 3.57 × 10−8 ± 7.23 × 10−7 | 0 **** | 0 **** | 0 **** | 0 **** | 0 **** | 0 **** | 0 **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordon, M.; Nawrotek, P.; Stachurska, X.; Mizielińska, M. Polyethylene Films Coated with Antibacterial and Antiviral Layers Based on CO2 Extracts of Raspberry Seeds, of Pomegranate Seeds and of Rosemary. Coatings 2021, 11, 1179. https://doi.org/10.3390/coatings11101179
Ordon M, Nawrotek P, Stachurska X, Mizielińska M. Polyethylene Films Coated with Antibacterial and Antiviral Layers Based on CO2 Extracts of Raspberry Seeds, of Pomegranate Seeds and of Rosemary. Coatings. 2021; 11(10):1179. https://doi.org/10.3390/coatings11101179
Chicago/Turabian StyleOrdon, Magdalena, Paweł Nawrotek, Xymena Stachurska, and Małgorzata Mizielińska. 2021. "Polyethylene Films Coated with Antibacterial and Antiviral Layers Based on CO2 Extracts of Raspberry Seeds, of Pomegranate Seeds and of Rosemary" Coatings 11, no. 10: 1179. https://doi.org/10.3390/coatings11101179
APA StyleOrdon, M., Nawrotek, P., Stachurska, X., & Mizielińska, M. (2021). Polyethylene Films Coated with Antibacterial and Antiviral Layers Based on CO2 Extracts of Raspberry Seeds, of Pomegranate Seeds and of Rosemary. Coatings, 11(10), 1179. https://doi.org/10.3390/coatings11101179








