Compositional Engineering of FAPbI3 Perovskite Added MACl with MAPbBr3 or FAPbBr3
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Preparation
2.3. Characterization and Device Measurement
3. Results and Discussion
3.1. FAPbI3 Perovskite Solar Cells with MACl
3.2. FAPbI3-MAPbBr3 Perovskite Solar Cells
3.3. FAPbI3-FAPbBr3 Perovskite Solar Cells
3.4. Comparison of FAPbI3-FAPbBr3 and FAPbI3-MAPbBr3 Perovskite Solar Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, Q.; Bae, S.H.; Sun, P.; Hsieh, Y.T.; Yang, Y.; Rim, Y.S.; Zhao, H.; Chen, Q.; Shi, W.; Li, G. Single crystal formamidinium lead iodide (FAPbI3): Insight into the structural, optical, and electrical properties. Adv. Mater. 2016, 28, 2253–2258. [Google Scholar] [CrossRef] [PubMed]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Su, J.; Zheng, X.; Lang, X.; Han, R.; Cai, H.; Ni, J.; Zhang, J.; Qiu, J. Effect of precursor solution ageing time on the photovoltaic performance of perovskite solar cells. Funct. Mater. Lett. 2021, 14, 2151025. [Google Scholar] [CrossRef]
- Koh, T.M.; Fu, K.; Fang, Y.; Chen, S.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G.; Boix, P.P.; Baikie, T. Formamidinium-containing metal-halide: An alternative material for near-IR absorption perovskite solar cells. J. Phys. Chem. C 2014, 118, 16458–16462. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Q.; Xu, F.; Fu, Y.; Wang, Y.; Yan, Y.; Zhang, L.; Zhao, Y. Spontaneous low-temperature crystallization of α-FAPbI3 for highly efficient perovskite solar cells. Sci. Bull. 2019, 64, 1608–1616. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, H.; Li, G.; Fang, G. Stabilizer-assisted growth of formamdinium-based perovskites for highly efficient and stable planar solar cells with over 22% efficiency. Nano Energy 2019, 63, 103835. [Google Scholar] [CrossRef]
- Kim, M.; Kim, G.-H.; Lee, T.K.; Choi, I.W.; Choi, H.W.; Jo, Y.; Yoon, Y.J.; Kim, J.W.; Lee, J.; Huh, D. Methylammonium chloride induces intermediate phase stabilization for efficient perovskite solar cells. Joule 2019, 3, 2179–2192. [Google Scholar] [CrossRef]
- Hanusch, F.C.; Wiesenmayer, E.; Mankel, E.; Binek, A.; Angloher, P.; Fraunhofer, C.; Giesbrecht, N.; Feckl, J.M.; Jaegermann, W.; Johrendt, D. Efficient planar heterojunction perovskite solar cells based on formamidinium lead bromide. J. Phys. Chem. Lett. 2014, 5, 2791–2795. [Google Scholar] [CrossRef]
- Smecca, E.; Numata, Y.; Deretzis, I.; Pellegrino, G.; Boninelli, S.; Miyasaka, T.; La Magna, A.; Alberti, A. Stability of solution-processed MAPbI3 and FAPbI3 layers. Phys. Chem. Chem. Phys. 2016, 18, 13413–13422. [Google Scholar] [CrossRef]
- Min, H.; Kim, M.; Lee, S.-U.; Kim, H.; Kim, G.; Choi, K.; Lee, J.H.; Seok, S.I. Efficient, stable solar cells by using inherent bandgap of α-phase formamidinium lead iodide. Science 2019, 366, 749–753. [Google Scholar] [CrossRef]
- Bu, T.; Li, J.; Li, H.; Tian, C.; Su, J.; Tong, G.; Ono, L.K.; Wang, C.; Lin, Z.; Chai, N.; et al. Lead halide–templated crystallization of methylamine-free perovskite for efficient photovoltaic modules. Science 2021, 372, 1327–1332. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Xie, F.; Chen, C.-C.; Wu, Y.; Li, X.; Cai, M.; Liu, X.; Yang, X.; Han, L. Vertical recrystallization for highly efficient and stable formamidinium-based inverted-structure perovskite solar cells. Energy Environ. Sci. 2017, 10, 1942–1949. [Google Scholar] [CrossRef]
- Reyna, Y.; Salado, M.; Kazim, S.; Pérez-Tomas, A.; Ahmad, S.; Lira-Cantu, M. Performance and stability of mixed FAPbI3(0.85) MAPbBr3(0.15) halide perovskite solar cells under outdoor conditions and the effect of low light irradiation. Nano Energy 2016, 30, 570–579. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Xu, F.; Wang, Y.; Li, G.; Yang, Y.; Zhao, Y. CH3NH3Cl assisted solvent engineering for highly crystallized and large grain size mixed-composition (FAPbI3)0.85(MAPbBr3)0.15 perovskites. Crystals 2017, 7, 272. [Google Scholar] [CrossRef]
- Mei, Y.; Liu, H.; Li, X.; Wang, S. Hollow TiO2 spheres as mesoporous layer for better efficiency and stability of perovskite solar cells. J. Alloys Compd. 2021, 866, 158079. [Google Scholar] [CrossRef]
- Mundhaas, N.; Yu, Z.J.; Bush, K.A.; Wang, H.P.; Häusele, J.; Kavadiya, S.; McGehee, M.D.; Holman, Z.C. Series resistance measurements of perovskite solar cells using Jsc–Voc measurements. Sol. RRL 2019, 3, 1800378. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Li, B.; Zhu, H.; Xu, Q.; Ouyang, J. High performance planar perovskite solar cells with a perovskite of mixed organic cations and mixed halides, MA1−xFAxPbI3−yCly. J. Mater. Chem. A 2016, 4, 12543–12553. [Google Scholar] [CrossRef]
- Fang, H.-H.; Adjokatse, S.; Shao, S.; Even, J.; Loi, M.A. Long-lived hot-carrier light emission and large blue shift in formamidinium tin triiodide perovskites. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gharibzadeh, S.; Abdollahi Nejand, B.; Jakoby, M.; Abzieher, T.; Hauschild, D.; Moghadamzadeh, S.; Schwenzer, J.A.; Brenner, P.; Schmager, R.; Haghighirad, A.A. Record open-circuit voltage wide-bandgap perovskite solar cells utilizing 2D/3D perovskite heterostructure. Adv. Energy Mater. 2019, 9, 1803699. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Tress, W.; Domanski, K.; Anaraki, E.H.; Turren-Cruz, S.-H.; Roose, B.; Boix, P.P.; Grätzel, M.; Saliba, M.; Abate, A. Identifying and suppressing interfacial recombination to achieve high open-circuit voltage in perovskite solar cells. Energy Environ. Sci. 2017, 10, 1207–1212. [Google Scholar] [CrossRef]
- Slimi, B.; Mollar, M.; Assaker, I.B.; Kriaa, A.; Chtourou, R.; Marí, B. Synthesis characterization of perovskite FAPbBr3− xIx thin films for solar cells. Mon. Für Chem. -Chem. Mon. 2017, 148, 835–844. [Google Scholar] [CrossRef]
- Chen, L.-C.; Chen, J.-C.; Chen, C.-C.; Wu, C.-G. Fabrication and properties of high-efficiency perovskite/PCBM organic solar cells. Nanoscale Res. Lett. 2015, 10, 1–5. [Google Scholar] [CrossRef][Green Version]
- Bao, X.; Wang, Y.; Zhu, Q.; Wang, N.; Zhu, D.; Wang, J.; Yang, A.; Yang, R. Efficient planar perovskite solar cells with large fill factor and excellent stability. J. Power Sources 2015, 297, 53–58. [Google Scholar] [CrossRef]
- Krishnan, U.; Kaur, M.; Kumar, M.; Kumar, A. Factors affecting the stability of perovskite solar cells: A comprehensive review. J. Photonics Energy 2019, 9, 021001. [Google Scholar]
- Kim, H.D.; Ohkita, H.; Benten, H.; Ito, S. Photovoltaic performance of perovskite solar cells with different grain sizes. Adv. Mater. 2016, 28, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Gedamu, D.; Asuo, I.M.; Benetti, D.; Basti, M.; Ka, I.; Cloutier, S.G.; Federico, R.; Nechache., R. Solvent-antisolvent ambient processed large grain size perovskite thin films for high-performance solar cells. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Habisreutinger, S.N.; Noel, N.K.; Snaith, H.J. Hysteresis index: A figure without merit for quantifying hysteresis in perovskite solar cells. ACS Energy Lett. 2018, 3, 2472–2476. [Google Scholar] [CrossRef]
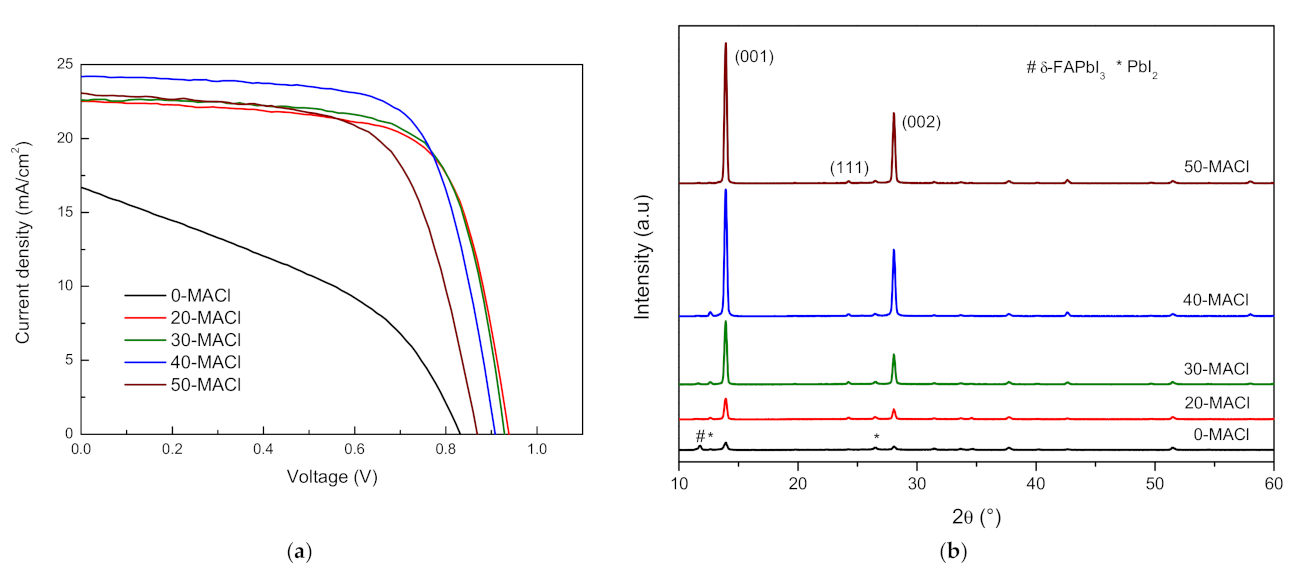
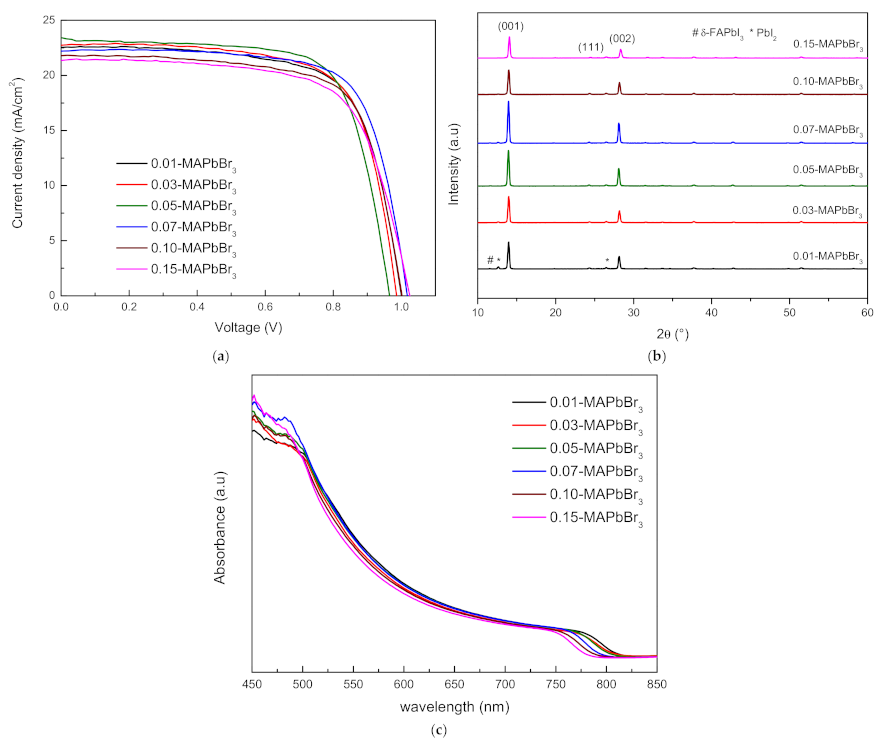
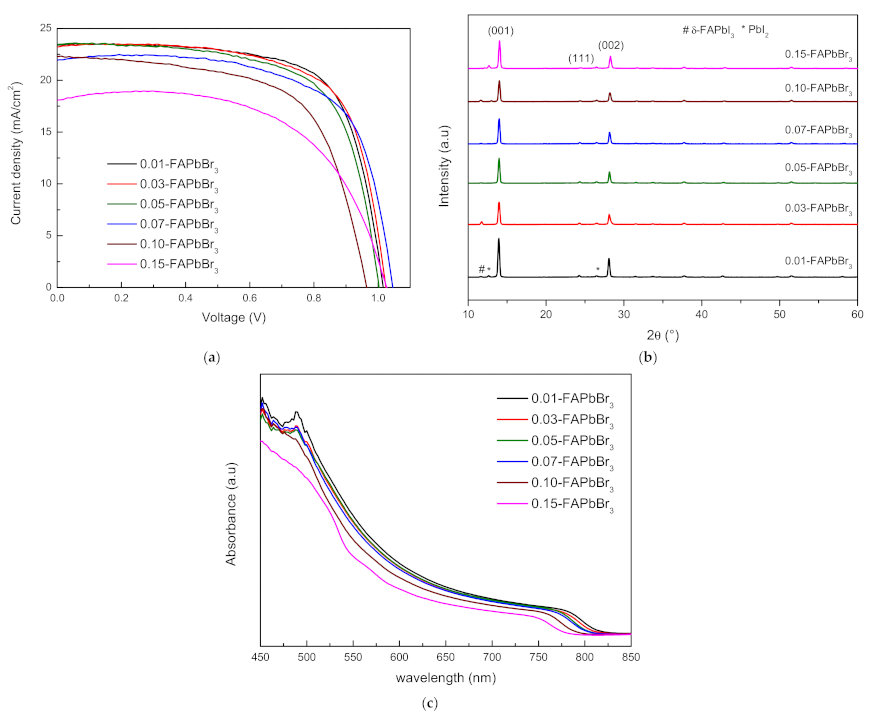

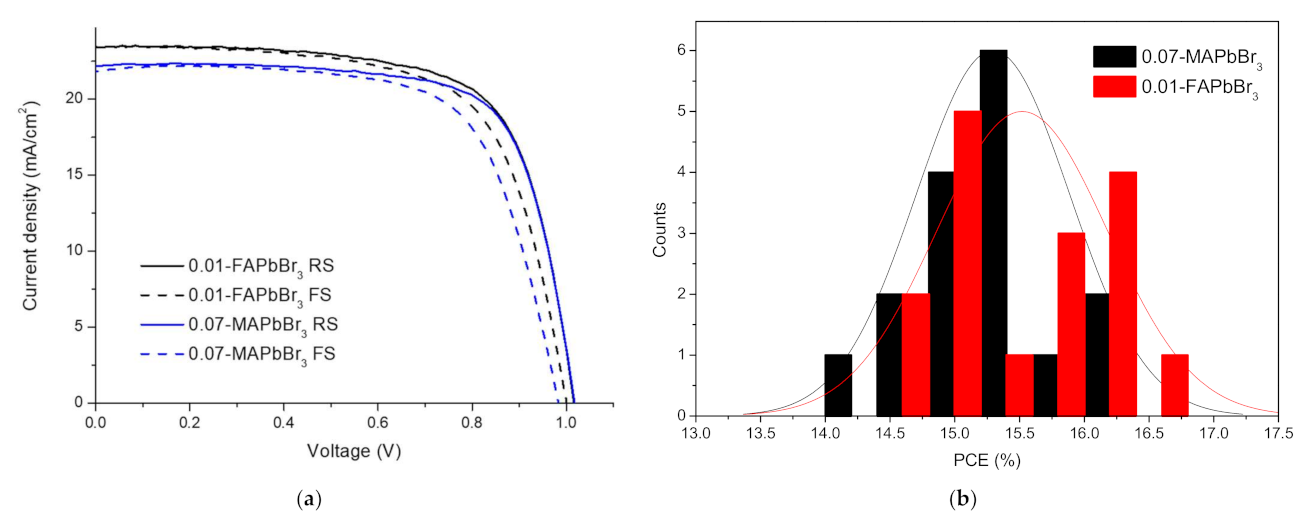
| Sample | VOC | JSC | FF | PCE (%) | Rs(Ω) |
|---|---|---|---|---|---|
| 0-MACl | 0.831 | 16.696 | 40.105 | 5.567 | 331.839 |
| 20-MACl | 0.938 | 22.550 | 68.982 | 14.596 | 119.269 |
| 30-MACl | 0.929 | 22.599 | 70.791 | 14.859 | 104.548 |
| 40-MACl | 0.908 | 24.181 | 70.037 | 15.379 | 109.747 |
| 50-MACl | 0.869 | 23.074 | 64.768 | 12.989 | 135.155 |
| Sample | VOC | JSC | FF | PCE (%) | Rs(Ω) |
|---|---|---|---|---|---|
| 0.01-MAPbBr3 | 1.000 | 22.538 | 69.594 | 15.691 | 120.472 |
| 0.03-MAPbBr3 | 0.986 | 22.729 | 70.188 | 15.730 | 113.333 |
| 0.05-MAPbBr3 | 0.965 | 23.407 | 70.878 | 16.014 | 109.144 |
| 0.07-MAPbBr3 | 1.017 | 22.196 | 72.176 | 16.301 | 116.721 |
| 0.10-MAPbBr3 | 1.002 | 21.785 | 70.414 | 15.372 | 122.829 |
| 0.15-MAPbBr3 | 1.025 | 21.651 | 67.764 | 14.826 | 147.174 |
| Sample | VOC | JSC | FF | PCE (%) | Rs (Ω) |
|---|---|---|---|---|---|
| 0.01-FAPbBr3 | 1.016 | 23.413 | 69.622 | 16.569 | 117.628 |
| 0.03-FAPbBr3 | 1.025 | 23.221 | 68.781 | 16.364 | 115.223 |
| 0.05-FAPbBr3 | 1.003 | 23.481 | 66.948 | 15.766 | 123.569 |
| 0.07-FAPbBr3 | 1.045 | 21.938 | 67.446 | 15.468 | 126.702 |
| 0.10-FAPbBr3 | 0.965 | 22.275 | 62.861 | 13.508 | 153.506 |
| 0.15-FAPbBr3 | 1.027 | 18.055 | 60.814 | 11.274 | 231.911 |
| Sample | Sweep Direction | VOC | JSC | FF | PCE (%) | RS(Ω) | HI |
|---|---|---|---|---|---|---|---|
| 0.01-FAPbBr3 | FS | 1.001 | 23.425 | 66.797 | 15.656 | 132.024 | 0.055 |
| RS | 1.016 | 23.413 | 69.622 | 16.569 | 117.628 | ||
| 0.07-MAPbBr3 | FS | 0.983 | 21.833 | 68.702 | 14.745 | 143.729 | 0.095 |
| RS | 1.017 | 22.196 | 72.176 | 16.301 | 116.721 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, S.H.; Choi, H.W. Compositional Engineering of FAPbI3 Perovskite Added MACl with MAPbBr3 or FAPbBr3. Coatings 2021, 11, 1184. https://doi.org/10.3390/coatings11101184
Joo SH, Choi HW. Compositional Engineering of FAPbI3 Perovskite Added MACl with MAPbBr3 or FAPbBr3. Coatings. 2021; 11(10):1184. https://doi.org/10.3390/coatings11101184
Chicago/Turabian StyleJoo, Sung Hwan, and Hyung Wook Choi. 2021. "Compositional Engineering of FAPbI3 Perovskite Added MACl with MAPbBr3 or FAPbBr3" Coatings 11, no. 10: 1184. https://doi.org/10.3390/coatings11101184
APA StyleJoo, S. H., & Choi, H. W. (2021). Compositional Engineering of FAPbI3 Perovskite Added MACl with MAPbBr3 or FAPbBr3. Coatings, 11(10), 1184. https://doi.org/10.3390/coatings11101184






