Electrochemical Dechlorination of 3-chlorophenol with Palladium-Loaded Carbon Felt Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Electroloading of Palladium on Carbon Felt
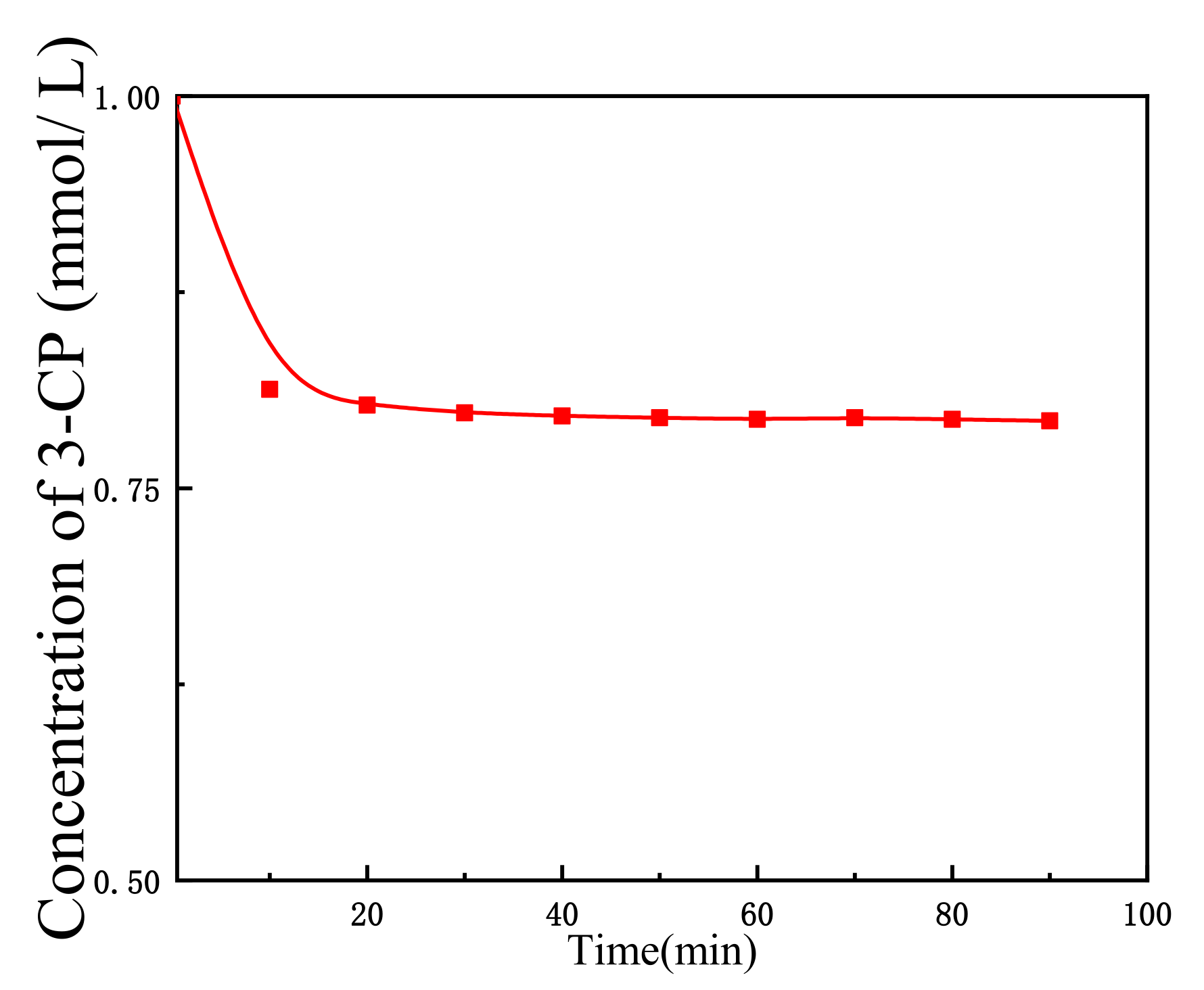
2.3. Electrolysis and Non-Electrolysis of 3-CP
3. Results and Discussion
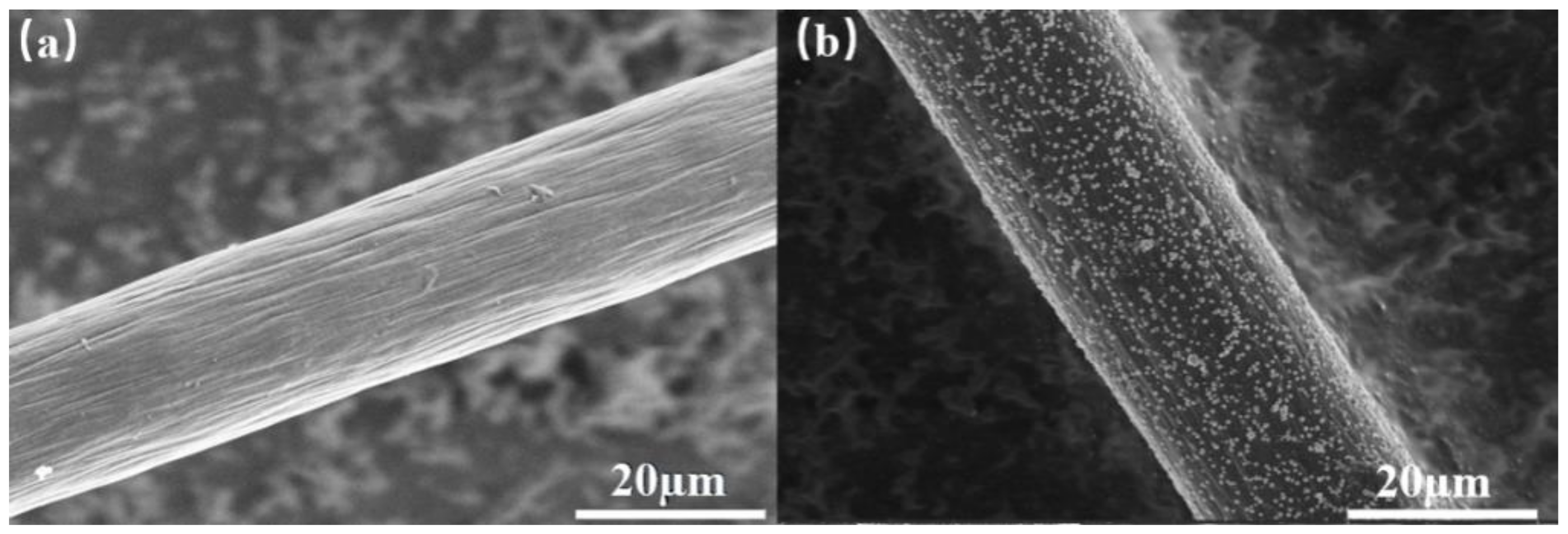
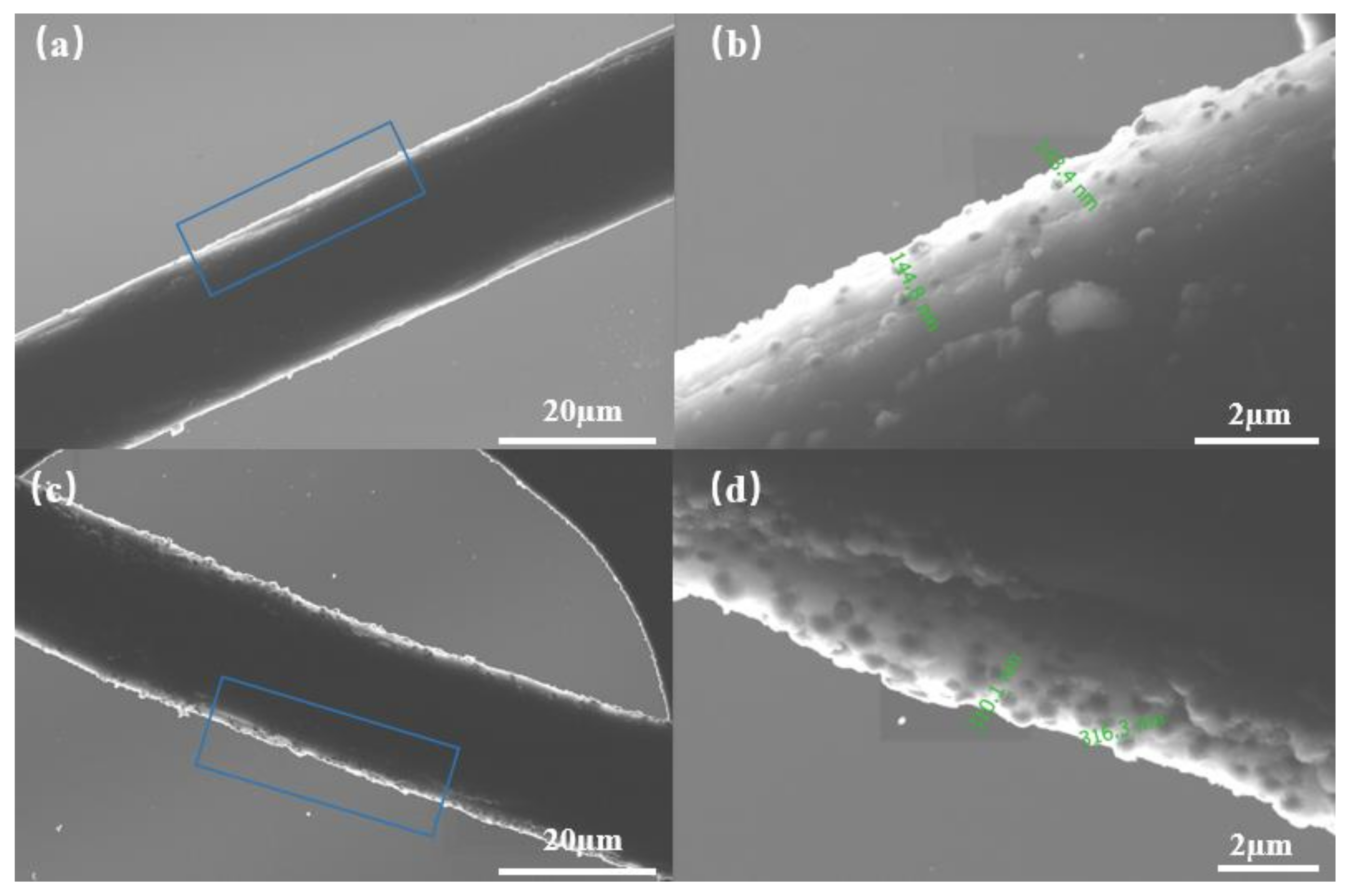
3.1. The Characterization of Pd/CF Cathode
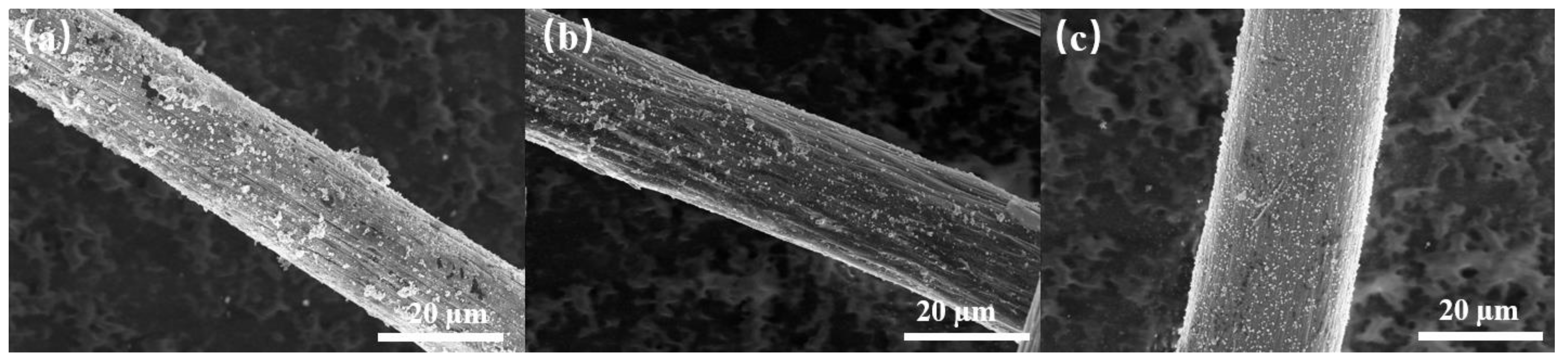
3.2. Influence of the Loaded Amount of Pd
3.3. Influence of the Loading Currents
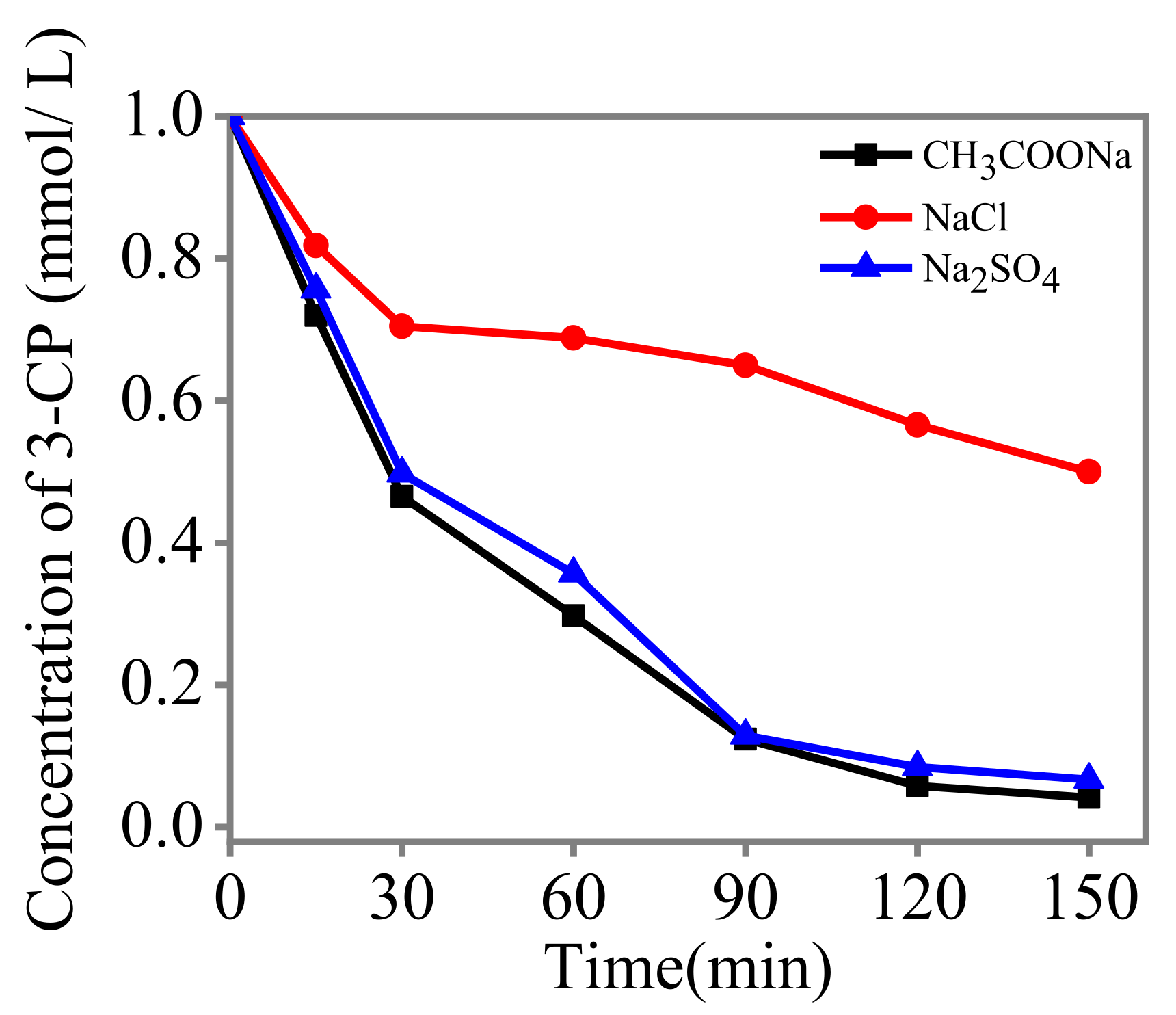
3.4. Influence of the Electrolytes
3.5. Influence of the Concentrations of Reduction Electrolyte
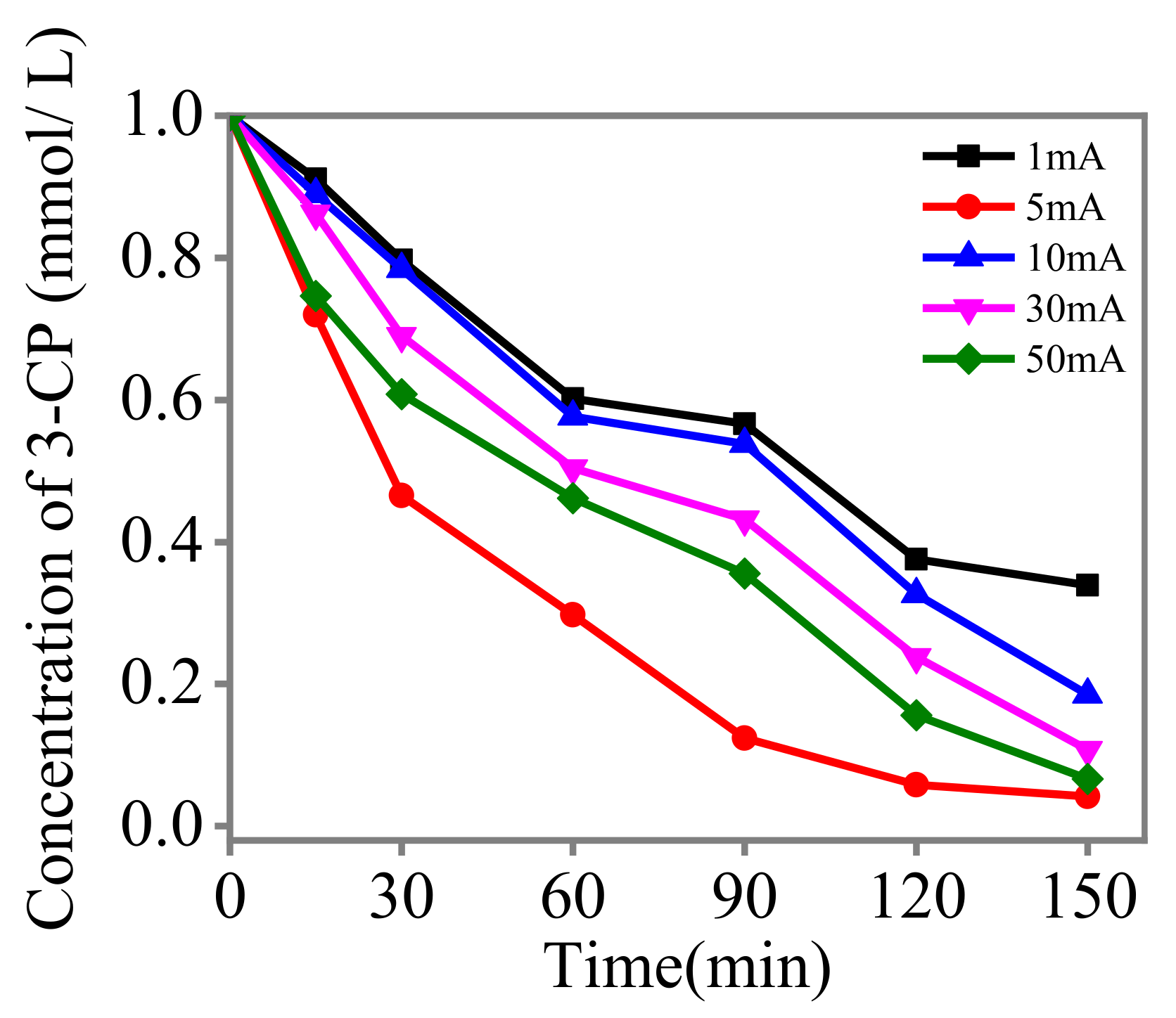
3.6. Influence of the Reducing Currents
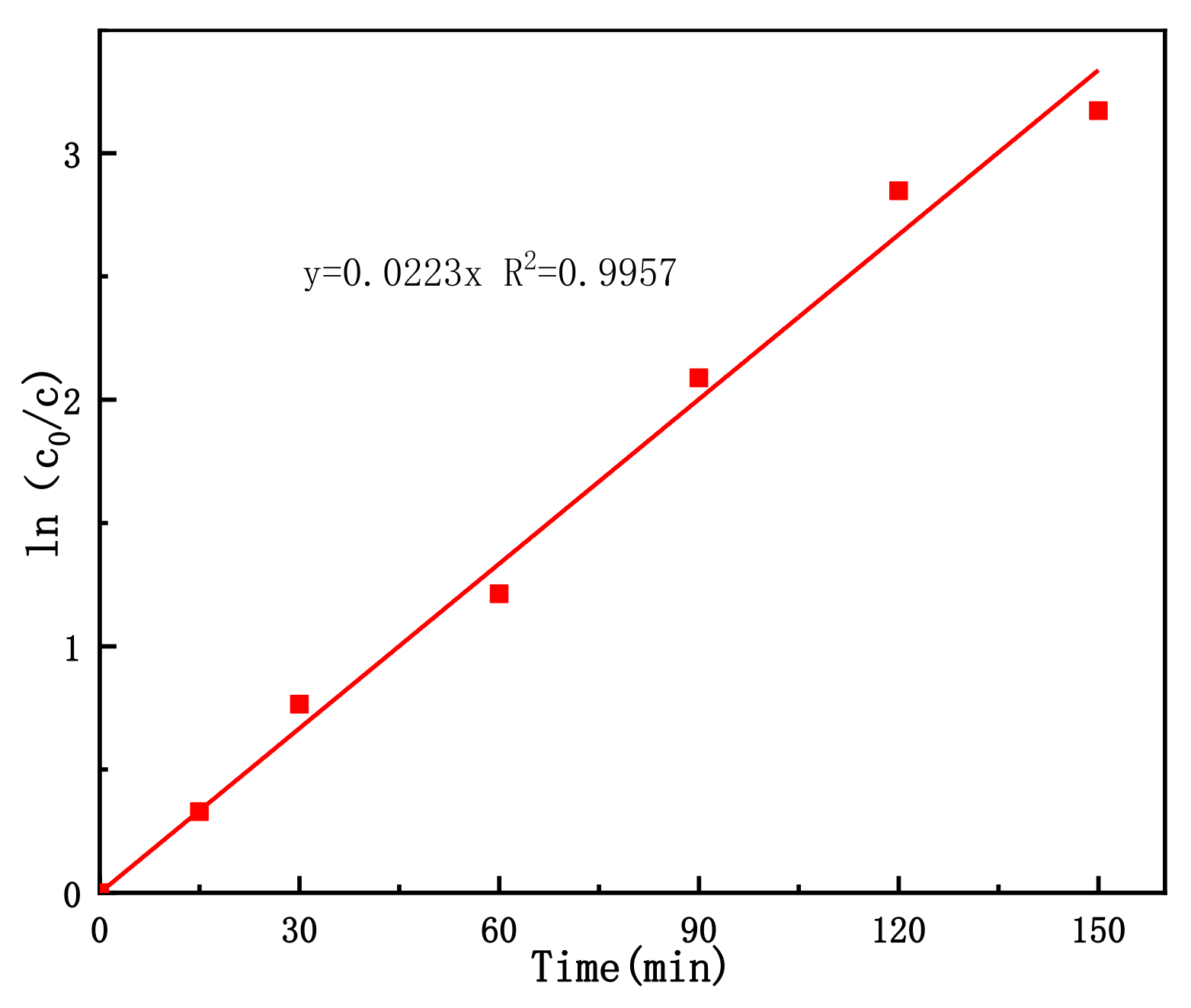
3.7. Kinetics of 3-CP Degradation
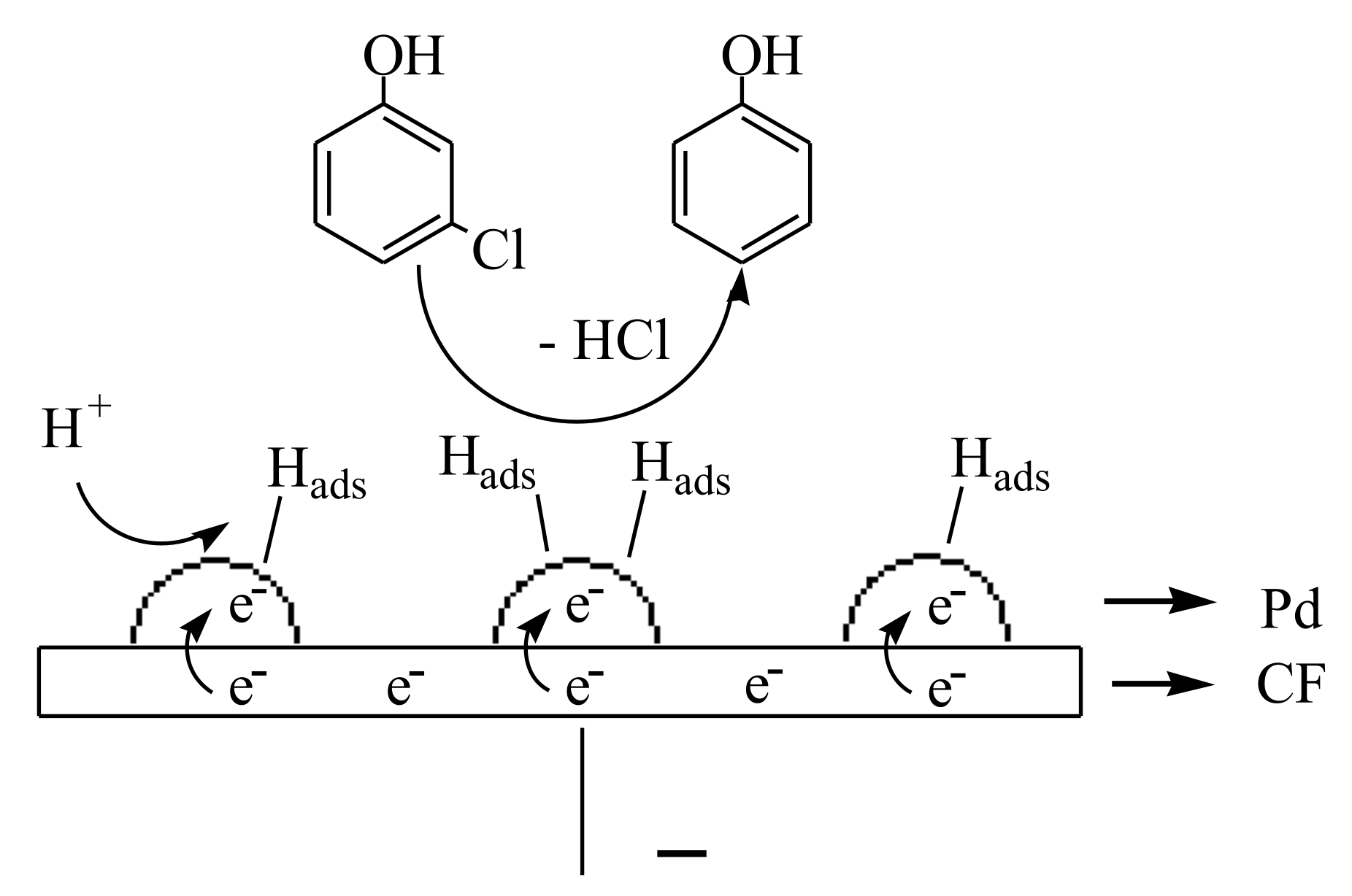
3.8. Mechanism and Possible Degradation Pathways
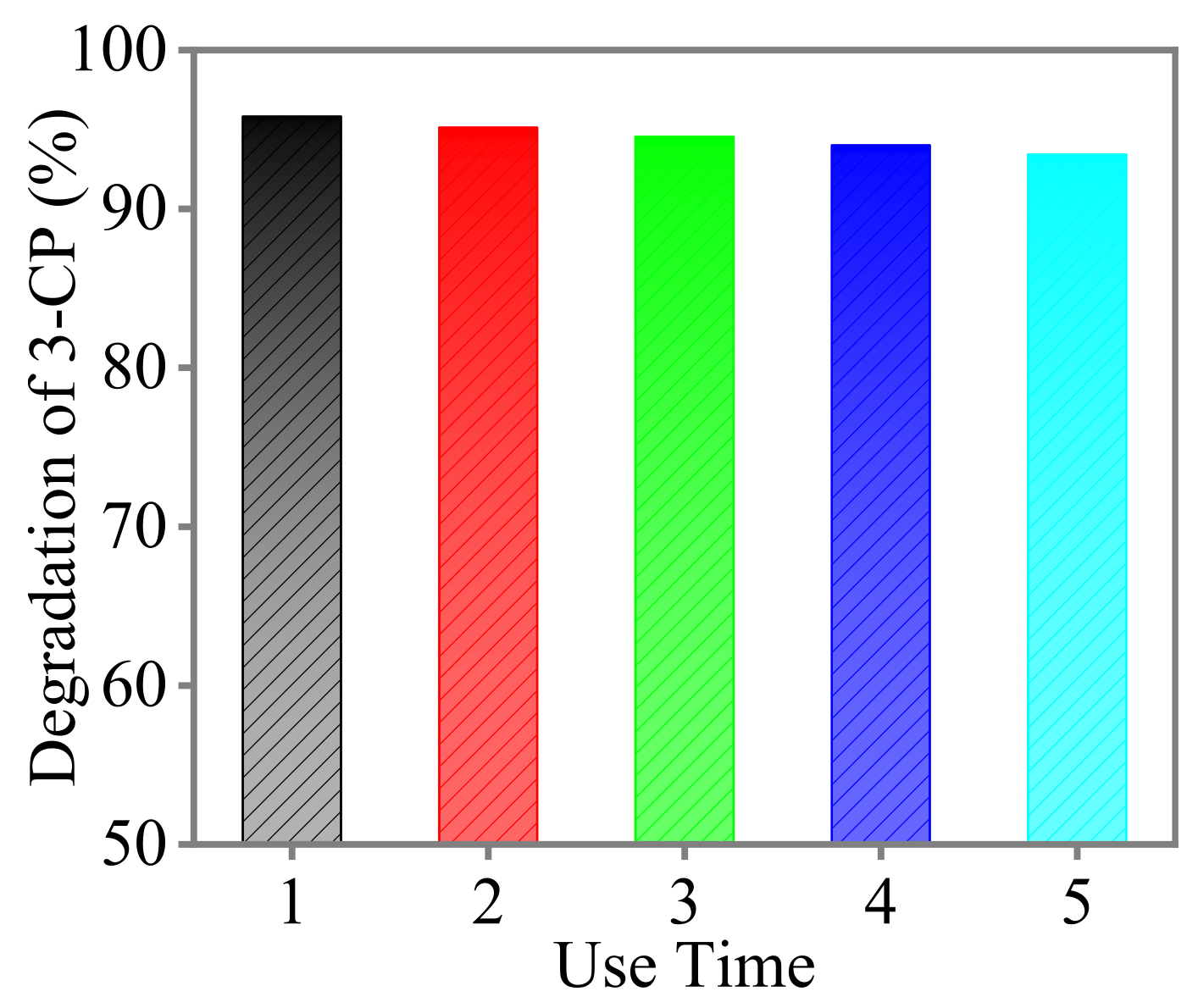
3.9. Reuse of Pd/CF
3.10. Comparison with Other Processes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marye, A.F.; Maria, T.D. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–342. [Google Scholar]
- Velasco, L.F.; Parra, J.B.; Ania, C.O. Role of activated carbon features on the photocatalytic degradation of phenol. Appl. Surf. Sci. 2010, 256, 5254–5258. [Google Scholar] [CrossRef] [Green Version]
- Aken, V.; Lambert, N.; Van den Broeck, R.; Degrève, J. Advances in ozonation and biodegradation processes to enhance chlorophenol abatement in multisubstrate wastewaters: A review. Environ. Sci. Water Res. Technol. 2019, 3, 444–481. [Google Scholar] [CrossRef]
- Garba, Z.N.; Zhou, W.M.; Lawan, I.; Xiao, W.; Zhang, M.X.; Wang, L.W.; Chen, L.H. An overview of chlorophenols as contaminants and their removal from wastewater by adsorption: A review, Review of biodegradation of chlorophenol organic pollutants. J. Environ. Manag. 2019, 241, 59–75. [Google Scholar] [CrossRef]
- Zouzelka, R.; Remzova, M.; Brabec, L.; Rathousky, J. Photocatalytic performance of porous TiO2 layers prepared by quantitative electrophoretic deposition from organic solvents. Appl. Catal. B Environ. 2018, 227, 70–78. [Google Scholar] [CrossRef]
- Zouzelka, R.; Kusumawati, Y.; Remzova, M.; Rathousky, J.; Pauporté, T. Photocatalytic activity of porous multiwalled carbon nanotube-TiO2 composite layers for pollutant degradation. J. Hazard. Mater. 2016, 317, 52–59. [Google Scholar] [CrossRef]
- Yang, B.; Yu, G. Electrocatalytic reductive dechlorination of 2,4,5-PCB in aqueous solution by palladium-modifified titanium mesh as the cathode. Acta Phys. Chim. Sin. 2006, 22, 306–311. [Google Scholar] [CrossRef]
- Ukisu, Y.; Iimura, S.; Uchida, R. Catalytic dechlorination of polychlorinated biphenyls with carbon-supported noble metal catalysts under mild conditions. Chemosphere 1996, 33, 1523–1530. [Google Scholar] [CrossRef]
- Arellano-González, M.A.; Texier, A.-C.; Lartundo-Rojas, L. Electrochemical Dechlorination of 2-Chlorophenol on Pd/Ti, Ni/Ti and Pd-Ni Alloy/Ti Electrodes. J. Electrochem. Soc. 2015, 162, E223–E230. [Google Scholar] [CrossRef] [Green Version]
- Bo, Y.; Shu, W.; Gang, Y.; Zhou, Y. Separation and Purification Technology, Electrocatalytic reduction of 2-chlorobiphenyl in contaminated water using palladium-modified electrode. Sep. Purif. Technol. 2008, 63, 353–359. [Google Scholar]
- Shu, X.Y.; Yang, Q.; Yao, F.B.; Zhong, Y.; Ren, W.C.; Chen, F. Electrocatalytic hydrodechlorination of 4-chlorophenol on Pd supported multi-walled carbon nanotubes particle electrodes. Chem. Eng. J. 2019, 358, 903–911. [Google Scholar] [CrossRef]
- Wu, Y.F.; Gan, L.; Zhang, S.P.; Song, H.O.; Lu, C. Carbon-nanotube-doped Pd-Ni bimetallic three-dimensional electrode for electrocatalytic hydrodechlorination of 4-chlorophenol: Enhanced activity and stability. J. Hazard. Mater. 2018, 356, 17–25. [Google Scholar] [CrossRef]
- Cheng, I.F.; Fernando, Q.; Korte, N. Electrochemical Dechlorination of 4-Chlorophenol to Phenol. Environ. Sci. Technol. 1997, 31, 1074–1078. [Google Scholar] [CrossRef]
- Luise, W.; Peter, P.; Monika, M. Determination of chlorophenols in soils using accelerated solvent extraction combined with solid-Phase microextraction. Anal. Chem. 2000, 72, 546–551. [Google Scholar]
- Grace, W.M.; Natasha, T.; Greg, M.S. Electro-oxidation and amperometric detection of chlorinated phenols at boron-doped diamond electrodes: A comparison of microcrystalline and nanocrystalline thin films. Environ. Sci. Technol. 2004, 38, 3674–3682. [Google Scholar]
- Zhu, R.Y.; Chen, X.M. Ultrasound assisted electrocatalytic oxidation of 3-chlorophenol in aqueous solution. J. Environ. Sci. Eng. 2008, 5, 27–30. [Google Scholar]
- Duan, X.Y.; Sui, X.Y.; Wang, W.Y.; Bai, W.H.; Chang, L.M. Fabrication of PbO2/SnO2 composite anode for electrochemical degradation of 3-chlorophenol in aqueous solution. Appl. Surf. Sci. 2019, 494, 211–222. [Google Scholar] [CrossRef]
- Aslama, M.; Tahir Soomroa, M.; Ismail Iqbal, M.I.; Salah, N.; Gondald, M.A.; Hameed, A. Sunlight mediated removal of chlorophenols over tungsten supported ZnO: Electrochemical and photocatalytic studies. J. Environ. Chem. Eng. 2015, 15, 1901–1911. [Google Scholar] [CrossRef]
- Mozia, S.; Bubacz, K.; Janus, M.; Morawski, A.W. Decomposition of 3-chlorophenol on nitrogen modifified TiO2 photocatalysts. J. Hazard. Mater. 2012, 203–204, 128–136. [Google Scholar] [CrossRef]
- Shen, X.Q.; Xiao, F.; Zhao, H.Y.; Chen, Y.; Fang, C. In Situ-Formed PdFe Nanoalloy and Carbon Defects in Cathode for Synergic Reduction−Oxidation of Chlorinated Pollutants in Electro Fenton Process. Environ. Sci. Technol. 2020, 54, 4564–4572. [Google Scholar] [CrossRef]
- Sun, Z.R.; Wei, X.F.; Zhang, H.; Hu, X. Dechlorination of pentachlorophenol (PCP) in aqueous solution on novel Pd-loaded electrode modified with PPy–SDBS composite film. Environ. Sci. Pollut. Res. 2015, 22, 3828–3837. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.J.; Yuan, S.H.; Mao, X.H.; Hu, W.; Liao, P.; Tong, M. Electrocatalytic activity of Pd-loaded Ti/TiO2 nanotubes cathode for TCE reduction in groundwater. Water Res. 2013, 4, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.R.; Zhang, J.W.; Wang, X.Y.; Hu, X. Electrochemically reductive dechlorination of 2,4,6-trichlorophenol on palladium loaded titanium cathode modified with graphene/polymeric pyrrole-sodium dodecyl benzene sulfonate. Desalin. Water Treat. 2017, 88, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Matsunaga, A.; Tezuka, M. Electroreductive dechlorination of chlorophenols with Pd catalyst supported on solid electrode. J. Environ. Sci. 2013, 25, 151–154. [Google Scholar]
- Yang, B.; Yu, G.; Liu, X.T. Electrochemical hydrodechlorination of 4-chlorobiphenyl in aqueous solution with the optimization of palladium-loaded cathode materials. Electrochim. Acta 2006, 52, 1075–1081. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, T.; Bao, J.; Tang, Y.; Li, C. Preparation method of an ultrafifine carbon supported Pd-catalyst as an anodic catalyst in a direct formic acid fuel cell. Electrochem. Commun. 2006, 8, 1625–1629. [Google Scholar] [CrossRef]
- Sun, Z.; Ge, H.; Hu, X.; Peng, Y. Electrocatalytic dechlorination of chloroform in aqueous solution on palladium/titanium electrode. Chem. Eng. Technol. 2009, 32, 134–139. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Yue, P.; Chen, G. Catalytic dechloronation of chlorophenols in water by palladium/iron. Water Res. 2001, 35, 1887–1890. [Google Scholar] [CrossRef] [Green Version]
- Andrey, I.; Yamanaka, I.; Otsuka, K. Pd-Loaded Carbon Felt as the Cathode for Selective Dechlorination of 2,4-Dichlorophenoxyacetic Acid in Aqueous Solution. J. Electrochem. Soc. 1998, 145, 3844–3850. [Google Scholar]
- Zhou, J.S.; Lou, Z.M.; Yang, K.L.; Xu, J. Electrocatalytic dechlorination of 2,4-dichlorobenzoic acid using different carbon-supported palladium moveable catalysts: Adsorption and dechlorination activity. Appl. Catal. B Environ. 2019, 44, 215–224. [Google Scholar] [CrossRef]
- Scialdone, O.; Corrado, E.; Galia, A.; Sirés, I. Electrochemical process and cell for the abatement of an unsaturated organic species from an aqueous solution. Electrochim. Acta 2014, 132, 15–19. [Google Scholar] [CrossRef]
- Hu, B.; Wu, C.; Zhang, Z.; Wang, L. Sonophotocatalytic degradation of trichloroacetic acid in aqueous solution. Ceram. Int. 2014, 40, 7015–7021. [Google Scholar] [CrossRef]
- Melnick, R.L.; Nyska, A.; Foster, P.M.; Roycroft, J.H.; Kissling, G.E. Toxicity and carcinogenicity of the water disinfection byproduct, dibromoacetic acid, in rats and mice. Toxicology 2007, 230, 126–233. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Min, Z.W.; Mok, C.; Kwon, H.; Kim, T.; Kim, D. Aqueous degradation of imidacloprid and fenothiocarb using contact glow discharge electrolysis: Degradation behavior and kinetics. Food Sci. Biotechnol. 2013, 22, 1773–1881. [Google Scholar] [CrossRef]







| C0/mM | Method | pH | (1)a | k/10−2 min−1 | Decrease in Removal Rate (%)/Reuse Times | References |
|---|---|---|---|---|---|---|
| 1.00 | electroreduction | 7.5 | 96/150 | 2.23 | 3/5 | This work |
| 1.00 | ultrasound assisted electrocatalytic oxidation | 9.1 | 35/60 | [16] | ||
| 0.39 | electrocatalytic oxidation | 5 | 98/120 | 3.84 | [17] | |
| 0.39 | electrochemical and photocatalytic process (tungsten supported ZnO/Solar) | 84/150 | 1.10 | [18] | ||
| 0.08 | photocatalytic TiO2 (0.2 g/L) UV energy | 7.0 | 77/300 | 4/5 | [19] | |
| 0.50 | electroreduction | 4.0 | 98/ | 3/4 | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Zhang, S.; Chen, Y.; Yang, H.; Geng, X.; Zhao, S.; Li, J.; Li, L. Electrochemical Dechlorination of 3-chlorophenol with Palladium-Loaded Carbon Felt Electrode. Coatings 2021, 11, 1188. https://doi.org/10.3390/coatings11101188
Li D, Zhang S, Chen Y, Yang H, Geng X, Zhao S, Li J, Li L. Electrochemical Dechlorination of 3-chlorophenol with Palladium-Loaded Carbon Felt Electrode. Coatings. 2021; 11(10):1188. https://doi.org/10.3390/coatings11101188
Chicago/Turabian StyleLi, Di, Siqi Zhang, Yingjia Chen, Haiming Yang, Xin Geng, Suying Zhao, Jiajun Li, and Lixiang Li. 2021. "Electrochemical Dechlorination of 3-chlorophenol with Palladium-Loaded Carbon Felt Electrode" Coatings 11, no. 10: 1188. https://doi.org/10.3390/coatings11101188





