Porous Carbonated Hydroxyapatite-Based Paraffin Wax Nanocomposite Scaffold for Bone Tissue Engineering: A Physicochemical Properties and Cell Viability Assay Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
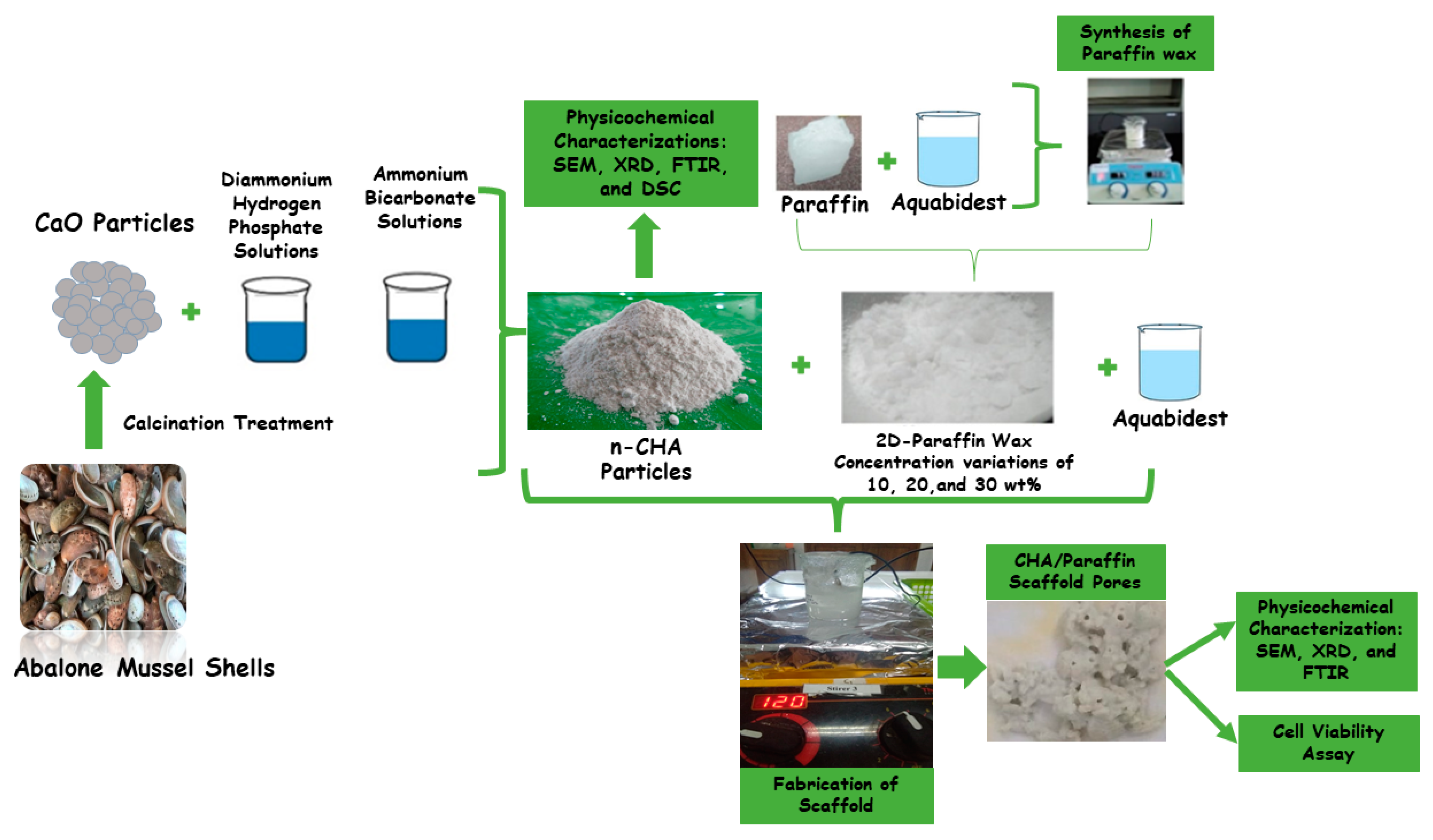
2.2. Synthesis of Carbonated Hydroxyapatite
2.3. Synthesis of CHA/Paraffin Wax Nanocomposite Scaffolds
2.4. Characterization of CHA Particles and CHA/Paraffin Wax Nanocomposite Scaffolds
2.5. Statistical Analysis
3. Results
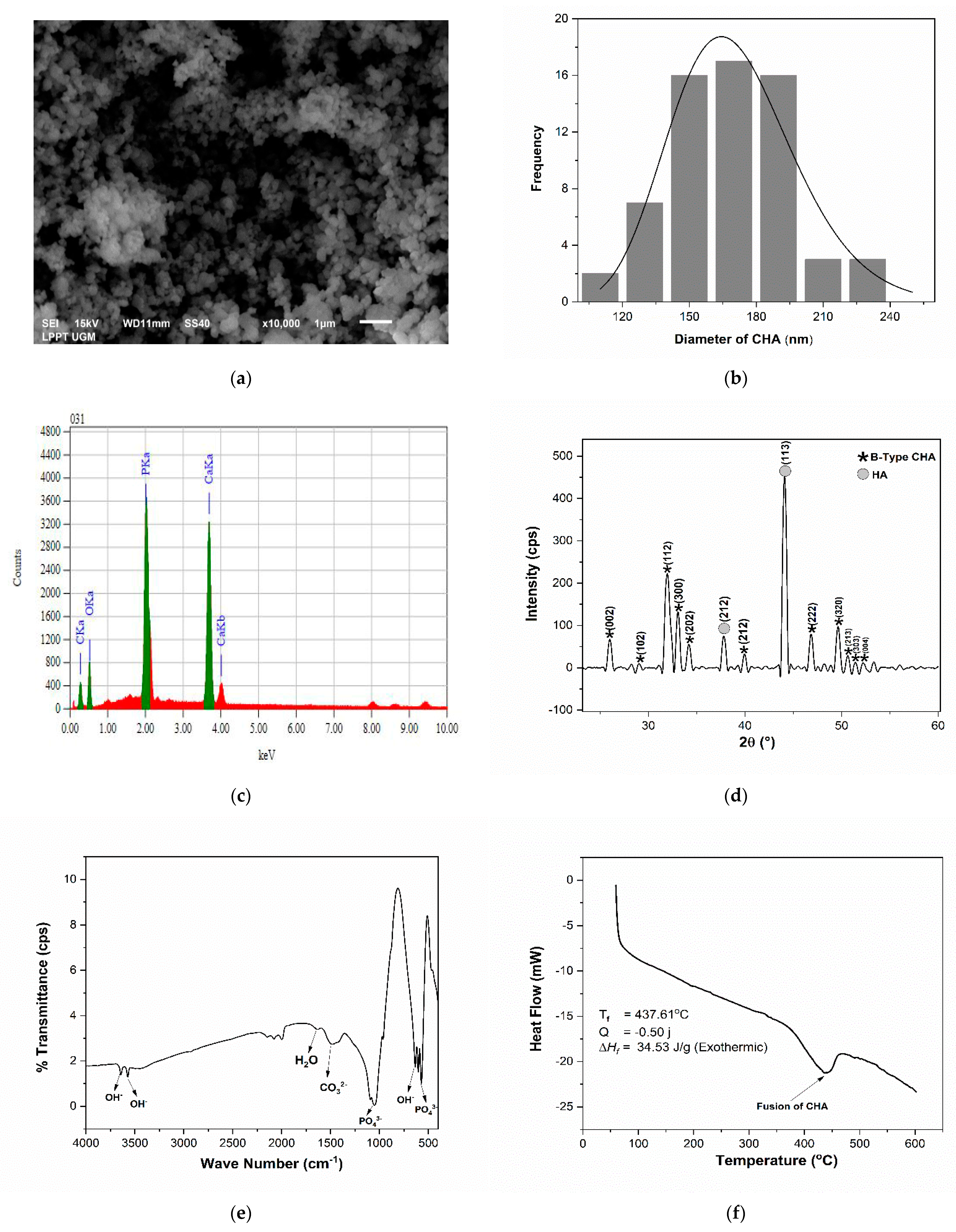
3.1. Analysis of the Physicochemical Properties of CHA
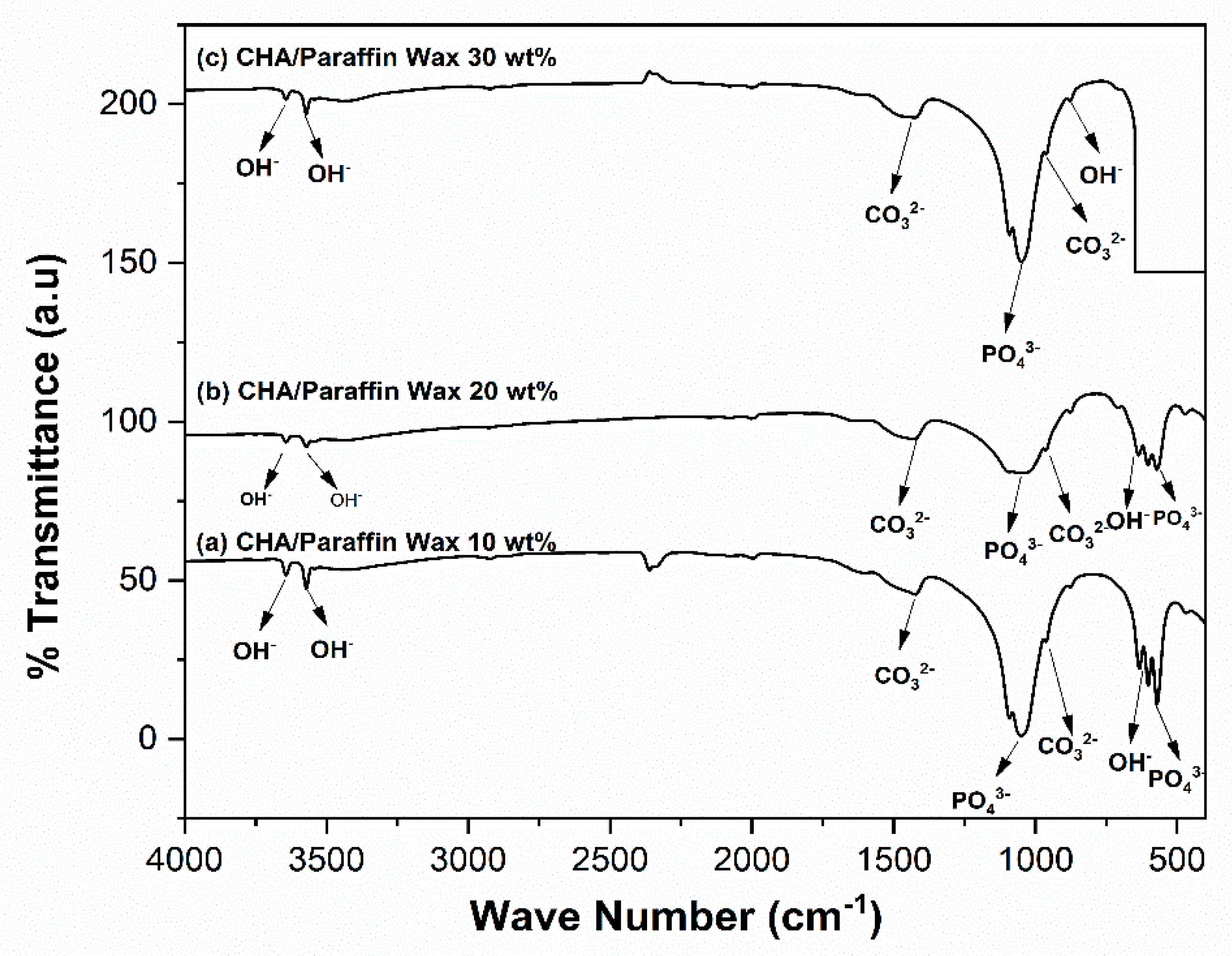
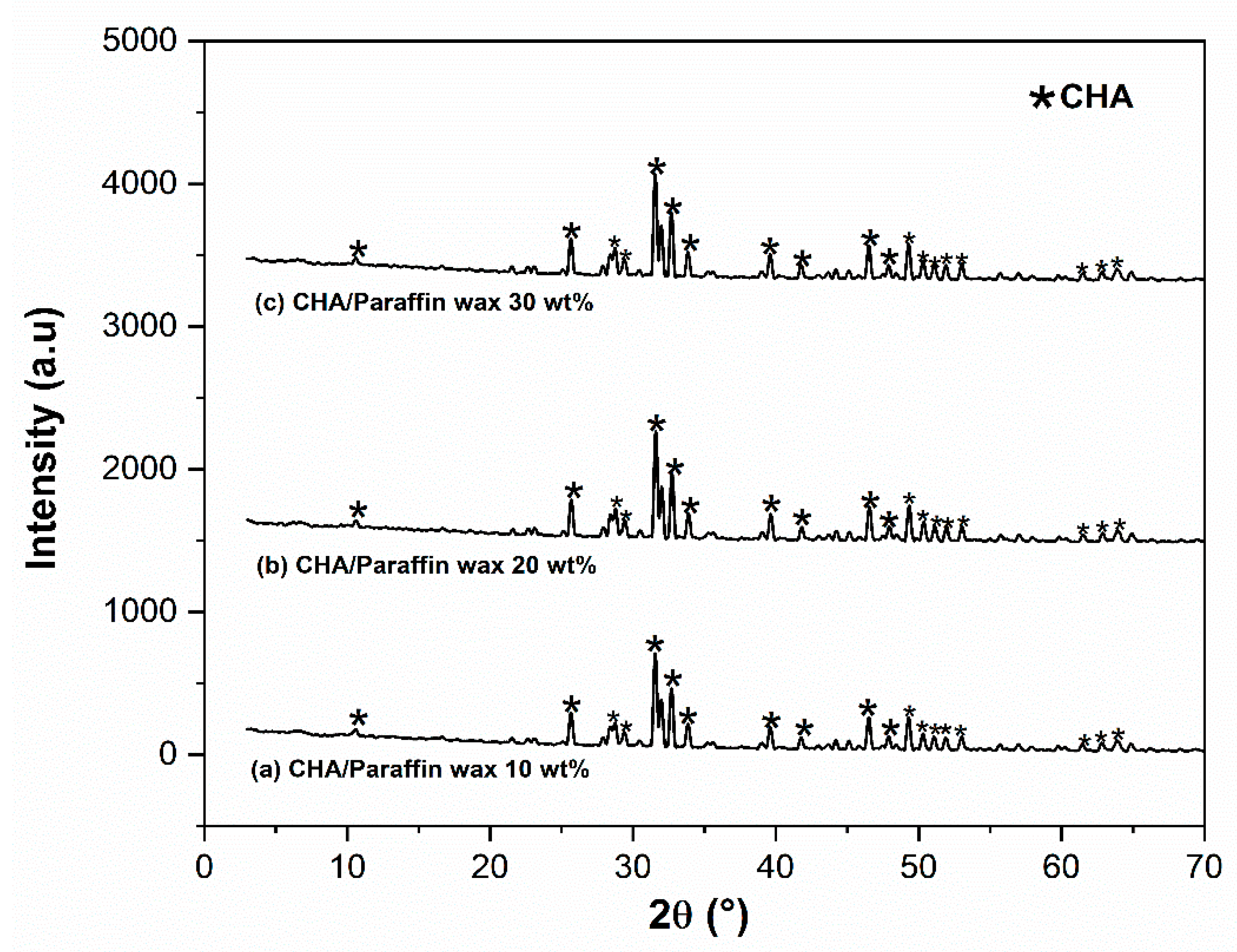
3.2. Analysis of the Physicochemical Properties of the CHA-Based Paraffin Wax Nanocomposite Scaffolds
4. Discussion
4.1. Analysis of the Physicochemical Properties of CHA
4.2. Analysis of the Physicochemical Properties of the CHA-Based Paraffin Wax Nanocomposite Scaffolds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mawuntu, V.J.; Yusuf, Y. Porous-structure engineering of hydroxyapatite-based scaffold synthesized from Pomacea canaliculata shell by using polyethylene oxide as polymeric porogen. IOP Conf. Ser. Mater. Sci. Eng. 2018, 432, 012045. [Google Scholar] [CrossRef] [Green Version]
- Sari, M.; Hening, P.; Chotimah; Ana, I.D.; Yusuf, Y. Bioceramic hydroxyapatite-based scaffold with a porous strructure using honeycomb as a natural polymeric porogen for bone tissue engineering. Biomater. Res. 2021, 25, 2. [Google Scholar] [CrossRef] [PubMed]
- Permatasari, H.A.; Supii, A.I.; Suparta, G.B.; Yusuf, Y. Characteristics of abalone mussel shells (Halioitis asinina) with calcination temperature variations as a basic material for synthesis of carbonated hydroxyapatite. Key Eng. Mater. 2019, 818, 31–36. [Google Scholar] [CrossRef]
- Sari, M.; Hening, P.; Chotimah; Ana, I.D.; Yusuf, Y. Porous structure of bioceramics carbonated hydroxyapatite-based honeycomb scaffold for bone tissue engineering. Mater. Today Commun. 2021, 26, 102135. [Google Scholar] [CrossRef]
- Youness, R.A.; Taha, M.A.; Ibrahim, M.A. Effect of sintering temperatures on the in vitro bioactivity, molecular structure and mechanical properties of titanium/carbonated hydroxyapatite nanobiocomposites. J. Mol. Struct. 2017, 1150, 188–195. [Google Scholar] [CrossRef]
- Laonapakul, T. Synthesis of hydroxyapatite from biogenic wastes. KKU Eng. J. 2015, 42, 269–275. [Google Scholar]
- Wati, R.; Yusuf, Y. Carbonated hydroxyapatite derived from Cerastoderma edule, Paphia undulata, and Meretrix meretrix shells. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042049. [Google Scholar] [CrossRef]
- Anggraini, R.M.; Supii, A.I.; Suparta, G.B.; Yusuf, Y. The Effect of pH on the characteristics of carbonate hydroxyapatite based on pearl shell (Pinctada maxima). Key Eng. Mater. 2019, 818, 44–49. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Wang, M.; Cheung, W.L.; Guo, B.C.; Jia, D.M. Synthesis of carbonated hydroxyapatite nanospheres through nanoemulsion. J. Mater. Sci. Mater. Med. 2008, 19, 103–110. [Google Scholar] [CrossRef]
- Ezekiel, I.; Kasim, S.R.; Ismail, Y.M.B.; Noor, A.F.M. Nanoemulsion synthesis of carbonated hydroxyapatite nanopowders: Effect of variant CO32−/PO43− molar ratios on phase, morphology, and bioactivity. Ceram. Int. 2018, 44, 13082–13089. [Google Scholar] [CrossRef]
- Rajabi-Zamani, A.H.; Behnamghader, A.; Kazemzadeh, A. Synthesis of nanocrystalline carbonated hydroxyapatite powder via nonalkoxide sol–gel method. Mater. Sci. Eng. C 2008, 28, 1326–1329. [Google Scholar] [CrossRef]
- Fathi, M.H.; Hanifi, A.; Mortazavi, V. Preparation and bioactivity evaluation of bone-like hydroxyapatite nanopow. J. Mater. Process. Technol. 2007, 202, 536–542. [Google Scholar] [CrossRef]
- Lala, S.; Brahmachari, S.; Das, P.K.; Das, D.; Kar, T.; Pradhan, S.K. Biocompatible nanocrystalline natural bonelike carbonated hydroxyapatite synthesized by mechanical alloying in a record minimum time. Mater. Sci. Eng. C 2014, 42, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Zhao, K.; Gao, C.; Zhu, P. Synthesis and characterization of carbonated hydroxyapatite with layered structure. Mater. Lett. 2019, 255, 126552. [Google Scholar] [CrossRef]
- Sari, M.; Yusuf, Y. Synthesis and characterization of hydroxyapatite based on green mussel shells (Perna viridis) with calcination temperature variation using the precipitation method. Int. J. Nanoelectron. Mater. 2018, 11, 357–370. [Google Scholar]
- Chen, P.; Liu, L.; Pan, J.; Mei, J.; Li, C.; Zheng, Y. Biomimetic composite scaffold of hydroxyapatite/gelatin-chitosan core-shell nanofibers for bone tissue engineering. Mater. Sci. Eng. C 2019, 9, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Mawuntu, V.J.; Yusuf, Y. Porous structure engineering of bioceramic hydroxyapatite-based scaffolds using PVA, PVP, and PEO as polymeric porogens. J. Asian Ceram. Soc. 2019, 7, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Legeros, R.Z.; Kijkowska, R.; Bautista, C.; Legeros, J.P. Synergistic effects of magnesium and carbonate on properties of biological and synthetic apatites. Connect. Tissue Res. 1995, 33, 203–209. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Le, T.Y.L.; Daly, S.; Wang, Y.; Maitz, P.K.; Schindeler, A.; Dehghani, F. Formation of porous biodegradable scaffolds based on poly (propylene carbonate) using gas foaming technology. Mater. Sci. Eng. C 2019, 96, 824–830. [Google Scholar] [CrossRef]
- Januariyasa, I.K.; Ana, I.D.; Yusuf, Y. Nanofibrous poly (vinyl alcohol)/chitosan contained carbonated hydroxyapatite nanoparticles scaffold for bone tissue engineering. Mater. Sci. Eng. C 2020, 107, 110347. [Google Scholar] [CrossRef]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Jo, I.; Shin, K.; Soon, Y.; Koh, Y.; Lee, J.; Kim, H. Highly porous hydroxyapatite scaffolds with elongated pores using stretched polymeric sponges as novel template. Mater. Lett. 2009, 63, 1702–1704. [Google Scholar] [CrossRef]
- Soltani, M.; Yousefpour, M.; Taherin, Z. Porous fluorhydroxyapatite-magnesium-gelatin novel composite scaffold based on freeze-drying mechanism for bone tissue engineering application. Mater. Lett. 2019, 244, 195–198. [Google Scholar] [CrossRef]
- Govindan, R.; Gu, F.L.; Karthi, S.; Girija, E.K. Effect of phosphate glass reinforcement on the mechanical and biological properties of freeze-dried gelatin composite scaffolds for bone tissue engineering applications. Mater. Today Commun. 2020, 22, 100765. [Google Scholar] [CrossRef]
- Januariyasa, I.K.; Yusuf, Y. Porous carbonated hydroxyapatite-based scaffold using simple gas foaming method. J. Asian Ceram. Soc. 2020, 8, 634–641. [Google Scholar] [CrossRef]
- Hayashi, K.; Munar, M.L.; Ishikawa, K. Effects of macropore size in carbonate apatite honeycomb scaffolds on bone regeneration. Mater. Sci. Eng. C 2020, 111, 110848. [Google Scholar] [CrossRef]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Honeycomb blocks composed of carbonate apatite, β-tricalcium phosphate, and hydroxyapatite for bone regeneration: Effects of composition on biological responses. Mater. Today Bio 2019, 4, 100031. [Google Scholar] [CrossRef]
- Ishikawa, K.; Munar, M.L.; Tsuru, K.; Miyamoto, Y. Fabrication of carbonate apatite honeycomb and its tissue response. J. Biomed. Mater. Res. Part A 2019, 107, 1014–1020. [Google Scholar] [CrossRef]
- Shao, Y.; Yang, F.; Qin, Q.; Zhou, Y.; Chen, C.; Liu, P.; He, X.; Guo, Z. Shaping and mechanical performance of gelcasting Ti6Al4V alloys with paraffin wax and stearic acid coated on powder surface. Mater. Today Commun. 2020, 25, 101533. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Duan, X. A novel composite phase change material with paraffin wax in tailings porous ceramics. Appl. Therm. Eng. 2019, 151, 115–123. [Google Scholar] [CrossRef]
- Zhao, J.; Duan, K.; Zhang, J.W.; Lu, X.; Weng, J. The influence of polymer concentrations on the structure and mechanical properties of porous polycaprolactone-coated hydroxyapatite scaffolds. Appl. Surf. Sci. 2010, 256, 4586–4590. [Google Scholar] [CrossRef]
- Ruiz-Aguilar, C.; Olivares-pinto, U.; Drew, R.A.L.; Aguilar-Reyes, E.A.; Alfonso, I. Porogen effect on structural and physical properties of β-TCP scaffolds for bone tissue regeneration. IRBM 2020, 42, 302–312. [Google Scholar] [CrossRef]
- Zhu, J.; Kong, Q.; Zheng, S.; Wang, Y.; Jiao, Z.; Nie, Y.; Liu, T.; Song, K. Toxicological evaluation of ionic liquid in a biological functional tissue construct model based on nano-hydroxyapatite/chitosan/gelatin hybrid scaffolds. Int. J. Biol. Macromol. 2020, 158, 800–810. [Google Scholar] [CrossRef]
- Desai, A.Y. Fabrication and Characterization of Titanium-Doped Hydroxyapatite Thin Films. Master’s Thesis, University of Cambridge, Cambridge, UK, 2007. [Google Scholar]
- Nejati, E.; Mirzadeh, H.; Zandi, M. Synthesis and characterization of nano-hydroxyapatite rods/poly (L-lactide acid) composite scaffolds for bone tissue engineering. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1589–1596. [Google Scholar] [CrossRef]
- Ana, I.D. Bone Substituting Materials in Dental Implantology; Springer: Cham, Switzerland, 2019; pp. 121–141. [Google Scholar]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Salcedo, S.; Arcos, D.; Vallet-Regi, M. Upgrading calcium phosphate scaffolds for tissue engineering applications. Key Eng. Mater. 2008, 377, 19–42. [Google Scholar] [CrossRef]
- Reinwald, Y.; Johal, R.K.; Ghaemmaghami, A.M.; Rose, F.R.A.J.; Howdle, S.M.; Shakesheff, K.M. Interconnectivity and permeability of supercritical fluid-foamed scaffolds and the effect of their structural properties on cell distribution. Polymer 2014, 55, 435–444. [Google Scholar] [CrossRef]
- Mehr, N.G.; Li, X.; Ariganello, M.B.; Hoemann, C.D.; Favis, B.D. Poly(ε-caprolactone) scaffolds of highly controlled porosity and interconnectivity derived from co-continuous polymer blends: Model bead and cell infiltration behavior. J. Mater. Sci. Mater. Med. 2014, 25, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Christy, P.N.; Basha, S.K.; Kumari, V.S.; Bashir, A.K.H.; Maaza, M.; Kaviyarasu, K.; Arasu, M.V.; Al-Dhabi, N.A.; Ignacimuthu, S. Biopolymeric nanocomposite scaffolds for bone tissue engineering applications—A review. J. Drug Deliv. Sci. Technol. 2020, 55, 101452. [Google Scholar] [CrossRef]
- Lou, T.; Wang, X.; Song, G.; Gu, Z.; Yang, Z. Structure and properties of PLLA/β-TCP nanocomposite scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2015, 26, 5366. [Google Scholar] [CrossRef]
- Scaglione, S.; Giannoni, P.; Bianchini, P.; Sandri, M.; Marotta, R.; Firpo, G.; Valbusa, U.; Tampieri, A.; Diaspora, A.; Bianco, P.; et al. Order versus Disorder: In vivo bone formation within osteoconductive scaffolds. Sci. Rep. 2011, 2, 274. [Google Scholar] [CrossRef] [Green Version]
- Kanimozhi, K.; Basha, S.K.; Kumari, V.S.; Kaviyarasu, K.; Maaza, M. In vitro cytocompatibility of chitosan/PVA/methylcellulose-nanocellulose nanocomposite scaffolds using L929 fibroblast cells. Appl. Surf. Sci. 2018, 449, 574–583. [Google Scholar] [CrossRef]
- Stocco, T.D.; Antonioli, E.; Elias, C.D.M.V.; Rodrigues, B.V.M.; Siqueira, I.A.W.D.B.; Ferretti, M.; Marciano, F.R.; Lobo, A.O. Cell viability of porous Poly (D,L-lactic acid)/vertically aligned carbon nanotubes/nanohydroxyapatite scaffolds for osteochondral tissue engineering. Materials 2019, 12, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heydari, Z.; Mohebbi-kalhori, D.; Afarani, M.S. Engineered electrospun polycaprolactone (PCL)/octacalcium phosphate (OCP) scaffold for bone tissue engineering. Mater. Sci. Eng. C 2017, 81, 127–132. [Google Scholar] [CrossRef] [PubMed]


| Scaffold with Paraffin Wax Concentrations Variation | CHA Mass (gr) | Paraffin Wax Mass (gr) |
|---|---|---|
| CHA/paraffin wax 10 | 0.4 | 0.044 |
| CHA/paraffin wax 20 | 0.4 | 0.100 |
| CHA/paraffin wax 30 | 0.4 | 0.171 |
| Scaffold with Paraffin Wax Concentrations Variation | Crystallite Size (nm) | Microstrain | |||
|---|---|---|---|---|---|
| a | c | c/a | |||
| 15 2 | 0.0086 | 9.79 | 6.93 | 0.707 | |
| 14 2 | 0.0088 | 9.76 | 6.92 | 0.709 | |
| 14 2 | 0.0093 | 9.67 | 6.84 | 0.707 | |
| Scaffold with Paraffin Wax Concentrations Variation | Porosity (%) | ||
|---|---|---|---|
| No pore | No pore | No pore | |
| 57 3 | 0.5 0.03 | 62.44 | |
| 80 3 | 0.6 0.02 | 75.93 | |
| 100 1 | 1.0 0.02 | 64.86 |
| Concentration Serial of Scaffold Solution | Cell Viability (%) | p-Value | |
|---|---|---|---|
| Mean SD | |||
| 24 h | 48 h | ||
| 15.625 | 91 12 | 110 24 | 0.002 |
| 31.25 | 88 15 | 115 12 | |
| 62.5 | 89 7 | 114 14 | |
| 125 | 86 12 | 110 8 | |
| 250 | 84 17 | 108 10 | |
| 500 | 83 2 | 98 9 | |
| 1000 | 75 6 | 81 4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sari, M.; Aminatun; Suciati, T.; Sari, Y.W.; Yusuf, Y. Porous Carbonated Hydroxyapatite-Based Paraffin Wax Nanocomposite Scaffold for Bone Tissue Engineering: A Physicochemical Properties and Cell Viability Assay Analysis. Coatings 2021, 11, 1189. https://doi.org/10.3390/coatings11101189
Sari M, Aminatun, Suciati T, Sari YW, Yusuf Y. Porous Carbonated Hydroxyapatite-Based Paraffin Wax Nanocomposite Scaffold for Bone Tissue Engineering: A Physicochemical Properties and Cell Viability Assay Analysis. Coatings. 2021; 11(10):1189. https://doi.org/10.3390/coatings11101189
Chicago/Turabian StyleSari, Mona, Aminatun, Tri Suciati, Yessie Widya Sari, and Yusril Yusuf. 2021. "Porous Carbonated Hydroxyapatite-Based Paraffin Wax Nanocomposite Scaffold for Bone Tissue Engineering: A Physicochemical Properties and Cell Viability Assay Analysis" Coatings 11, no. 10: 1189. https://doi.org/10.3390/coatings11101189
APA StyleSari, M., Aminatun, Suciati, T., Sari, Y. W., & Yusuf, Y. (2021). Porous Carbonated Hydroxyapatite-Based Paraffin Wax Nanocomposite Scaffold for Bone Tissue Engineering: A Physicochemical Properties and Cell Viability Assay Analysis. Coatings, 11(10), 1189. https://doi.org/10.3390/coatings11101189






