Engineering Nanostructured Antimony-Based Anode Materials for Sodium Ion Batteries
Abstract
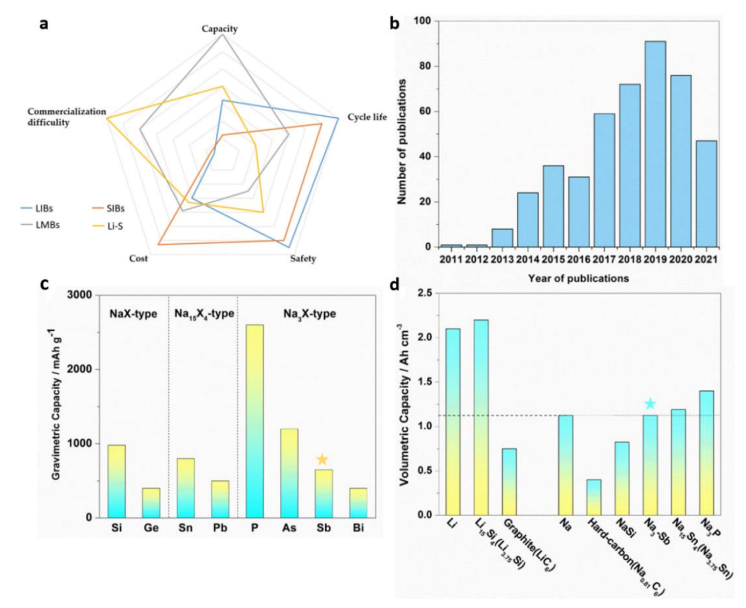
:1. Introduction
2. Sb-Based Anode
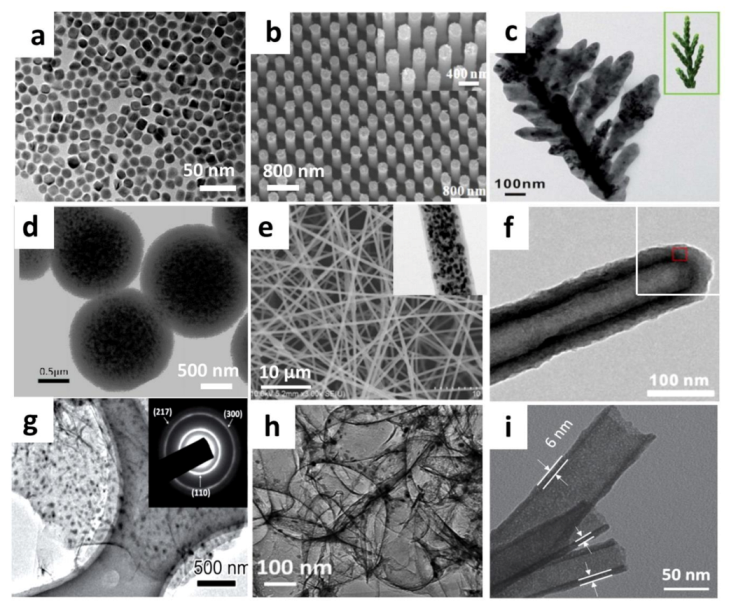
2.1. Pure Sb Anode
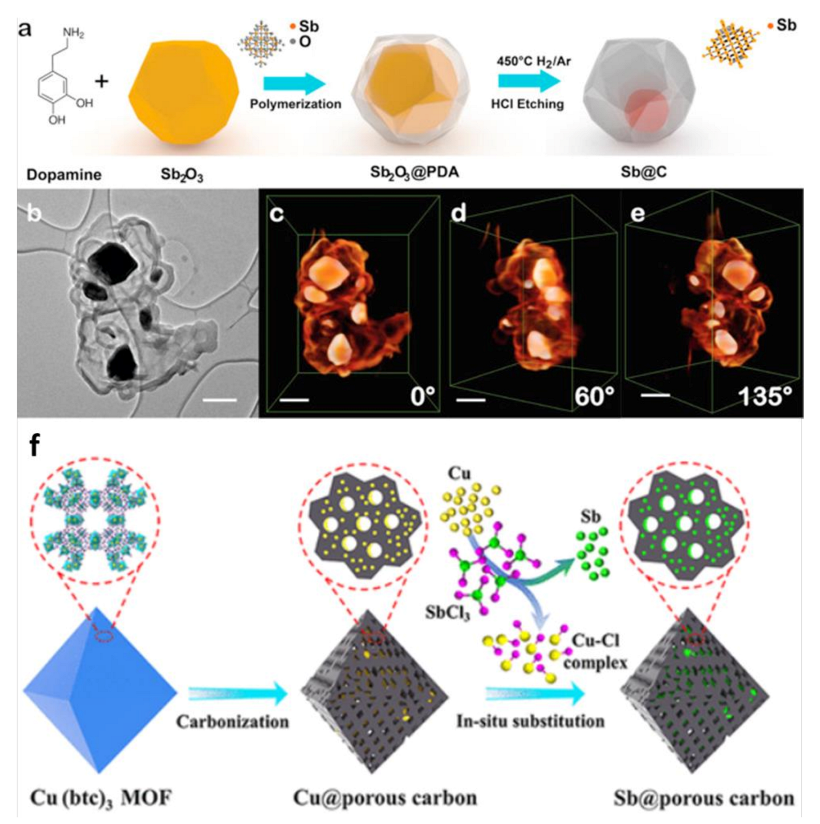
2.2. Sb/Carbenecous Composite
2.3. Sb/Oxide Composite
2.4. Other Sb Hybrid Composites
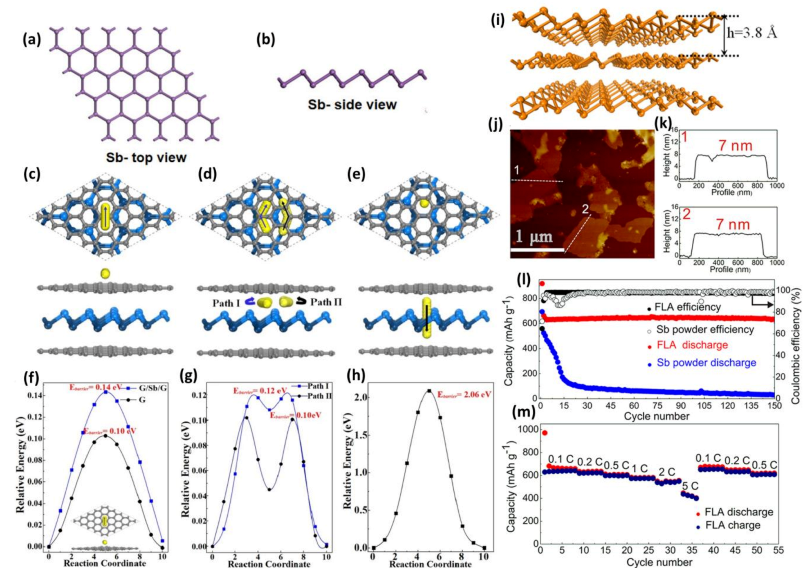
3. Sb-Based Chalcogenide Anode
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abraham, K.M. How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Usiskin, R.; Lu, Y.; Popovic, J.; Law, M.; Balaya, P.; Hu, Y.-S.; Maier, J. Fundamentals, status and promise of sodium-based batteries. Nat. Rev. Mater. 2021, 1–16. [Google Scholar] [CrossRef]
- Luo, W.; Gaumet, J.-J.; Mai, L.-Q. Antimony-based intermetallic compounds for lithium-ion and sodium-ion batteries: Synthesis, construction and application. Rare Met. 2017, 36, 321–338. [Google Scholar] [CrossRef]
- Lao, M.; Zhang, Y.; Luo, W.; Yan, Q.; Sun, W.; Dou, S.X. Alloy-Based Anode Materials toward Advanced Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1700622. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhang, Y.; Xu, M. A review on pyrophosphate framework cathode materials for sodium-ion batteries. J. Mater. Chem. A 2019, 7, 15006–15025. [Google Scholar] [CrossRef]
- Chayambuka, K.; Mulder, G.; Danilov, D.; Notten, P.H.L. Sodium-Ion Battery Materials and Electrochemical Properties Reviewed. Adv. Energy Mater. 2018, 8, 1800079. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Chen, M.; Zou, C.; Jin, H.; Wang, S.; Chou, S.; Liu, Y.; Dou, S. The Cathode Choice for Commercialization of Sodium-Ion Batteries: Layered Transition Metal Oxides versus Prussian Blue Analogs. Adv. Funct. Mater. 2020, 30, 1909530. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Q.; Yao, Z.; Wang, J.; Sánchez-Lengeling, B.; Ding, F.; Qi, X.; Lu, Y.; Bai, X.; Li, B.; et al. Rational design of layered oxide materials for sodium-ion batteries. Science 2020, 370, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Roy, S.; Zhao, Y.; Zhang, J. Recent advances in semimetallic pnictogen (As, Sb, Bi) based anodes for sodium-ion batteries: Structural design, charge storage mechanisms, key challenges and perspectives. Nano Res. 2021, 1–34. [Google Scholar] [CrossRef]
- Liang, S.; Cheng, Y.; Zhu, J.; Xia, Y.; Müller-Buschbaum, P. A Chronicle Review of Nonsilicon (Sn, Sb, Ge)-Based Lithium/Sodium-Ion Battery Alloying Anodes. Small Methods 2020, 4, 2000218. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, J.; Li, S.; Chen, Z.; Guo, Z. Phosphorus-Based Alloy Materials for Advanced Potassium-Ion Battery Anode. J. Am. Chem. Soc. 2017, 139, 3316–3319. [Google Scholar] [CrossRef] [Green Version]
- Darwiche, A.; Marino, C.; Sougrati, M.T.; Fraisse, B.; Stievano, L.; Monconduit, L. Better Cycling Performances of Bulk Sb in Na-Ion Batteries Compared to Li-Ion Systems: An Unexpected Electrochemical Mechanism. J. Am. Chem. Soc. 2013, 135, 20805–20811. [Google Scholar] [CrossRef]
- Baggetto, L.; Ganesh, P.; Sun, C.-N.; Meisner, R.A.; Zawodzinski, T.A.; Veith, G.M. Intrinsic thermodynamic and kinetic properties of Sb electrodes for Li-ion and Na-ion batteries: Experiment and theory. J. Mater. Chem. A 2013, 1, 7985–7994. [Google Scholar] [CrossRef]
- Wu, J.; Ihsan-Ul-Haq, M.; Ciucci, F.; Huang, B.; Kim, J.-K. Rationally designed nanostructured metal chalcogenides for advanced sodium-ion batteries. Energy Storage Mater. 2021, 34, 582–628. [Google Scholar] [CrossRef]
- He, M.; Kravchyk, K.; Walter, M.; Kovalenko, M. Monodisperse Antimony Nanocrystals for High-Rate Li-ion and Na-ion Battery Anodes: Nano versus Bulk. Nano Lett. 2014, 14, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Xu, Y.; Wang, C.; Wen, L.; Fang, Y.; Mi, Y.; Zhou, M.; Zhao, H.; Lei, Y. Large-scale highly ordered Sb nanorod array anodes with high capacity and rate capability for sodium-ion batteries. Energy Environ. Sci. 2015, 8, 2954–2962. [Google Scholar] [CrossRef]
- Liu, S.; Feng, J.; Bian, X.; Liu, J.; Xu, H. The morphology-controlled synthesis of a nanoporous-antimony anode for high-performance sodium-ion batteries. Energy Environ. Sci. 2016, 9, 1229–1236. [Google Scholar] [CrossRef]
- Zhou, L.; Zhuang, Z.; Zhao, H.; Lin, M.; Zhao, D.; Mai, L. Intricate Hollow Structures: Controlled Synthesis and Applications in Energy Storage and Conversion. Adv. Mater. 2017, 29, 1602914. [Google Scholar] [CrossRef]
- Hou, H.; Jing, M.; Yang, Y.; Zhang, Y.; Zhu, Y.; Song, W.; Yang, X.; Ji, X. Sb porous hollow microspheres as advanced anode materials for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 2971–2977. [Google Scholar] [CrossRef]
- Hou, H.; Jing, M.; Yang, Y.; Zhu, Y.; Fang, L.; Song, W.; Pan, C.; Yang, X.; Ji, X. Sodium/Lithium Storage Behavior of Antimony Hollow Nanospheres for Rechargeable Batteries. ACS Appl. Mater. Interfaces 2014, 6, 16189–16196. [Google Scholar] [CrossRef]
- Hou, H.; Jing, M.; Zhang, Y.; Chen, J.; Huang, Z.; Ji, X. Cypress leaf-like Sb as anode material for high-performance sodium-ion batteries. J. Mater. Chem. A 2015, 3, 17549–17552. [Google Scholar] [CrossRef]
- Selvaraj, B.; Wang, C.-C.; Song, Y.-F.; Sheu, H.-S.; Liao, Y.-F.; Wu, N.-L. Remarkable microstructural reversibility of antimony in sodium ion battery anodes. J. Mater. Chem. A 2020, 8, 22620–22625. [Google Scholar] [CrossRef]
- Wang, Y.; Brezesinski, T.; Antonietti, M.; Smarsly, B. Ordered Mesoporous Sb-, Nb-, and Ta-Doped SnO2 Thin Films with Adjustable Doping Levels and High Electrical Conductivity. ACS Nano 2009, 3, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Yang, H.; Fu, C.; Sun, M.; Wang, L.; Liu, T. In-situ synthesis of microspherical Sb@C composite anode with high tap density for lithium/sodium-ion batteries. Compos. Commun. 2020, 17, 177–181. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Y.; Lu, Y.; Han, X.; Cheng, F.; Chen, J. Spherical nano-Sb@C composite as a high-rate and ultra-stable anode material for sodium-ion batteries. Nano Res. 2015, 8, 3384–3393. [Google Scholar] [CrossRef]
- Wang, G.; Xiong, X.; Lin, Z.; Yang, C.; Lin, Z.; Liu, M. Sb/C composite as a high-performance anode for sodium ion batteries. Electrochim. Acta 2017, 242, 159–164. [Google Scholar] [CrossRef]
- Ramireddy, T.; Sharma, N.; Xing, T.; Chen, Y.; Leforestier, J.; Glushenkov, A.M. Size and Composition Effects in Sb-Carbon Nanocomposites for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 30152–30164. [Google Scholar] [CrossRef] [PubMed]
- Kalisvaart, W.P.; Olsen, B.C.; Luber, E.J.; Buriak, J.M. Sb-Si Alloys and Multilayers for Sodium-Ion Battery Anodes. ACS Appl. Energy Mater. 2019, 2, 2205–2213. [Google Scholar] [CrossRef]
- Liang, S.; Cheng, Y.-J.; Wang, X.; Xu, Z.; Ma, L.; Xu, H.; Ji, Q.; Zuo, X.; Müller-Buschbaum, P.; Xia, Y. Impact of CO2 activation on the structure, composition, and performance of Sb/C nanohybrid lithium/sodium-ion battery anodes. Nanoscale Adv. 2021, 3, 1942–1953. [Google Scholar] [CrossRef]
- Hou, H.; Yang, Y.; Zhu, Y.; Jing, M.; Pan, C.; Fang, L.; Song, W.; Yang, X.; Ji, X. An Electrochemical Study of Sb/Acetylene Black Composite as Anode for Sodium-Ion Batteries. Electrochim. Acta 2014, 146, 328–334. [Google Scholar] [CrossRef]
- Bodenes, L.; Darwiche, A.; Monconduit, L.; Martinez, H. The Solid Electrolyte Interphase a key parameter of the high performance of Sb in sodium-ion batteries: Comparative X-ray Photoelectron Spectroscopy study of Sb/Na-ion and Sb/Li-ion batteries. J. Power Sources 2015, 273, 14–24. [Google Scholar] [CrossRef]
- Lu, H.; Wu, L.; Xiao, L.; Ai, X.; Yang, H.; Cao, Y. Investigation of the Effect of Fluoroethylene Carbonate Additive on Electrochemical Performance of Sb-Based Anode for Sodium-Ion Batteries. Electrochim. Acta 2016, 190, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, O.; Cochrane, M.; Li, M.; Yan, Y.; Ma, K.; Fu, J.; Wang, Z.; Tolbert, S.H.; Shenoy, V.B.; Detsi, E. Enhanced Cycling Stability of Macroporous Bulk Antimony-Based Sodium-Ion Battery Anodes Enabled through Active/Inactive Composites. Adv. Energy Mater. 2018, 8, 1801781. [Google Scholar] [CrossRef]
- Chen, B.; Qin, H.; Li, K.; Zhang, B.; Liu, E.; Zhao, N.; Shi, C.; He, C. Yolk-shelled Sb@C nanoconfined nitrogen/sulfur co-doped 3D porous carbon microspheres for sodium-ion battery anode with ultralong high-rate cycling. Nano Energy 2019, 66, 104133. [Google Scholar] [CrossRef]
- Yang, C.; Chen, J.; Ji, X.; Pollard, T.P.; Lu, X.; Sun, C.-J.; Hou, S.; Liu, Q.; Liu, C.; Qing, T.; et al. Aqueous Li-ion battery enabled by halogen conversion-intercalation chemistry in graphite. Nat. Cell Biol. 2019, 569, 245–250. [Google Scholar] [CrossRef]
- Dong, S.; Li, C.; Li, Z.; Zhang, L.; Yin, L. Mesoporous Hollow Sb/ZnS@C Core-Shell Heterostructures as Anodes for High-Performance Sodium-Ion Batteries. Small 2018, 14, 1704517. [Google Scholar] [CrossRef]
- Song, J.; Yan, P.; Luo, L.; Qi, X.; Rong, X.; Zheng, J.; Xiao, B.; Feng, S.; Wang, C.; Hu, Y.-S.; et al. Yolk-shell structured Sb@C anodes for high energy Na-ion batteries. Nano Energy 2017, 40, 504–511. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W.; Peng, J.; Zhang, W.; Liang, Z.; Wu, J.; Feng, J.; Li, H.; Huang, S. Metal-Organic Framework Derived Ultrafine Sb@Porous Carbon Octahedron via In Situ Substitution for High-Performance Sodium-Ion Batteries. ACS Nano 2021, 15, 15104–15113. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, L.; Fu, J.; Yun, J.; Kim, K.H. Hierarchical Tiny-Sb encapsulated in MOFs derived-carbon and TiO2 hollow nanotubes for enhanced Li/Na-Ion half-and full-cell batteries. Chem. Eng. J. 2021, 417, 129106. [Google Scholar] [CrossRef]
- Palanisamy, M.; Pol, V.G.; Evans, S.F.; Jackson, K.; Jafta, C.J.; Bridges, C.A.; Dai, S.; Levine, A.M.; Lee, R.J.; Paranthaman, M.P. Encapsulated Sb and Sb2O3 particles in waste-tire derived carbon as stable composite anodes for sodium-ion batteries. Sustain. Energy Fuels 2020, 4, 3613–3622. [Google Scholar] [CrossRef]
- Xu, X.; Si, L.; Zhou, X.; Tu, F.; Zhu, X.; Bao, J. Chemical bonding between antimony and ionic liquid-derived nitrogen-doped carbon for sodium-ion battery anode. J. Power Sources 2017, 349, 37–44. [Google Scholar] [CrossRef]
- Mun, Y.S.; Yoon, Y.; Hur, J.; Park, M.S.; Bae, J.; Kim, J.H.; Yoon, Y.S.; Yoo, I.S.; Lee, S.G.; Kim, I.T. Copper-antimony-red phosphorus composites as promising anode materials for sodium-ion batteries. J. Power Sources 2017, 362, 115–122. [Google Scholar] [CrossRef]
- Xu, A.; Huang, C.; Li, G.; Zou, K.; Sun, H.; Fu, L.; Ju, J.; Song, Y.; Wu, S.; Xu, Z.; et al. Sb2O3@Sb nanoparticles impregnated in N-doped carbon microcages for ultralong life and high-rate sodium ion batteries. J. Mater. Chem. A 2021, 9, 12169–12178. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, L.; Hu, G.; Mai, L.; Cui, Y. Nanowires for Electrochemical Energy Storage. Chem. Rev. 2019, 119, 11042–11109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Han, X.; Xu, Y.; Liu, Y.; Zheng, S.; Xu, K.; Hu, L.; Wang, C. Electrospun Sb/C Fibers for a Stable and Fast Sodium-Ion Battery Anode. ACS Nano 2013, 7, 6378–6386. [Google Scholar] [CrossRef]
- Wu, L.; Hu, X.; Qian, J.; Pei, F.; Wu, F.; Mao, R.; Ai, X.; Yang, H.; Cao, Y. Sb-C nanofibers with long cycle life as an anode material for high-performance sodium-ion batteries. Energy Environ. Sci. 2014, 7, 323–328. [Google Scholar] [CrossRef]
- Yang, K.; Tang, J.; Liu, Y.; Kong, M.; Zhou, B.; Shang, Y.; Zhang, W.-H. Controllable Synthesis of Peapod-like Sb@C and Corn-like C@Sb Nanotubes for Sodium Storage. ACS Nano 2020, 14, 5728–5737. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, X.-Y.; Lou, X.W.; Paik, U. Sb@C coaxial nanotubes as a superior long-life and high-rate anode for sodium ion batteries. Energy Environ. Sci. 2016, 9, 2314–2318. [Google Scholar] [CrossRef]
- Jing, W.T.; Zhang, Y.; Gu, Y.; Zhu, Y.F.; Yang, C.C.; Jiang, Q. N-Doped Carbon Nanonecklaces with Encapsulated Sb as a Sodium-Ion Battery Anode. Matter 2019, 1, 720–733. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Bai, Z.; Qian, Y.; Yang, J. One-Dimensional Yolk-Shell Sb@Ti-O-P Nanostructures as a High-Capacity and High-Rate Anode Material for Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 447–454. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, B.; Liu, S.; Ma, Q.; Zhang, W.-H. Galvanic Replacement Synthesis of Highly Uniform Sb Nanotubes: Reaction Mechanism and Enhanced Sodium Storage Performance. ACS Nano 2019, 13, 5885–5892. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Li, F.; Gaumet, J.-J.; Magri, P.; Diliberto, S.; Zhou, L.; Mai, L. Bottom-Up Confined Synthesis of Nanorod-in-Nanotube Structured Sb@N-C for Durable Lithium and Sodium Storage. Adv. Energy Mater. 2018, 8, 1703237. [Google Scholar] [CrossRef]
- Zhou, X.; Dai, Z.; Bao, J.; Guo, Y.-G. Wet milled synthesis of an Sb/MWCNT nanocomposite for improved sodium storage. J. Mater. Chem. A 2013, 1, 13727–13731. [Google Scholar] [CrossRef]
- Li, X.; Qu, J.; Hu, Z.; Xie, H.; Yin, H. Electrochemically converting Sb2S3/CNTs to Sb/CNTs composite anodes for sodium-ion batteries. Int. J. Hydrogen Energy 2021, 46, 17071–17083. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Wu, Z.; Zhang, S.; Ge, S.; Guo, P.; Hua, M.; Lu, X.; Wang, S.; Zhang, J. Direct tuning of meso-/micro-porous structure of carbon nanofibers confining Sb nanocrystals for advanced sodium and potassium storage. J. Alloy. Compd. 2020, 833, 155127. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, C.; Xue, H.; Wang, S.; Shen, Y.; Wang, Z.; Chang, L.; Liu, W.; Cheng, Y.; Wang, L. High-rate lithium/sodium storage capacities of nitrogen-enriched porous antimony composite prepared from organic-inorganic ligands. Appl. Surf. Sci. 2021, 563, 150297. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, Z.; Lin, X.; Shen, Y.; Li, Q. The Sb/SbPO4@3D-G composite as a promising anode material for sodium-ion batteries. Inorg. Chem. Front. 2020, 7, 3448–3455. [Google Scholar] [CrossRef]
- Li, X.; Sun, M.; Ni, J.; Li, L. Template-Free Construction of Self-Supported Sb Prisms with Stable Sodium Storage. Adv. Energy Mater. 2019, 9, 1901096. [Google Scholar] [CrossRef]
- Wang, G.-Z.; Feng, J.-M.; Dong, L.; Li, X.-F.; Li, D.-J. Porous graphene anchored with Sb/SbOx as sodium-ion battery anode with enhanced reversible capacity and cycle performance. J. Alloy. Compd. 2017, 693, 141–149. [Google Scholar] [CrossRef]
- Gong, S.; Lee, J.; Kim, H. Development of electrode architecture using Sb-rGO composite and CMC binder for high-performance sodium-ion battery anodes. J. Korean Ceram. Soc. 2020, 57, 91–97. [Google Scholar] [CrossRef]
- Li, X.; Qu, J.; Xie, H.; Song, Q.; Fu, G.; Yin, H. An electro-deoxidation approach to co-converting antimony oxide/graphene oxide to antimony/graphene composite for sodium-ion battery anode. Electrochim. Acta 2020, 332, 135501. [Google Scholar] [CrossRef]
- Liu, X.; Gao, M.; Yang, H.; Zhong, X.; Yu, Y. 2D sandwich-like nanosheets of ultrafine Sb nanoparticles anchored to graphene for high-efficiency sodium storage. Nano Res. 2017, 10, 4360–4367. [Google Scholar] [CrossRef]
- Hwang, C.; Choi, S.; Jung, G.Y.; Yang, J.; Kwak, S.K.; Park, S.; Song, H.-K. Graphene-wrapped Porous Sb Anodes for Sodium-Ion Batteries by Mechanochemical Compositing and Metallomechanical Reduction of Sb2O. Ele. Acta 2017, 252, 25–32. [Google Scholar] [CrossRef]
- Wu, F.; Guo, X.; Li, M.; Xu, H. One-step hydrothermal synthesis of Sb2S3/reduced graphene oxide nanocomposites for high-performance sodium ion batteries anode materials. Ceram. Int. 2017, 43, 6019–6023. [Google Scholar] [CrossRef]
- Liu, J.-H.; Li, Y.-F.; Shi, Y.-H.; Guo, J.-Z.; Yang, J.; Wu, X.-L.; Zhang, J.-P.; Hu, W.; Sun, H.-Z. Boron-doped Sb/SbO2@rGO composites with tunable components and enlarged lattice spacing for high-rate sodium-ion batteries. J. Phys. D Appl. Phys. 2021, 54, 315505. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Wang, T.; Notten, P.H.; Ma, H.; Liu, Z.; Wang, C.; Wang, G. Novel hybrid of amorphous Sb/N-doped layered carbon for high-performance sodium-ion batteries. Chem. Eng. J. 2021, 407, 127169. [Google Scholar] [CrossRef]
- Guo, L.; Vafakhah, S.; Ding, M.; Pam, M.E.; Wang, Y.; Shang, Y.; Huang, S.; Gu, C.; Von Lim, Y.; Yang, H.Y. Direct antimony recovery from wastewater as anode materials for sodium-ion batteries. Mater. Today Energy 2020, 16, 100403. [Google Scholar] [CrossRef]
- Ld, A.; Dd, B.; Ps, A. Binder-free electrophoretic deposition of Sb/rGO on Cu foil for superior electrochemical performance in Li-ion and Na-ion batteries. Electrochim. Acta 2020, 358, 136948. [Google Scholar]
- Zhang, W.; Liu, Y.; Chen, C.; Li, Z.; Huang, Y.; Hu, X. Flexible and binder-free electrodes of Sb/rGO and Na3V2(PO4)3/rGO nanocomposites for sodium-ion batteries. Small 2015, 11, 3822–3829. [Google Scholar] [CrossRef] [PubMed]
- Ares, P.; Palacios, J.J.; Abellán, G.; Gómez-Herrero, J.; Zamora, F. Recent Progress on Antimonene: A New Bidimensional Material. Adv. Mater. 2018, 30, 2. [Google Scholar] [CrossRef]
- Wang, G.; Pandey, R.; Karna, S.P. Atomically Thin Group V Elemental Films: Theoretical Investigations of Antimonene Allotropes. ACS Appl. Mater. Interfaces 2015, 7, 11490–11496. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Yan, Z.; Li, Y.; Chen, Z.; Zeng, H. Atomically Thin Arsenene and Antimonene: Semimetal-Semiconductor and Indirect-Direct Band-Gap Transitions. Angew. Chem. Int. Ed. 2015, 127, 3155–3158. [Google Scholar] [CrossRef]
- Ares, P.; Aguilar-Galindo, F.; Rodríguez-San-Miguel, D.; Aldave, D.A.; Díaz-Tendero, S.; Alcamí, M.; Martín, F.; Gómez-Herrero, J.; Zamora, F. Mechanical Isolation of Highly Stable Antimonene under Ambient Conditions. Adv. Mater. 2016, 28, 6332–6336. [Google Scholar] [CrossRef]
- Gibaja, C.; Rodriguez-San-Miguel, D.; Ares, P.; Gómez-Herrero, J.; Zamora, F. Few-Layer Antimonene by Liquid-Phase Exfoliation. Angew. Chem. Int. Ed. 2016, 128, 14557–14561. [Google Scholar] [CrossRef] [Green Version]
- GusmãO, R.; Sofer, Z.; BoušA, D.; Pumera, M. Pnictogen (As, Sb, Bi) Nanosheets for Electrochemical Applications Are Produced by Shear Exfoliation Using Kitchen Blenders. Angew. Chem. Int. Ed. 2017, 129, 14609–14614. [Google Scholar] [CrossRef]
- Chen, H.-A.; Sun, H.; Wu, C.-R.; Wang, Y.-X.; Lee, P.-H.; Pao, C.-W.; Lin, S.-Y. Single-Crystal Antimonene Films Prepared by Molecular Beam Epitaxy: Selective Growth and Contact Resistance Reduction of the 2D Material Heterostructure. ACS Appl. Mater. Interfaces 2018, 10, 15058–15064. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Song, X.; Liu, J.; Yan, Z.; Huo, C.; Zhang, S.; Su, M.; Liao, L.; Wang, W.; Ni, Z.; et al. Two-dimensional antimonene single crystals grown by van der Waals epitaxy. Nat. Commun. 2016, 7, 13352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, S.; Srivastava, P. Modelling of antimonene as an anode material in sodium-ion battery: A first-principles study. Mater. Chem. Phys. 2020, 241, 122381. [Google Scholar] [CrossRef]
- Sengupta, A.; Frauenheim, T. Lithium and sodium adsorption properties of monolayer antimonene. Mater. Today Energy 2017, 5, 347–354. [Google Scholar] [CrossRef]
- Su, J.; Duan, T.; Li, W.; Xiao, B.; Zhou, G.; Pei, Y.; Wang, X. A first-principles study of 2D antimonene electrodes for Li ion storage. Appl. Surf. Sci. 2018, 462, 270–275. [Google Scholar] [CrossRef]
- Su, J.; Li, W.; Duan, T.; Xiao, B.; Wang, X.; Pei, Y.; Zeng, X.C. Graphene/antimonene/graphene heterostructure: A potential anode for sodium-ion batteries. Carbon 2019, 153, 767–775. [Google Scholar] [CrossRef]
- Gao, Y.; Tian, W.; Huo, C.; Zhang, K.; Guo, S.; Zhang, S.; Song, X.; Jiang, L.; Huo, K.; Zeng, H. Tailoring natural layered β-phase antimony into few layer antimonene for Li storage with high rate capabilities. J. Mater. Chem. A 2019, 7, 3238–3243. [Google Scholar] [CrossRef]
- Martínez-Periñán, E.; Down, M.P.; Gibaja, C.; Lorenzo, E.; Zamora, F.; Banks, C.E. Antimonene: A Novel 2D Nanomaterial for Supercapacitor Applications. Adv. Energy Mater. 2018, 8, 1702606. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, J.; Yao, P.; Xie, H.; Zhang, M.; Ji, M.; Liu, H.; Liu, Q.; Zhu, C.; Xu, J. Antimonene Engineered Highly Deformable Freestanding Electrode with Extraordinarily Improved Energy Storage Performance. Adv. Energy Mater. 2019, 9, 1902462. [Google Scholar] [CrossRef]
- Yang, Y.; Leng, S.; Shi, W. Electrochemical exfoliation of porous antimonene as anode materials for sodium-ion batteries. Electrochem. Commun. 2021, 126, 107025. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, S.; Huo, C.; Zhu, D.; Li, Q.; Wang, L.; Ren, X.; Xie, L.; Guo, S.; Chu, P.K.; et al. Few-Layer Antimonene: Anisotropic Expansion and Reversible Crystalline-Phase Evolution Enable Large-Capacity and Long-Life Na-Ion Batteries. ACS Nano 2018, 12, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Xu, J.; Zhang, Y.; Wei, Z.; Mao, M.; Lian, X.; Wang, S.; Yang, C.; Fan, X.; Ma, J.; et al. Antimony Nanorod Encapsulated in Cross-Linked Carbon for High-Performance Sodium Ion Battery Anodes. Nano Lett. 2019, 19, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yang, L.; Bai, X.; Wu, Q.; Liang, M.; Wang, Y.; Zhao, N.; Shi, C.; Zhou, B.; He, C. Heterostructure Engineering of Core-Shelled Sb@Sb2O3 Encapsulated in 3D N-Doped Carbon Hollow-Spheres for Superior Sodium/Potassium Storage. Small 2021, 17, 2006824. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhang, P.; Wang, X.; Li, Q.; Dong, Y.; Hua, J.; Zhou, L.; Mai, L. Antimony nanoparticles anchored in three-dimensional carbon network as promising sodium-ion battery anode. J. Power Sources 2016, 304, 340–345. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, W.; Wu, C.; Fan, Q.; Zhang, W.; Hu, X.; Huang, Y. Self-wrapped Sb/C nanocomposite as anode material for High-performance sodium-ion batteries. Nano Energy 2015, 16, 479–487. [Google Scholar] [CrossRef]
- Fan, X.-Y.; Jiang, Z.; Huang, L.; Wang, X.; Han, J.; Sun, R.; Gou, L.; Li, D.-L.; Ding, Y.-L. 3D Porous Self-Standing Sb Foam Anode with a Conformal Indium Layer for Enhanced Sodium Storage. ACS Appl. Mater. Interfaces 2020, 12, 20344–20353. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, W.; Yang, Z.; Gu, L.; Yu, Y. Nanoconfined antimony in sulfur and nitrogen co-doped three-dimensionally (3D) interconnected macroporous carbon for high-performance sodium-ion batteries. Nano Energy 2015, 18, 12–19. [Google Scholar] [CrossRef]
- Wan, F.; Guo, J.-Z.; Zhang, X.-H.; Zhang, J.-P.; Sun, H.-Z.; Yan, Q.; Han, D.-X.; Niu, L.; Wu, X.-L. In Situ Binding Sb Nanospheres on Graphene via Oxygen Bonds as Superior Anode for Ultrafast Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 7790–7799. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Jiang, Y.; Wei, Q.; Huang, Q.; Dai, Y.; Xiong, F.; Li, Q.; An, Q.; Xu, X.; Zhu, Z.; et al. Multidimensional Synergistic Nanoarchitecture Exhibiting Highly Stable and Ultrafast Sodium-Ion Storage. Adv. Mater. 2018, 30, e1707122. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Zhou, M.; Li, W.; Tao, H.; Wang, R.; Cheng, S.; Jiang, K. Facile Tailoring of Multidimensional Nanostructured Sb for Sodium Storage Applications. ACS Nano 2019, 13, 9533–9540. [Google Scholar] [CrossRef]
- Wang, N.; Bai, Z.; Qian, Y.; Yang, J. Double-Walled Sb@TiO2x Nanotubes as a Superior High-Rate and Ultralong-Lifespan Anode Material for Na-Ion and Li-Ion Batteries. Adv. Mater. 2016, 28, 4126–4133. [Google Scholar] [CrossRef]
- Li, P.; Guo, X.; Wang, S.; Zang, R.; Li, X.; Man, Z.; Li, P.; Liu, S.; Wu, Y.; Wang, G. Two-dimensional Sb@TiO2−x nanoplates as a high-performance anode material for sodium-ion batteries. J. Mater. Chem. A 2019, 7, 2553–2559. [Google Scholar] [CrossRef]
- Zhou, L.; Cheng, Y.; Sun, Q.; Sun, L.; Wang, C.; Wang, X.; Yin, D.; Wang, L.; Ming, J. High alkaline ion storage capacity of hollow interwoven structured Sb/TiO2 particles: The galvanic replacement formation mechanism and volumetric buffer effect. Chem. Commun. 2018, 54, 4049–4052. [Google Scholar] [CrossRef]
- Kong, M.; Liu, Y.; Zhou, B.; Yang, K.; Zhang, W.H. Rational Design of Sb@C@TiO2 Triple-Shell Nanoboxes for High-Performance Sodium-Ion Batteries. Small 2020, 16, e2001976. [Google Scholar] [CrossRef]
- Ye, J.; Xia, G.; Zheng, Z.; Hu, C. Facile controlled synthesis of coral-like nanostructured Sb2O3@Sb anode materials for high performance sodium-ion batteries. Int. J. Hydrogen Energy 2020, 45, 9969–9978. [Google Scholar] [CrossRef]
- Pan, J.; Wang, N.; Zhou, Y.; Yang, X.; Zhou, W.; Qian, Y.; Yang, J. Simple synthesis of a porous Sb/Sb2O3 nanocomposite for a high-capacity anode material in Na-ion batteries. Nano Res. 2017, 10, 1794–1803. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, K.Y.; Choi, W. One-Pot Synthesis of Antimony-Embedded Silicon Oxycarbide Materials for High-Performance Sodium-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1702607. [Google Scholar] [CrossRef]
- Li, N.; Liao, S.; Sun, Y.; Song, H.W.; Wang, C.X. Uniformly dispersed self-assembled growth of Sb2O3/Sb@graphene nanocomposites on a 3D carbon sheet network for high Na-storage capacity and excellent stability. J. Mater. Chem. A 2015, 3, 5820–5828. [Google Scholar] [CrossRef]
- Hu, M.; Jiang, Y.; Sun, W.; Wang, H.; Jin, C.; Yan, M. Reversible Conversion-Alloying of Sb2O3 as a High-Capacity, High-Rate, and Durable Anode for Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 19449–19455. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, H.; Wang, G. Sb2O3 Nanowires as Anode Material for Sodium-Ion Battery. Arab. J. Sci. Eng. 2014, 39, 6589–6593. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Xu, Y.; Liu, Y.; Dai, Z.; Bao, J. An SbOx/Reduced Graphene Oxide Composite as a High-Rate Anode Material for Sodium-Ion Batteries. J. Phys. Chem. C 2014, 118, 23527–23534. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Cheng, S.; Jiang, K. A two-dimensional hybrid of SbOx nanoplates encapsulated by carbon flakes as a high performance sodium storage anode. J. Mater. Chem. A 2017, 5, 1160. [Google Scholar] [CrossRef]
- Liu, S.; Cai, Z.; Zhou, J.; Zhu, M.; Pan, A.; Liang, S. High-performance sodium-ion batteries and flexible sodium-ion capacitors based on Sb2×3(X = O, S)/carbon fiber cloth. J. Mater. Chem. A 2017, 5, 9169–9176. [Google Scholar] [CrossRef]
- Kalubarme, R.S.; Park, C.-J.; Kale, B.B.; Gosavi, S.W. Highly crystalline antimony oxide octahedron: An efficient anode for sodium-ion batteries. J. Mater. Sci. Mater. Electron. 2021, 32, 3809–3818. [Google Scholar] [CrossRef]
- Chen, X.Y.; Huh, H.S.; Lee, S.W. Hydrothermal synthesis of antimony oxychloride and oxide nanocrystals: Sb4O5Cl2, Sb8O11Cl2, and Sb2O. J. Solid State Chem. 2008, 181, 2127–2132. [Google Scholar] [CrossRef]
- Lin, Y.; Feng, W.; Li, Z.; Xu, T.; Fei, H. Facile synthesis of phase-pure Sb8O11Cl2 microrods as anode materials for sodium-ion batteries with high capacity. Ionics 2017, 23, 3197–3202. [Google Scholar] [CrossRef]
- Xie, J.; Pei, Y.; Liu, L.; Guo, S.-P.; Xia, J.; Li, M.; Ouyang, Y.; Zhang, X.; Wang, X. Hydrothermal synthesis of antimony oxychlorides submicron rods as anode materials for lithium-ion batteries and sodium-ion batteries. Electrochim. Acta 2017, 254, 246–254. [Google Scholar] [CrossRef]
- Tang, J.-J.; Wang, Y.; Jiao, Z.; Wu, M. Self-assembly nanostructures of one-dimensional antimony oxide and oxychloride. Mater. Lett. 2009, 63, 1481–1484. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, H.; Li, L.; Tian, M.; Han, J.; Zhang, L.; Guo, L. One-step synthesis and flame retardancy of sheaf-like microcrystal antimony oxychloride. J. Nanosci. Nanotechnol. 2011, 11, 8504–8509. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, K.; Janas, K.; Shaijumon, M. Antimony oxychloride/graphene aerogel composite as anode material for sodium and lithium ion batteries. Carbon 2018, 131, 86–93. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Qin, B.; Lu, C.; Butt, H.A.; Zheng, T.; Zhang, D. Polyaniline-coated nanoporous antimony with improved performance for sodium-ion battery anodes. J. Alloy. Compd. 2021, 861, 158647. [Google Scholar] [CrossRef]
- Luo, X.; Tan, H.; Ma, T.; Wang, H.; Lv, M.; Yu, Z.; Fu, C.; Chang, X.; Jin, S. Nitrogen doped porous carbon coated antimony as high performance anode material for sodium-ion batteries. Nanotechnology 2021, 32, 315401. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Kalisvaart, W.P.; Olsen, B.C.; Luber, E.J.; Mitlin, D.; Buriak, J.M. Sn-Bi-Sb alloys as anode materials for sodium ion batteries. J. Mater. Chem. A 2017, 5, 9661–9670. [Google Scholar] [CrossRef]
- Zhao, Y.; Manthiram, A. High-Capacity, High-Rate Bi-Sb Alloy Anodes for Lithium-Ion and Sodium-Ion Batteries. Chem. Mater. 2015, 27, 3096–3101. [Google Scholar] [CrossRef]
- Yu, D.-K.; Park, C.-M. Sb-based intermetallics and nanocomposites as stable and fast Na-ion battery anodes. Chem. Eng. J. 2021, 409, 127380. [Google Scholar] [CrossRef]
- Guo, S.; Li, H.; Lu, Y.; Liu, Z.; Hu, X. Lattice softening enables highly reversible sodium storage in anti-pulverization Bi-Sb alloy/carbon nanofibers. Energy Storage Mater. 2020, 27, 270–278. [Google Scholar] [CrossRef]
- Kalisvaart, W.P.; Xie, H.; Olsen, B.C.; Luber, E.J.; Buriak, J.M. Understanding the Mechanism of Enhanced Cycling Stability in Sn-Sb Composite Na-Ion Battery Anodes: Operando Alloying and Diffusion Barriers. ACS Appl. Energy Mater. 2019, 2, 5133–5139. [Google Scholar] [CrossRef]
- Sarkar, S.; Peter, S.C. An overview on Sb-based intermetallics and alloys for sodium-ion batteries: Trends, challenges and future prospects from material synthesis to battery performance. J. Mater. Chem. A 2021, 9, 5164–5196. [Google Scholar] [CrossRef]
- Xie, H.; Tan, X.; Luber, E.J.; Olsen, B.C.; Kalisvaart, W.P.; Jungjohann, K.L.; Mitlin, D.; Buriak, J.M. β-SnSb for Sodium Ion Battery Anodes: Phase Transformations Responsible for Enhanced Cycling Stability Revealed by In Situ TEM. ACS Energy Lett. 2018, 3, 1670–1676. [Google Scholar] [CrossRef]
- Sarkar, S.; Chaupatnaik, A.; Ramarao, S.D.; Subbarao, U.; Barpanda, P.; Peter, S.C. Operando Sodiation Mechanistic Study of a New Antimony-Based Intermetallic CoSb as a High-Performance Sodium-Ion Battery Anode. J. Phys. Chem. C 2020, 124, 15757–15768. [Google Scholar] [CrossRef]
- Li, C.; Pei, Y.R.; Zhao, M.; Yang, C.C.; Jiang, Q. Sodium storage performance of ultrasmall SnSb nanoparticles. Chem. Eng. J. 2021, 420, 129617. [Google Scholar] [CrossRef]
- Luo, W.; Calas, A.; Tang, C.; Li, F.; Zhou, L.; Mai, L. Ultralong Sb2Se3 Nanowire-Based Free-Standing Membrane Anode for Lithium/Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 35219–35226. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Li, F.; Li, Q.; Wang, X.; Yang, W.; Zhou, L.; Mai, L. Heterostructured Bi2S3-Bi2O3 Nanosheets with a Built-In Electric Field for Improved Sodium Storage. ACS Appl. Mater. Interfaces 2018, 10, 7201–7207. [Google Scholar] [CrossRef]
- Kravchyk, K.V.; Kovalenko, M.V.; Bodnarchuk, M.I. Colloidal Antimony Sulfide Nanoparticles as a High-Performance Anode Material for Li-ion and Na-ion Batteries. Sci. Rep. 2020, 10, 2554. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, M.-H.; Choi, H.-Y.; Park, C.-M.; Lee, S.-M.; Choi, J.-H. Investigation of electrochemical reaction mechanism for antimony selenide nanocomposite for sodium-ion battery electrodes. J. Appl. Electrochem. 2018, 49, 207–216. [Google Scholar] [CrossRef]
- Ge, P.; Cao, X.-Y.; Hou, H.; Li, S.; Ji, X. Rodlike Sb2Se3 Wrapped with Carbon: The Exploring of Electrochemical Properties in Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 34979–34989. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.-H.; Park, C.-M. 2D layered Sb2Se3-based amorphous composite for high-performance Li- and Na-ion battery anodes. J. Power Sources 2019, 433, 126639. [Google Scholar] [CrossRef]
- Wen, J.; Pei, Y.; Liu, L.; Su, D.; Yang, M.; Wang, Q.; Zhang, W.; Dai, J.; Feng, Y.; Wu, T.; et al. Fully encapsulated Sb2Se3/Sb/C nanofibers: Towards high-rate, ultralong-lifespan lithium-ion batteries. J. Alloy. Compd. 2021, 874, 159961. [Google Scholar] [CrossRef]
- Guo, L.; Cao, L.; Huang, J.; Li, J.; Chen, S. Carbon capsule confined Sb2Se3 for fast Na+ extraction in sodium-ion batteries. Sustain. Energy Fuels 2020, 4, 797–808. [Google Scholar] [CrossRef]
- Zhai, H.; Jiang, H.; Qian, Y.; Cai, X.; Liu, H.; Qiu, Y.; Jin, M.; Xiu, F.; Liu, X.; Lai, L. Sb2S3 nanocrystals embedded in multichannel N-doped carbon nanofiber for ultralong cycle life sodium-ion batteries. Mater. Chem. Phys. 2020, 240, 122139. [Google Scholar] [CrossRef]
- Wen, S.; Zhao, J.; Zhao, Y.; Xu, T.; Xu, J. Reduced graphene oxide (RGO) decorated Sb2S3 nanorods as anode material for sodium-ion batteries. Chem. Phys. Lett. 2019, 716, 171–176. [Google Scholar] [CrossRef]
- Zhang, Q.; Zeng, Y.; Wang, X.; Wang, L.; Wang, H.; Xiao, J.; Li, X. Sb2S3 nanoparticles anchored on N-doped 3D carbon nanofibers as anode material for sodium ion batteries with improved electrochemical performance. J. Alloy. Compd. 2021, 881, 160594. [Google Scholar] [CrossRef]
- Ge, P.; Hou, H.; Ji, X.; Huang, Z.; Li, S.; Huang, L. Enhanced stability of sodium storage exhibited by carbon coated Sb2S3 hollow spheres. Mater. Chem. Phys. 2018, 203, 185–192. [Google Scholar] [CrossRef]
- Dashairya, L.; Das, D.; Saha, P. Elucidating the role of graphene and porous carbon coating on nanostructured Sb2S3 for superior lithium and sodium storage. J. Alloy. Compd. 2021, 883, 160906. [Google Scholar] [CrossRef]
- Hameed, A.S.; Reddy, M.V.; Chen, J.L.T.; Chowdari, B.V.R.; Vittal, J.J. RGO/Stibnite Nanocomposite as a Dual Anode for Lithium and Sodium Ion Batteries. ACS Sustain. Chem. Eng. 2016, 4, 2479–2486. [Google Scholar] [CrossRef]
- Guo, L.; Cao, L.; Huang, J.; Wang, Y.; Li, W.; Qi, H.; Chen, S.; Li, J. Design of an ultra-stable Sb2Se3 anode with excellent Na storage performance. J. Alloy. Compd. 2019, 810, 151930. [Google Scholar] [CrossRef]
- Zheng, T.; Li, G.; Zhao, L.; Shen, Y. Flowerlike Sb2S3/PPy Microspheres Used as Anode Material for High-Performance Sodium-Ion Batteries. Eur. J. Inorg. Chem. 2018, 2018, 1224–1228. [Google Scholar] [CrossRef]
- Shi, Y.; Li, F.; Zhang, Y.; He, L.; Ai, Q.; Luo, W. Sb₂S₃@PPy Coaxial Nanorods: A Versatile and Robust Host Material for Reversible Storage of Alkali Metal Ions. Nanomaterials 2019, 9, 560. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Yuan, S.; Yin, Y.-B.; Zhu, Y.; Zhang, X.; Yan, J.-M. Green and Facile Fabrication of MWNTs@Sb2S3@PPy Coaxial Nanocables for High-Performance Na-Ion Batteries. Part. Part. Syst. Charact. 2016, 33, 493–499. [Google Scholar] [CrossRef]
- Pan, Z.-Z.; Yan, Y.; Cui, N.; Xie, J.-C.; Zhang, Y.-B.; Mu, W.-S.; Hao, C. Ionic Liquid-Assisted Preparation of Sb2S3/Reduced Graphene Oxide Nanocomposite for Sodium-Ion Batteries. Adv. Mater. Interfaces 2018, 5, 1701481. [Google Scholar] [CrossRef]
- Luo, W.; Gaumet, J.-J.; Magri, P.; Diliberto, S.; Li, F.; Franchetti, P.; Ghanbaja, J.; Mai, L. Fast, green microwave-assisted synthesis of single crystalline Sb2Se3 nanowires towards promising lithium storage. J. Energy Chem. 2019, 30, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Cui, J.; Deng, Y.; Chong, W.G.; Wu, J.; Haq, M.I.U.; Mai, Y.-W.; Kim, J.-K. Ultrathin Sb2S3 nanosheet anodes for exceptional pseudocapacitive contribution to multi-battery charge storage. Energy Storage Mater. 2019, 20, 36–45. [Google Scholar] [CrossRef]
- Hou, H.; Jing, M.; Huang, Z.; Yang, Y.; Zhang, Y.; Chen, J.; Wu, Z.; Ji, X. One-Dimensional Rod-Like Sb2S3-Based Anode for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 19362–19369. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, D.; Zhang, X.; Hou, S.; Li, D.; Lu, T.; Yao, Y.; Pan, L. In situ growth of Sb2S3 on multiwalled carbon nanotubes as high-performance anode materials for sodium-ion batteries. Electrochim. Acta 2017, 228, 436–446. [Google Scholar] [CrossRef]
- Xie, J.; Xia, J.; Yuan, Y.; Liu, L.; Zhang, Y.; Nie, S.; Yan, H.; Wang, X. Sb2S3 embedded in carbon-silicon oxide nanofibers as high-performance anode materials for lithium-ion and sodium-ion batteries. J. Power Sources 2019, 435, 226762. [Google Scholar] [CrossRef]
- Xia, L.; Yang, Z.; Tang, B.; Li, F.; Wei, J.; Zhou, Z. Carbon Nanofibers with Embedded Sb2Se3 Nanoparticles as Highly Reversible Anodes for Na-Ion Batteries. Small 2021, 17, e2006016. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Hu, M.; Zhang, Z.; Zapien, J.A.; Wang, X.; Lee, J.-M.; Zhang, W. Nitrogen-Doped Carbon-Encapsulated Antimony Sulfide Nanowires Enable High Rate Capability and Cyclic Stability for Sodium-Ion Batteries. ACS Appl. Nano Mater. 2019, 2, 1457–1465. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, W.-Q.; Zhao, J.-C.; Rao, P.-H.; Mao, J.-F. Superior sodium and lithium storage in strongly coupled amorphous Sb2S3 spheres and carbon nanotubes. Int. J. Miner. Met. Mater. 2021, 28, 1194–1203. [Google Scholar] [CrossRef]
- Zhao, W.; Li, M.; Qi, Y.; Tao, Y.; Shi, Z.; Liu, Y.; Cheng, J. Ultrasound sonochemical synthesis of amorphous Sb2S3-graphene composites for sodium-ion batteries. J. Colloid Interface Sci. 2021, 586, 404–411. [Google Scholar] [CrossRef]
- Li, M.; Huang, F.; Pan, J.; Li, L.; Zhang, Y.; Yao, Q.; Zhou, H.; Deng, J. Amorphous Sb2S3 Nanospheres In-Situ Grown on Carbon Nanotubes: Anodes for NIBs and KIBs. Nanomater. 2019, 9, 1323. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Manthiram, A. Amorphous Sb2S3 embedded in graphite: A high-rate, long-life anode material for sodium-ion batteries. Chem. Commun. 2015, 51, 13205–13208. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Yu, X.; Wen, X. Formation of Polypyrrole-Coated Sb2Se3 Microclips with Enhanced Sodium-Storage Properties. Angew. Chem. Int. Ed. 2018, 57, 9859–9863. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, L.; Gu, Q.; Chao, D.; Jaroniec, M.; Qiao, S.Z. Multi-shell hollow structured Sb2S3 for sodium-ion batteries with enhanced energy density. Nano Energy 2019, 60, 591–599. [Google Scholar] [CrossRef]
- Cao, L.; Gao, X.; Zhang, B.; Ou, X.; Zhang, J.; Luo, W.-B. Bimetallic Sulfide Sb2S3@FeS2 Hollow Nanorods as High-Performance Anode Materials for Sodium-Ion Batteries. ACS Nano 2020, 14, 3610–3620. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, D.; Zhang, K.; Kang, W.; Wang, X.; Ma, P.; Wan, Y.; Cao, D.; Sun, D. Cation-exchange construction of ZnSe/Sb2Se3 hollow microspheres coated by nitrogen-doped carbon with enhanced sodium ion storage capability. Nanoscale 2020, 12, 17915–17924. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Li, C.; Ge, X.; Li, Z.; Miao, X.; Yin, L. ZnS-Sb2S3@C Core-Double Shell Polyhedron Structure Derived from Metal-Organic Framework as Anodes for High Performance Sodium Ion Batteries. ACS Nano 2017, 11, 6474–6482. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhang, X.; Yang, Y.; Wang, X.; Yao, J. Electrospinning fabrication of flexible, foldable, and twistable Sb2S3/TiO2/C nanofiber anode for lithium ion batteries. Chem. Eng. J. 2021, 413, 127400. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, G.; Lin, Y.; Wang, Y.; Ou, X.; Zheng, F.; Yang, C.; Wang, J.-H.; Liu, M. Enhancing Sodium Ion Battery Performance by Strongly Binding Nanostructured Sb2S3 on Sulfur-Doped Graphene Sheets. ACS Nano 2016, 10, 10953–10959. [Google Scholar] [CrossRef]
- Zhao, W.; Li, C.M. Mesh-structured N-doped graphene@Sb2Se3 hybrids as an anode for large capacity sodium-ion batteries. J. Colloid Interface Sci. 2017, 488, 356–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, G.; Yin, X.; Shi, S.; Zhao, Y.; Zhang, J. Sb2S3@YP Nanostructured Anode Material Synthesized by a Novel Vaporization-Condensation Method for Long Cycle-Life Sodium-Ion Battery. J. Electrochem. Soc. 2020, 167, 140531. [Google Scholar] [CrossRef]
- Jaramillo-Quintero, O.A.; Barrera-Peralta, R.V.; El Hachimi, A.G.; Guillén-López, A.; Pérez, O.; Reguera, E.; Rincón, M.E.; Muñiz, J. Understanding the interaction between heteroatom-doped carbon matrix and Sb2S3 for efficient sodium-ion battery anodes. J. Colloid Interface Sci. 2021, 585, 649–659. [Google Scholar] [CrossRef]
- Zhan, W.; Zhu, M.; Lan, J.; Wang, H.; Yuan, H.; Yang, X.; Sui, G. 1D Sb2S3@nitrogen-doped carbon coaxial nanotubes uniformly encapsulated within 3D porous graphene aerogel for fast and stable sodium storage. Chem. Eng. J. 2021, 408, 128007. [Google Scholar] [CrossRef]
- El Hachimi, A.G.; Guillén-López, A.; Jaramillo-Quintero, O.A.; Rincón, M.E.; Sevilla-Camacho, P.Y.; Muñiz, J. Exploring the enhanced performance of Sb2S3/doped-carbon composites as potential anode materials for sodium-ion batteries: A density functional theory approach. Int. J. Quantum Chem. 2021, 121, e26779. [Google Scholar] [CrossRef]
- He, F.; Tang, C.; Zhu, G.; Liu, Y.; Du, A.; Zhang, Q.; Wu, M.; Zhang, H. Leaf-inspired design of mesoporous Sb2S3/N-doped Ti3C2Tx composite towards fast sodium storage. Sci. China Ser. B Chem. 2021, 64, 964–973. [Google Scholar] [CrossRef]
- Wu, Y.; Nie, P.; Dou, H.; Jiang, J.; Zhu, Y.; Zhang, X. Graphene scrolls coated Sb2S3 nanowires as anodes for sodium and lithium ion batteries. Nano-Struct. Nano-Objects 2018, 15, 197–204. [Google Scholar] [CrossRef]
- Wang, D.; Cao, L.; Luo, D.; Gao, R.; Li, H.; Wang, D.; Sun, G.; Zhao, Z.; Li, N.; Zhang, Y.; et al. Chain mail heterostructured hydrangea-like binary metal sulfides for high efficiency sodium ion battery. Nano Energy 2021, 87, 106185. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.; Qin, Y.; Zhao, Z.; Xu, Y.; Yi, Y.; Guan, H.; Fu, Y.; Liu, P.; Li, D. Sb2S3-Bi2S3 microrods with the combined action of carbon encapsulation and rGO confinement for improving high cycle stability in sodium/potassium storage. Chem. Eng. J. 2021, 414, 128787. [Google Scholar] [CrossRef]
- Lin, J.; Yao, L.; Zhang, C.; Ding, H.; Wu, Y.; Li, S.; Han, J.; Yue, G.; Peng, D. Construction of Sb2S3@SnS@C Tubular Heterostructures as High-Performance Anode Materials for Sodium-Ion Batteries. ACS Sustain. Chem. Eng. 2021, 9, 11280–11289. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, J.; Xu, M.; Wang, H.; Gong, Y.; Xu, J. Facile synthesis of Sb2S3/MoS2 heterostructure as anode material for sodium-ion batteries. Nanotechnol. 2018, 29, 335401. [Google Scholar] [CrossRef]
- Bera, S.; Roy, A.; Guria, A.K.; Mitra, S.; Pradhan, N. Insights of Diffusion Doping in Formation of Dual-Layered Material and Doped Heterostructure SnS-Sn:Sb2S3 for Sodium Ion Storage. J. Phys. Chem. Lett. 2019, 10, 1024–1030. [Google Scholar] [CrossRef]
- Li, X.; Liang, H.; Liu, X.; Sun, R.; Qin, Z.; Fan, H.; Zhang, Y. Ion-exchange strategy of CoS2/Sb2S3 hetero-structured nanocrystals encapsulated into 3D interpenetrating dual-carbon framework for high-performance Na+/K+ batteries. Chem. Eng. J. 2021, 425, 130657. [Google Scholar] [CrossRef]
- Yan, C.; Zhu, Y.; Li, Y.; Fang, Z.; Peng, L.; Zhou, X.; Chen, G.; Yu, G. Local Built-In Electric Field Enabled in Carbon-Doped Co3O4 Nanocrystals for Superior Lithium-Ion Storage. Adv. Funct. Mater. 2018, 28, 166–173. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, T.; Zhao, X.; Pang, W.K.; Gao, H.; Li, S.; Zhou, Z.; Liu, H.K.; Guo, Z. Atomic Interface Engineering and Electric-Field Effect in Ultrathin Bi2MoO6 Nanosheets for Superior Lithium Ion Storage. Adv. Mater. 2017, 29, 1700396. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, S.; Li, X.; Li, C.; Zang, R.; Man, Z.; Wu, Y.; Li, P.; Wang, G. SnS2/Sb2S3 Heterostructures Anchored on Reduced Graphene Oxide Nanosheets with Superior Rate Capability for Sodium-Ion Batteries. Chem.-A Eur. J. 2018, 24, 3873–3881. [Google Scholar] [CrossRef]
- Ren, M.; Cao, D.; Jiang, W.; Su, K.; Pan, L.; Jiang, Y.; Yan, S.; Qiu, T.; Yang, M.; Yang, J.; et al. Hierarchical Composite of Sb2S3 decorated on highly crumpled Ti3C2Tx nanosheets for enhanced sodium storage properties. Electrochim. Acta 2021, 373, 137835. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, M.; Jiang, W.; Yao, J.; Pan, L.; Yang, J. Hierarchical Sb2S3@m-Ti3C2Tx composite anode with enhanced Na-ion storage properties. J. Alloy. Compd. 2021, 887, 161318. [Google Scholar] [CrossRef]
- Ihsan-Ul-Haq, M.; Huang, H.; Wu, J.; Mubarak, N.; Susca, A.; Luo, Z.; Huang, B.; Kim, J.-K. Unveiling solid electrolyte interface morphology and electrochemical kinetics of amorphous Sb2Se3/CNT composite anodes for ultrafast sodium storage. Carbon 2021, 171, 119–129. [Google Scholar] [CrossRef]
- Ma, X.; Luo, W.; Yan, M.; He, L.; Mai, L. In situ characterization of electrochemical processes in one dimensional nanomaterials for energy storages devices. Nano Energy 2016, 24, 165–188. [Google Scholar] [CrossRef]
- Yao, S.; Cui, J.; Lu, Z.; Xu, Z.-L.; Qin, L.; Huang, J.; Sadighi, Z.; Ciucci, F.; Kim, J.-K. Unveiling the Unique Phase Transformation Behavior and Sodiation Kinetics of 1D van der Waals Sb2S3Anodes for Sodium Ion Batteries. Adv. Energy Mater. 2017, 7, 1602149. [Google Scholar] [CrossRef]
- Ou, X.; Yang, C.; Xiong, X.; Zheng, F.; Pan, Q.; Jin, C.; Liu, M.; Huang, K. A New rGO-Overcoated Sb2Se3 Nanorods Anode for Na+ Battery: In Situ X-Ray Diffraction Study on a Live Sodiation/Desodiation Process. Adv. Funct. Mater. 2017, 27, 1606242. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, W.; Gao, P.; Zhu, C.; Hu, X.; Qu, K.; Chen, J.; Wang, Y.; Sun, L.; Mai, L.; et al. Unveiling the microscopic origin of asymmetric phase transformations in (de)sodiated Sb2Se3 with in situ transmission electron microscopy. Nano Energy 2020, 77, 105299. [Google Scholar] [CrossRef]
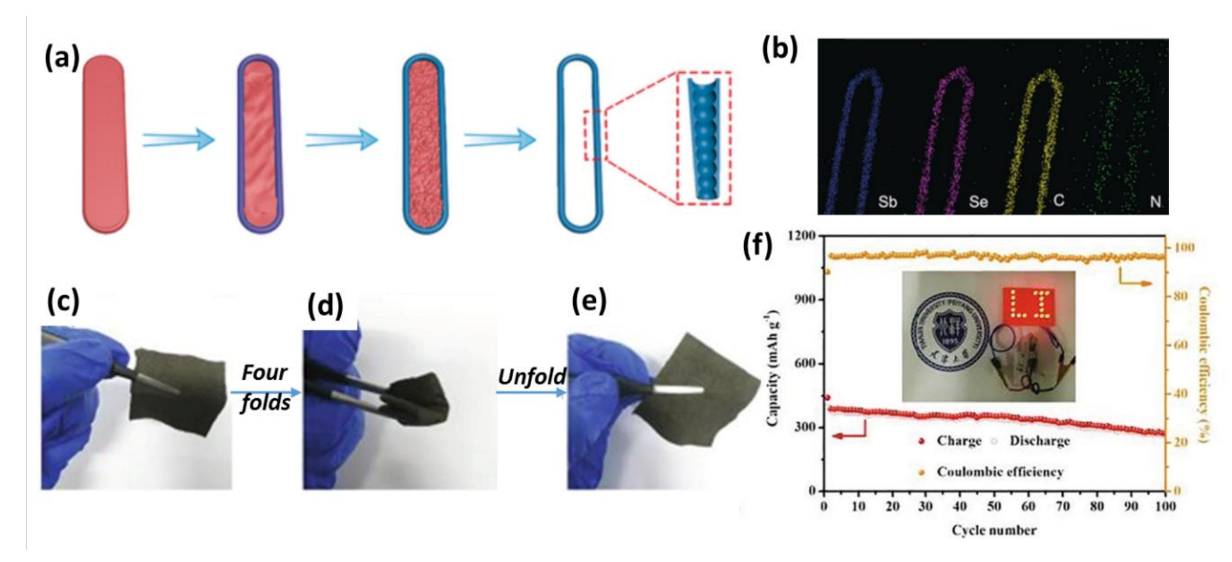
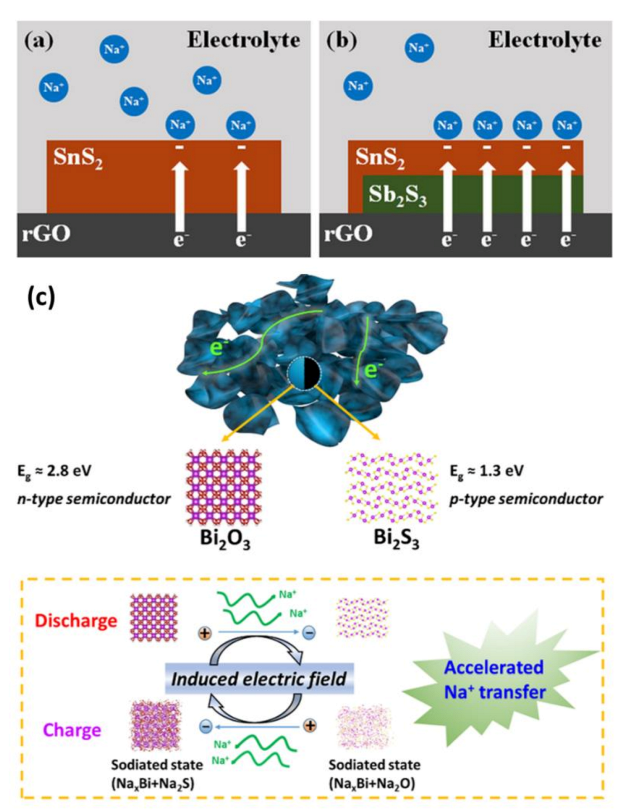
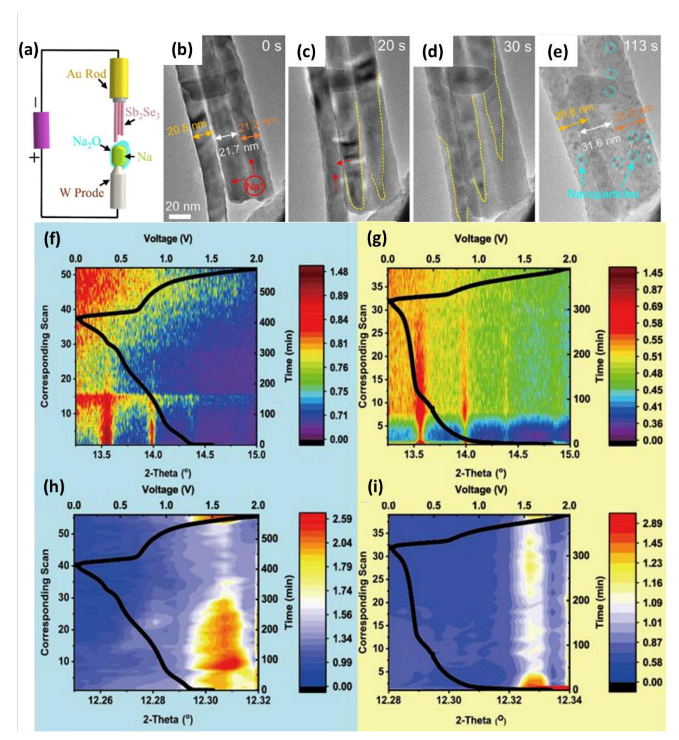
| Materials | Preparation Methods | mAg−1 | Capacity and Cycle Life | Ref. |
|---|---|---|---|---|
| Monodisperse Antimony Nanocrystals | One-pot colloidal synthesis | 330 | 500 mAhg−1 after 50 cycle | [15] |
| Highly ordered Sb nanorod array | Electrodeposition & template | 200 | 620 mAh g−1 at the 100 cycle | [16] |
| Nanoporous-antimony Anode | Template method | 100 | 573.8 mAh g−1 after 200 cycles | [17] |
| Sb porous hollow microspheres | Zinc balls are etched as templates | 100 | 617 mAh g−1 after 100 cycles | [19] |
| Sb Hollow Nanospheres | Nickel spheres are etched as templates | 50 | 622.2 mAh g−1 after 50 cycles | [20] |
| Cypress leaf-like Sb | Chemical replacement reaction | 100 | 629 mAh g−1 after 120 cycles | [21] |
| Sb nanoparticles/matrix | Aerosol spray pyrolysis technique | 100 | 385 mAh g−1 after 500 cycles | [25] |
| Microporous Sb/MgF2 | Ball milling and heat treatment | 330 | 551 mAh g−1 after 300 cycles | [33] |
| Yolk-shelled Sb@C | Spray drying and heat treatment | 20,000 | 331 mAh g−1 after 10,000 cycles | [34] |
| Sb/ZnS@C core-shell heterostructure | Hydrothermal and heat treatment | 100 | 554.8 mAh g−1 after 150 cycles | [36] |
| Sb@ porous carbon octahedron | In situ substitution method | 100 | 634.6 mAh g−1 after 200 cycles | [38] |
| Sb2O3@Sb nanoparticles | Spray drying and heating treatment | 10,000 | 245.2 mAh g−1 after 10,000 cycles | [43] |
| Electrospun Sb/C Fibers | Electrospinning method | 100 | 350 mAh g−1 after 300 cycles | [45] |
| Peapod-like Sb@C | Sintering and chemical replacement | 100 | 559 mAh g−1 after 200 cycles | [47] |
| Sb@C coaxial nanotubes | Thermal-reduction | 100 | 407 mAh g−1 after 240 cycles | [48] |
| N-Doped Carbon Nanonecklaces | Electrostatic spinning | 1000 | 401 mAh g−1 after 6000 cycles | [49] |
| Yolk@Shell Sb@Ti-O-P | Chemical synthesis | 500 | 760 mAh g−1 after 200 cycles | [50] |
| Self-Supported Sb Prisms | Electrochemical deposition | 330 | 531 mAh g−1 after 100 cycles | [58] |
| Porous antimonene | Electrochemical exfoliation | 100 | 569.1 mAh g−1 after 200 cycles | [85] |
| Few-Layer Antimonene | Liquid-phase exfoliation | 330 | 620 mAh g−1 after 150th cycle | [86] |
| 3D Porous Sb Foam Anode | Electrodepositing strategy | 300 | 456.5 mAh g−1 after 300 cycles | [91] |
| Double-Walled Sb@TiO2−x Nanotubes | Chemical synthesis and calcination | 2640 | 300 mAh g−1 after 1000 cycles | [96] |
| Sb@TiO2−x nanoplates | Salt-template method | 100 | 568 mAh g−1 after 100 cycles | [97] |
| Sb@C@TiO2 Triple-Shell Nanoboxes | Template method | 1000 | 193 mAh g−1 after 4000 cycles | [99] |
| Polyaniline-coated antimony | In situ oxidative polymerization | 330 | 412.4 mAh g−1 after 250 cycles | [116] |
| Sn-Bi-Sb alloys | Sputtering to get alloy film | 200 | 621 mA h g−1 after 100 cycles | [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, W.; Ren, J.; Feng, W.; Chen, X.; Yan, Y.; Zahir, N. Engineering Nanostructured Antimony-Based Anode Materials for Sodium Ion Batteries. Coatings 2021, 11, 1233. https://doi.org/10.3390/coatings11101233
Luo W, Ren J, Feng W, Chen X, Yan Y, Zahir N. Engineering Nanostructured Antimony-Based Anode Materials for Sodium Ion Batteries. Coatings. 2021; 11(10):1233. https://doi.org/10.3390/coatings11101233
Chicago/Turabian StyleLuo, Wen, Jingke Ren, Wencong Feng, Xingbao Chen, Yinuo Yan, and Noura Zahir. 2021. "Engineering Nanostructured Antimony-Based Anode Materials for Sodium Ion Batteries" Coatings 11, no. 10: 1233. https://doi.org/10.3390/coatings11101233
APA StyleLuo, W., Ren, J., Feng, W., Chen, X., Yan, Y., & Zahir, N. (2021). Engineering Nanostructured Antimony-Based Anode Materials for Sodium Ion Batteries. Coatings, 11(10), 1233. https://doi.org/10.3390/coatings11101233







