Oxygen Barrier Performance of Poly(vinyl alcohol) Coating Films with Different Induced Crystallinity and Model Predictions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVOH Coating Films
2.3. Characterization
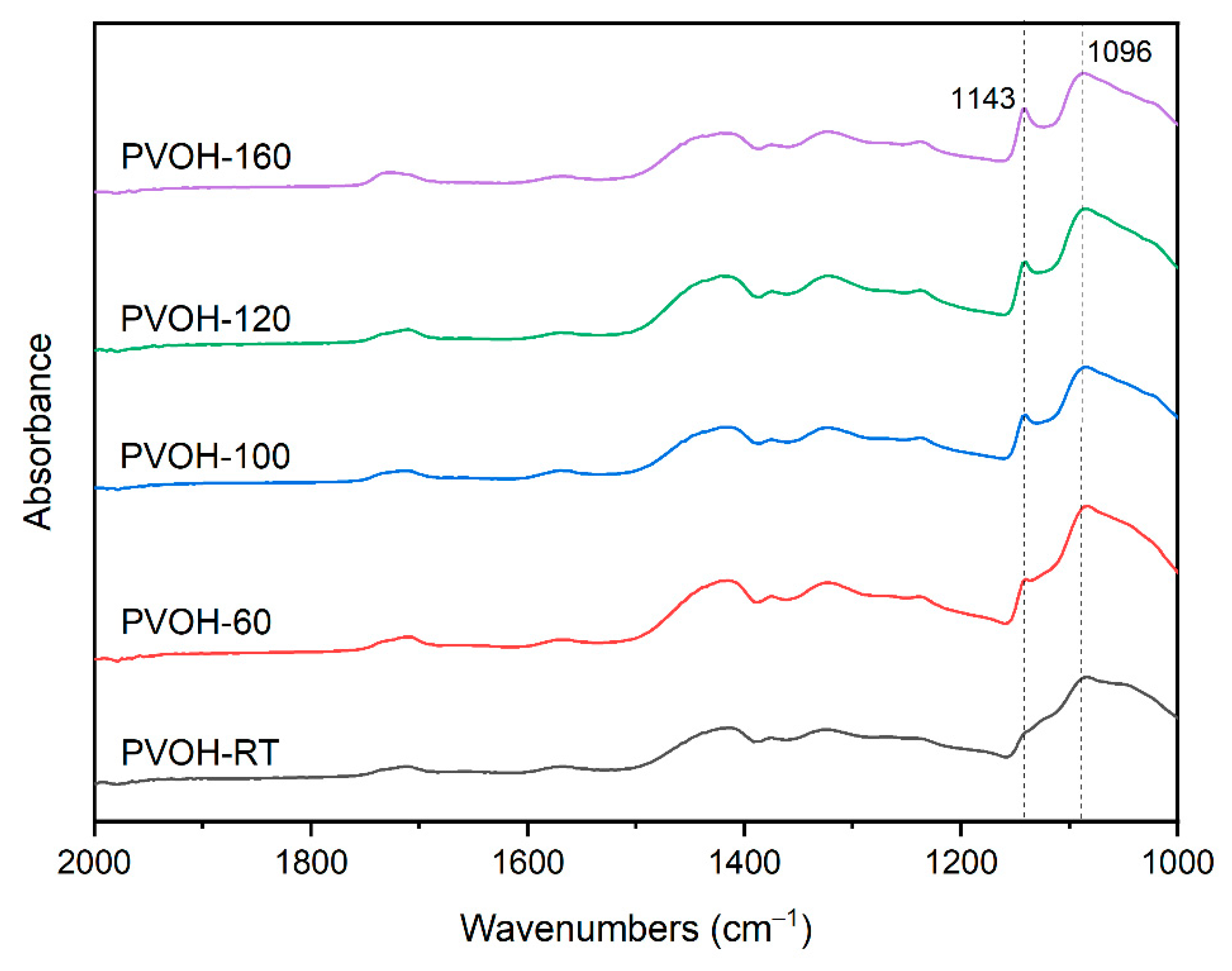
2.3.1. Fourier Transform Infrared Spectroscopy
2.3.2. Differential Scanning Calorimetry
2.3.3. X-ray Diffraction
2.4. Barrier Performance Measurements
2.5. Proposed Models
3. Results and Discussion
3.1. Degree of Crystallinity of PVOH
3.2. Barrier Performance
3.3. Model Predictions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben Halima, N. Poly(vinyl alcohol): Review of its promising applications and insights into biodegradation. RSC Adv. 2016, 6, 39823–39832. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.; Mohamad, A.B.; Al-Amiery, A.A. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, J.G. Poly (Vinyl Alcohol): Basic Properties and Uses; Macdonald: New York, NY, USA, 1970. [Google Scholar]
- Musetti, A.; Paderni, K.; Fabbri, P.; Pulvirenti, A.; Al-Moghazy, M.; Fava, P. Poly(vinyl alcohol)-Based Film Potentially Suitable for Antimicrobial Packaging Applications. J. Food Sci. 2014, 79, E577–E582. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Teleky, B.-E.; Mitrea, L.; Călinoiu, L.-F.; Martău, G.-A.; Simon, E.; Varvara, R.-A.; Vodnar, D.C. Active Packaging—Poly(Vinyl Alcohol) Films Enriched with Tomato By-Products Extract. Coatings 2020, 10, 141. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Development of ultrasound treated polyvinyl alcohol/tea polyphenol composite films and their physicochemical properties. Ultrason. Sonochem. 2019, 51, 386–394. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Guo, X.; Wang, H.; Qian, F.; Lv, Y. Active Biodegradable Polyvinyl Alcohol–Hemicellulose/Tea Polyphenol Films with Excellent Moisture Resistance Prepared via Ultrasound Assistance for Food Packaging. Coatings 2021, 11, 219. [Google Scholar] [CrossRef]
- Toda, A. Small angle X-ray scattering from finite sequence of lamellar stacks of crystalline polymers. Polymer 2020, 211, 123110. [Google Scholar] [CrossRef]
- Mallapragada, S.K.; Narasimhan, B. Infrared Spectroscopy in Analysis of Polymer Crystallinity. In Encyclopedia of Analytical Chemistry; Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 1–14. [Google Scholar] [CrossRef]
- Hagemann, H.; Snyder, R.G.; Peacock, A.J.; Mandelkern, L. Quantitative Infrared Methods for the Measurement of Crystallinity and its Temperature Dependence- Polyethylene. Macromolecules 1989, 22, 3600–3606. [Google Scholar] [CrossRef]
- Desmaisons, J.; Rueff, M.; Bras, J.; Dufresne, A. Impregnation of paper with cellulose nanocrystal reinforced polyvinyl alcohol: Synergistic effect of infrared drying and CNC content on crystallinity. Soft Matter 2018, 14, 9425–9435. [Google Scholar] [CrossRef]
- Kong, Y.; Hay, J.N. The measurement of the crystallinity of polymers by DSC. Polymers 2002, 43, 3873–3878. [Google Scholar] [CrossRef]
- Shen, X.; Hu, W.; Russell, T.P. Measuring the Degree of Crystallinity in Semicrystalline Regioregular Poly(3-hexylthiophene). Macromolecules 2016, 49, 4501–4509. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Weeks, J.J. Specific Volume and Degree of Crystallinity of Semicrystalline Poly (chlorotrifluoroethylene), and Estimated Specific Volumes of the Pure Amorphous and Crystalline Phases. J. Res. Natl. Bur. Stand. 1958, 6, 465–479. [Google Scholar] [CrossRef]
- Peterson, J.; TenCate, J.; Proffen, T.; Darling, T.; Nakotte, H.; Page, K. Quantifying amorphous and crystalline phase content with the atomic pair distribution function. J. Appl. Crystallogr. 2013, 46, 332–336. [Google Scholar] [CrossRef]
- Tashiro, K.; Kitai, H.; Saharin, S.M.; Shimazu, A.; Itou, T. Quantitative Crystal Structure Analysis of Poly(vinyl Alcohol)–Iodine Complexes on the Basis of 2D X-ray Diffraction, Raman Spectra, and Computer Simulation Techniques. Macromolecules 2015, 48, 2138–2148. [Google Scholar] [CrossRef]
- Tanaka, M.; Young, R.J. Molecular Orientation Distributions in the Crystalline and Amorphous Regions of Uniaxially Oriented Isotactic Polypropylene Films Determined by Polarized Raman Spectroscopy. J. Macromol. Sci. Part B 2007, 44, 967–991. [Google Scholar] [CrossRef]
- Grunlan, J.C.; Grigorian, A.; Hamilton, C.B.; Mehrabi, A.R. Effect of clay concentration on the oxygen permeability and optical properties of a modified poly(vinyl alcohol). J. Appl. Polym. Sci. 2004, 93, 1102–1109. [Google Scholar] [CrossRef]
- Vieth, W.R. Diffusion in and through Polymers: Principles and Applications; Hanser Publishers: New York, NY, USA, 1991. [Google Scholar]
- Picard, E.; Espuche, E.; Fulchiron, R. Effect of an organo-modified montmorillonite on PLA crystallization and gas barrier properties. Appl. Clay Sci. 2011, 53, 58–65. [Google Scholar] [CrossRef]
- Duan, Z.; Thomas, N.L. Water vapour permeability of poly(lactic acid): Crystallinity and the tortuous path model. J. Appl. Phys. 2014, 115, 064903. [Google Scholar] [CrossRef] [Green Version]
- Tretinnikov, O.N.; Zagorskaya, S.A. Determination of the degree of crystallinity of poly(vinyl alcohol) by FTIR spectroscopy. J. Appl. Spectrosc. 2012, 79, 521–526. [Google Scholar] [CrossRef]
- Nyflött, Å.; Moons, E.; Bonnerup, C.; Carlsson, G.; Järnström, L.; Lestelius, M. The influence of clay orientation and crystallinity on oxygen permeation in dispersion barrier coatings. Appl. Clay Sci. 2016, 126, 17–24. [Google Scholar] [CrossRef]
- Carrera, M.C.; Erdmann, E.; Destéfanis, H.A. Barrier Properties and Structural Study of Nanocomposite of HDPE/Montmorillonite Modified with Polyvinylalcohol. J. Chem. 2013, 2013, 679567. [Google Scholar] [CrossRef]
- Mallapragada, S.K.; Peppas, N.A. Dissolution mechanism of semicrystalline poly(vinyl alcohol) in water. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 1339–1346. [Google Scholar] [CrossRef]
- Peppas, N.A.; Merrill, E.W. Differential scanning calorimetry of crystillized PVA hydrogels. J. Appl. Polym. Sci. 1976, 20, 1457–1465. [Google Scholar] [CrossRef]
- Gaidukov, S.; Danilenko, I.; Gaidukova, G. Characterization of Strong and Crystalline Polyvinyl Alcohol/Montmorillonite Films Prepared by Layer-by-Layer Deposition Method. Int. J. Polym. Sci. 2015, 2015, 123469. [Google Scholar] [CrossRef] [Green Version]
- Aziz, S.B.; Marf, A.S.; Dannoun, E.M.A.; Brza, M.A.; Abdullah, R.M. The Study of the Degree of Crystallinity, Electrical Equivalent Circuit, and Dielectric Properties of Polyvinyl Alcohol (PVA)-Based Biopolymer Electrolytes. Polymers 2020, 12, 2184. [Google Scholar] [CrossRef]
- International, A. Standard Test Method for Oxygen Gas Transmission Rate through Plastic Film and Sheeting Using a Coulometric Sensor. In ASTM D3985-05; ASTM: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Introzzi, L.; Blomfeldt, T.O.; Trabattoni, S.; Tavazzi, S.; Santo, N.; Schiraldi, A.; Piergiovanni, L.; Farris, S. Ultrasound-assisted pullulan/montmorillonite bionanocomposite coating with high oxygen barrier properties. Langmuir 2012, 28, 11206–11214. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Freeman, B.D. Gas solubility, diffusivity and permeability in poly(ethylene oxide). J. Membr. Sci. 2004, 239, 105–117. [Google Scholar] [CrossRef]
- Nielsen, L.E. Models for the Permeability of Filled Polymer Systems. J. Macromol. Sci. Part A Chem. 1967, 1, 929–942. [Google Scholar] [CrossRef]
- Zid, S.; Zinet, M.; Espuche, E. Modeling diffusion mass transport in multiphase polymer systems for gas barrier applications: A review. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 621–639. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, Z.W.; Dong, Y.; Han, N.; Liu, S. Water and gas barrier properties of polyvinyl alcohol (PVA)/starch (ST)/glycerol (GL)/halloysite nanotube (HNT) bionanocomposite films: Experimental characterisation and modelling approach. Compos. Part B Eng. 2019, 174, 107033. [Google Scholar] [CrossRef]
- Idris, A.; Man, Z.; Maulud, A.S.; Uddin, F. Modified Bruggeman models for prediction of CO2 permeance in polycarbonate/silica nanocomposite membranes. Can. J. Chem. Eng. 2017, 95, 2398–2409. [Google Scholar] [CrossRef]
- Kenney, J.F.; Willcockson, G.W. Structure-Property relationships of poly(vinyl alcohol). III. Relationships between stereo-regularity, crystallinity, and water resistance in poly(vinyl alcohol). J. Appl. Polym. Sci. A-1 Polym. Chem. 1966, 4, 679–698. [Google Scholar] [CrossRef]
- Peppas, N.A. Infrared Spectroscopy of Semicrystalline Poly(vinyl alcohol) Networks. Makromol. Chem 1977, 178, 595–601. [Google Scholar] [CrossRef]
- Minus, M.L.; Chae, H.G.; Kumar, S. Single wall carbon nanotube templated oriented crystallization of poly(vinyl alcohol). Polymer 2006, 47, 3705–3710. [Google Scholar] [CrossRef]




| S. No. | Sample Name | Annealing Temperature (°C) | Annealing Time (min) |
|---|---|---|---|
| 1 | PVOH-RT | 25 | 10 |
| 2 | PVOH-60 | 60 | 10 |
| 3 | PVOH-100 | 100 | 10 |
| 4 | PVOH-120 | 120 | 10 |
| 5 | PVOH-160 | 160 | 10 |
| S. No | Sample Names | Crystallinity | ||
|---|---|---|---|---|
| 1 | PVOH-RT | 0.31 | 0.31 | 0.32 |
| 2 | PVOH-60 | 0.37 | 0.35 | 0.34 |
| 3 | PVOH-100 | 0.45 | 0.41 | 0.40 |
| 4 | PVOH-120 | 0.47 | 0.45 | 0.45 |
| 5 | PVOH-160 | 0.54 | 0.52 | 0.56 |
| S. No. | Sample Names | OTR (cc/[m2·day]) | Thickness (μm) | Oxygen Permeability (cc·μm/[m2·h·atm]) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | Average | CI | |||
| 1 | PET Sub. | 15.90 | 15.78 | 118.0 | 78.15 | 77.58 | 77.87 | - |
| 2 | PVOH-RT | 1.88 | 1.58 | 128.2 | 10.03 | 8.44 | 9.24 | 5.00 |
| 3 | PVOH-60 | 1.42 | 1.25 | 128.1 | 7.56 | 6.68 | 7.12 | 2.78 |
| 4 | PVOH-100 | 1.08 | 1.18 | 127.8 | 5.75 | 6.25 | 6.00 | 1.58 |
| 5 | PVOH-120 | 0.90 | 0.71 | 128.0 | 4.79 | 3.81 | 4.30 | 3.11 |
| 6 | PVOH-160 | 0.21 | 0.47 | 127.9 | 1.12 | 2.51 | 1.81 | 4.41 |
| S. No. | Crystallinity Data Source | Aspect Ratio (α = L/W) | AARE (%) | Crystallites Distribution |
|---|---|---|---|---|
| 1 | FTIR Data | 3.502 | 5.18 | Orderly |
| 2 | DSC Data | 3.350 | 4.61 | |
| 3 | XRD Data | 3.350 | 5.79 | |
| 4 | FTIR Data | 10.90 | 4.66 | Randomly |
| 5 | DSC Data | 10.21 | 4.45 | |
| 6 | XRD Data | 10.05 | 5.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idris, A.; Muntean, A.; Mesic, B.; Lestelius, M.; Javed, A. Oxygen Barrier Performance of Poly(vinyl alcohol) Coating Films with Different Induced Crystallinity and Model Predictions. Coatings 2021, 11, 1253. https://doi.org/10.3390/coatings11101253
Idris A, Muntean A, Mesic B, Lestelius M, Javed A. Oxygen Barrier Performance of Poly(vinyl alcohol) Coating Films with Different Induced Crystallinity and Model Predictions. Coatings. 2021; 11(10):1253. https://doi.org/10.3390/coatings11101253
Chicago/Turabian StyleIdris, Alamin, Adrian Muntean, Beko Mesic, Magnus Lestelius, and Asif Javed. 2021. "Oxygen Barrier Performance of Poly(vinyl alcohol) Coating Films with Different Induced Crystallinity and Model Predictions" Coatings 11, no. 10: 1253. https://doi.org/10.3390/coatings11101253
APA StyleIdris, A., Muntean, A., Mesic, B., Lestelius, M., & Javed, A. (2021). Oxygen Barrier Performance of Poly(vinyl alcohol) Coating Films with Different Induced Crystallinity and Model Predictions. Coatings, 11(10), 1253. https://doi.org/10.3390/coatings11101253








