Interfacial Microstructure and Corrosion Behaviour of Mild Steel Coated with Alumina Nanoparticles Doped Tin Composite via Direct Tinning Route
Abstract
:1. Introduction
2. Experimental
2.1. Coating Process
2.2. Microstructure Characterization
2.3. Corrosion Resistance Test
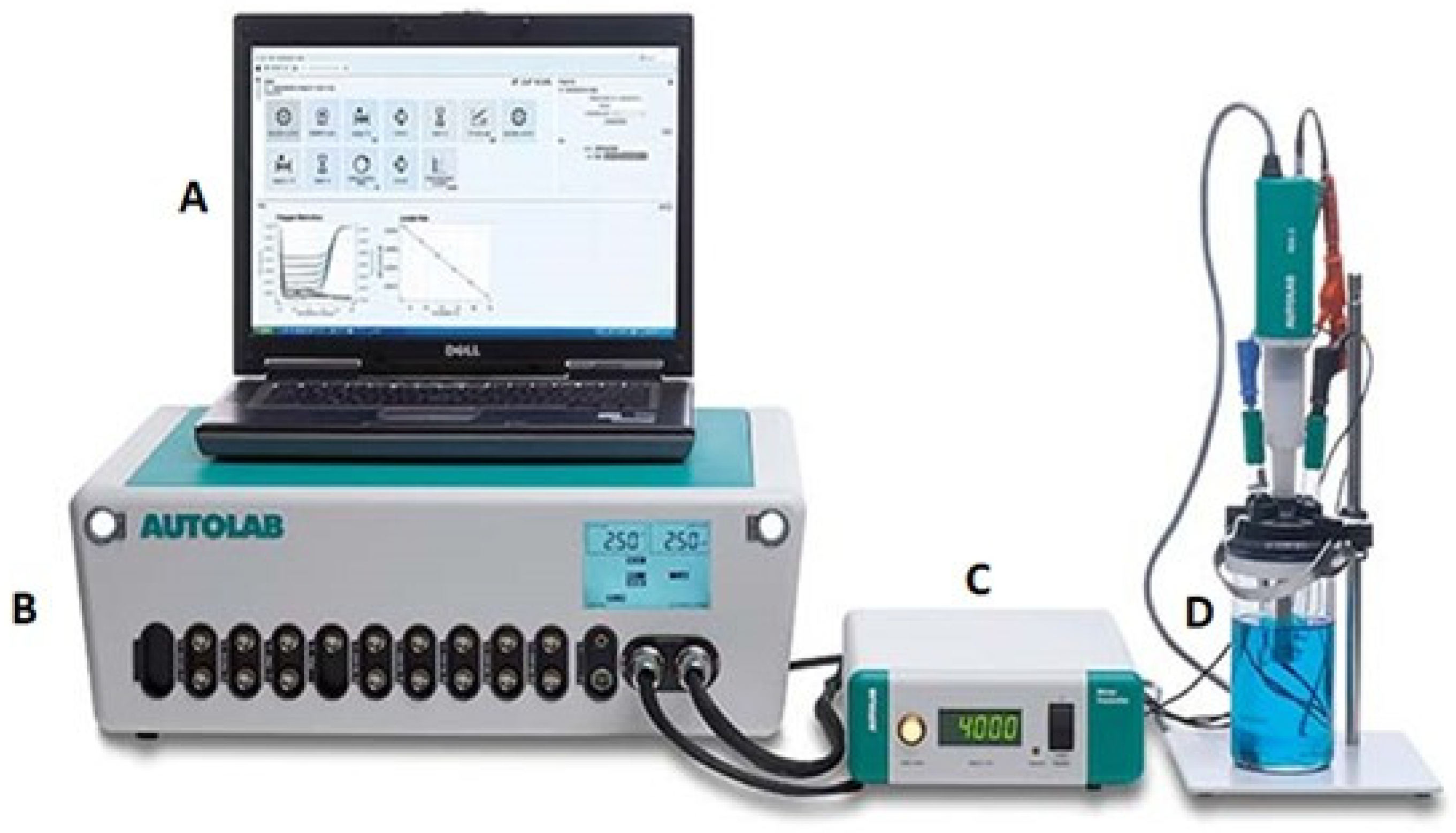
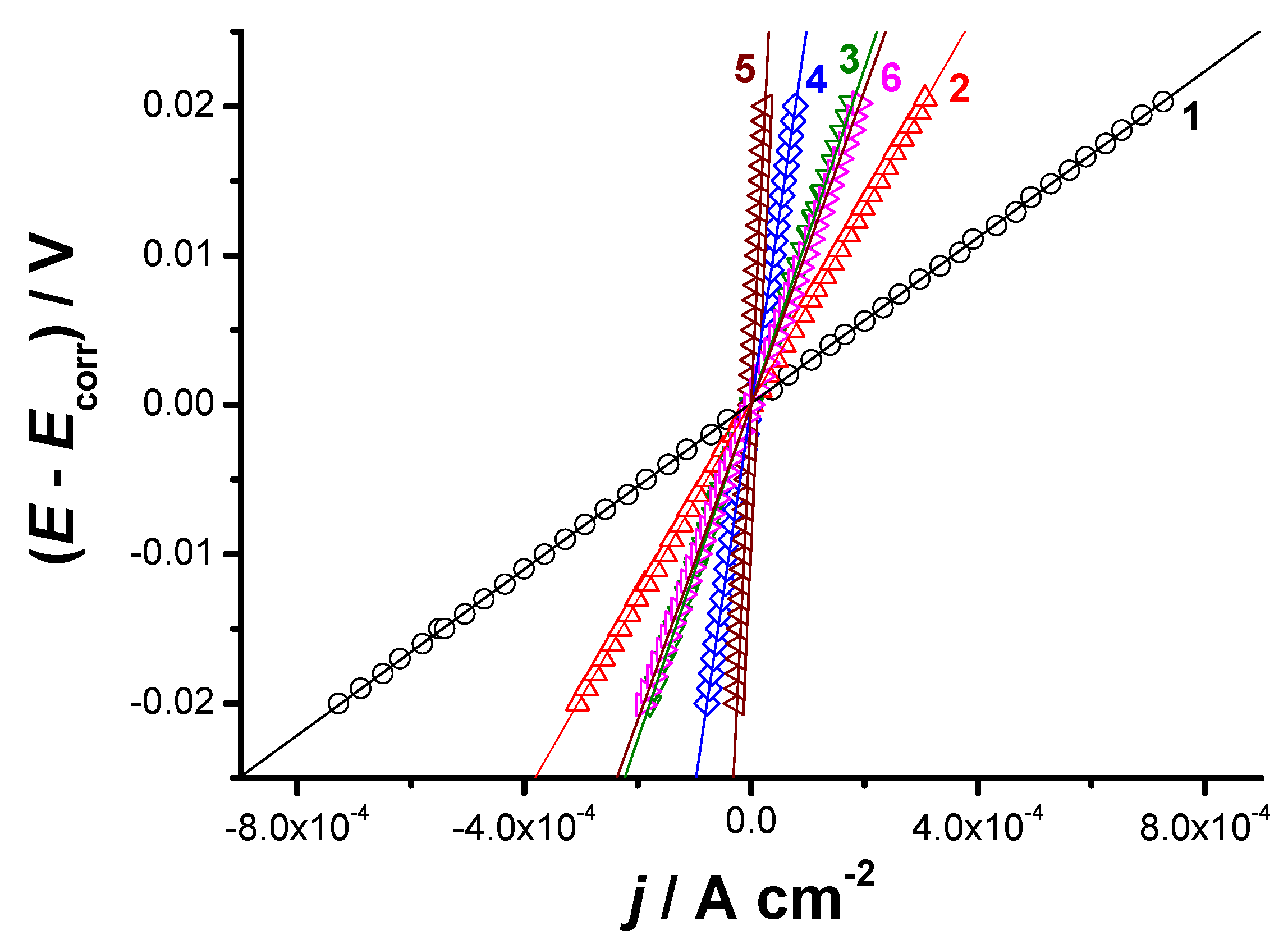
2.3.1. Electrochemical Setup
2.3.2. Uniform Corrosion Studies
3. Results and Discussion
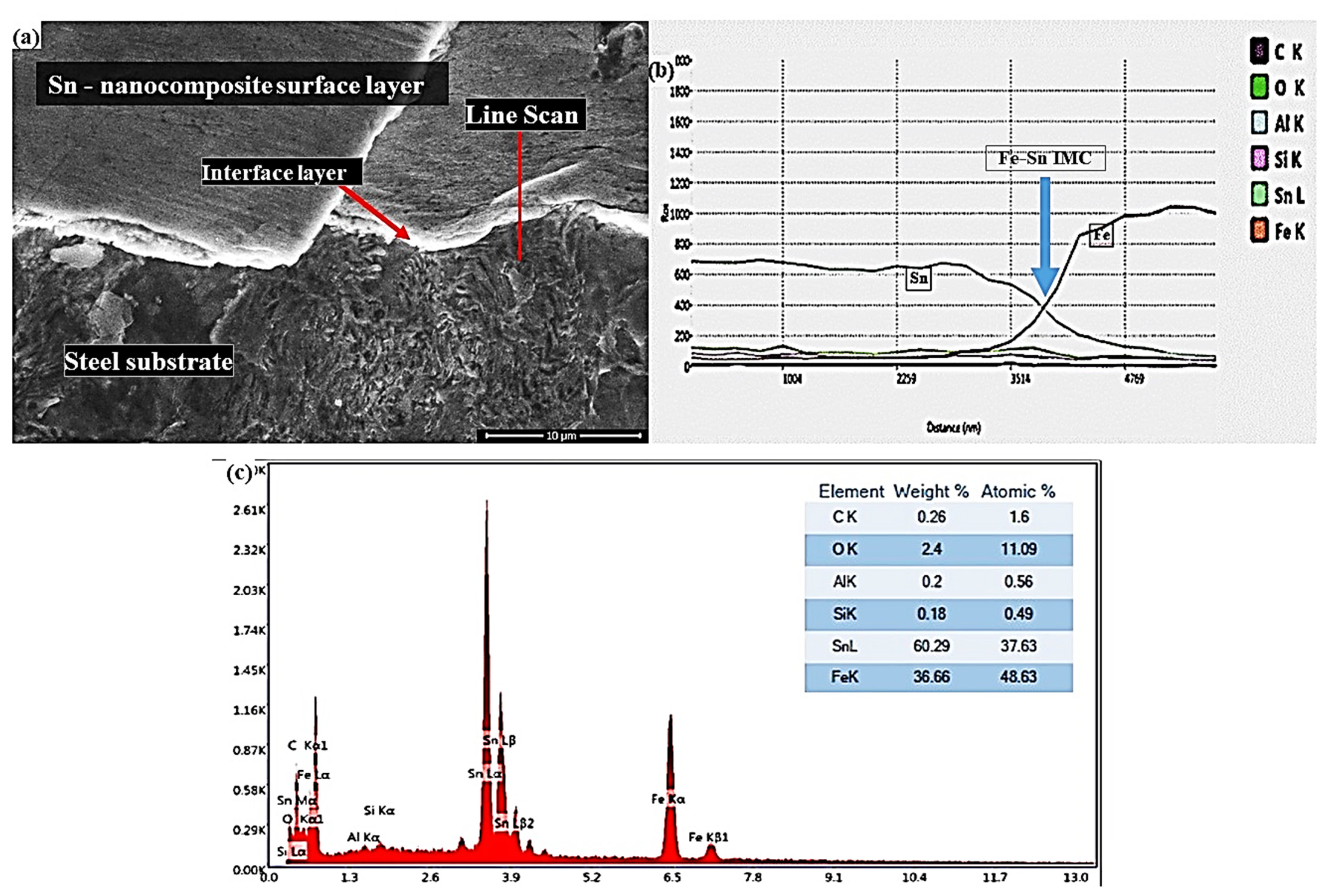
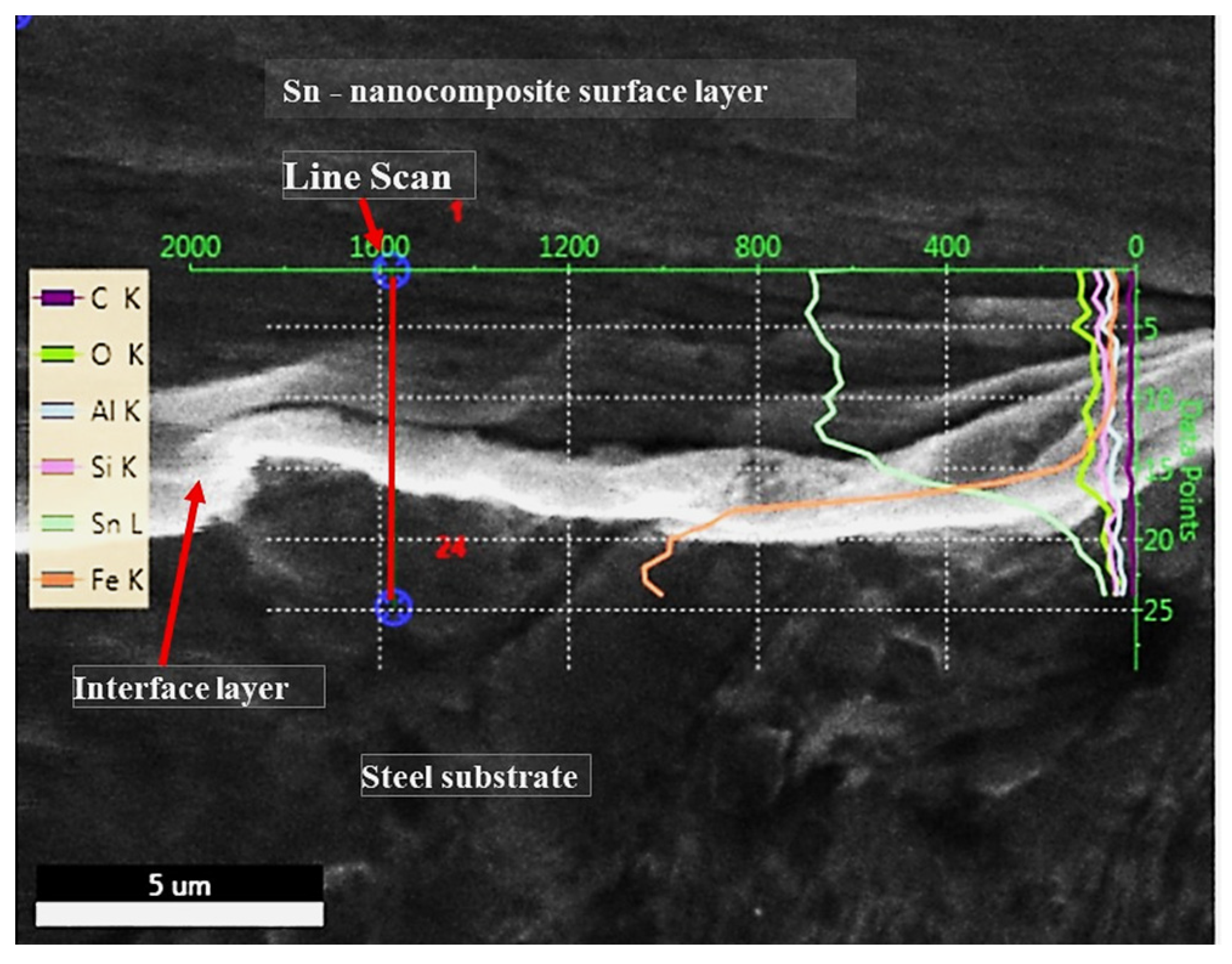
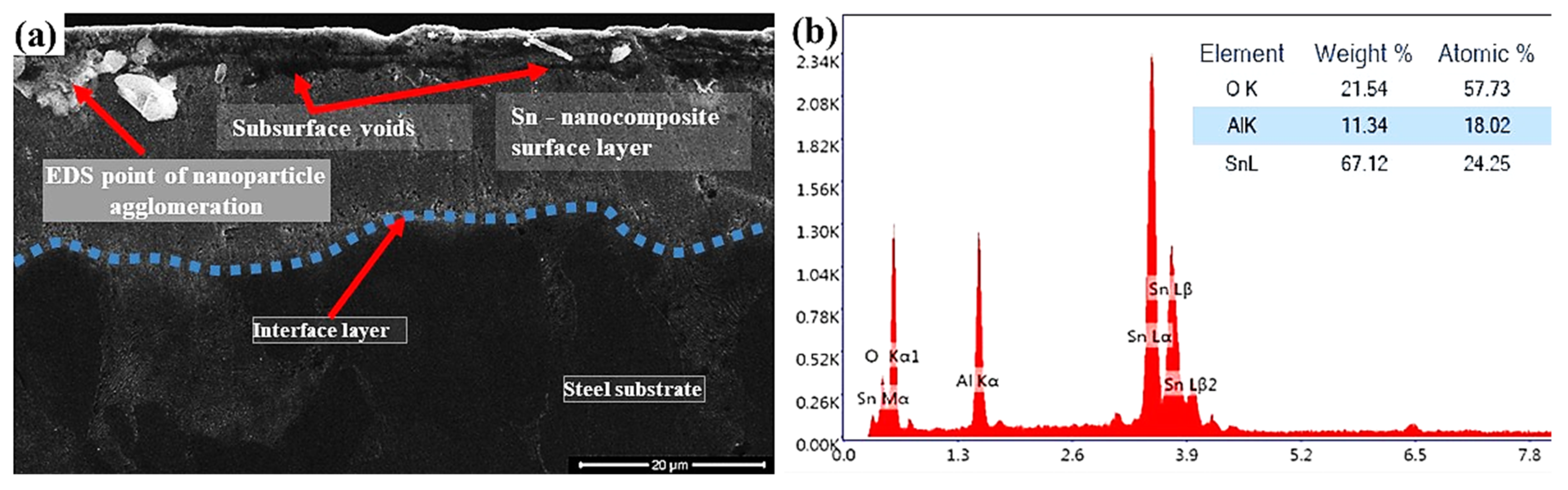
3.1. Microstructural Changes Accompanying Coating Process
3.2. Corrosion Resistance Enhancement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Wang, Y.; Xiao, L.; Zhang, L.; Su, Y.; Lin, J.S. High-temperature oxidation of hot-dip aluminizing coatings on a Ti3Al–Nb alloy nd the effects of element additions. J. Corros. Sci. 2012, 64, 137–144. [Google Scholar] [CrossRef]
- Li, Y.J.; Liang, T.Q.; Ao, R.; Zhao, H.; Chen, X.; Zeng, J. Oxidation resistance of iron-based coatings by supersonic arc spraying at high temperature. J. Surf. Coat. Technol. 2018, 347, 99–112. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, H.; Zhang, Y.; Sun, W.; Zhang, J.M. Effect of Oxide Film Thickness on Electrical and Thermal Conductivity of Micro-arc Oxidize Aluminium Substrates. J. Surf. Technol. 2017, 46, 23–27. [Google Scholar]
- Fan, X.M.; Li, L.; Wen, H.Y.; Liu, S.F.; Li, Z.Q. Study on hot-diffusion aluminzing of cast iron and its high temperature oxidation resistance. Mater. Prot. 2005, 38, 48–50. [Google Scholar]
- Bozza, F.; Bolelli, G.; Giolli, C.; Giorgetti, A.; Lusvarghi, L.; Sassatelli, P.; Scrivani, A.; Candeli, A.; Thoma, M. Diffusion mechanisms and microstructure development in pack aluminizing of Ni-based alloys. Surf. Coat. Technol. 2014, 239, 147–159. [Google Scholar] [CrossRef]
- Amaya, A.; Piamba, O.; Olaya, J. Improvement of Corrosion Resistance for Gray Cast Iron in Palm Biodiesel Application Using Thermoreactive Diffusion Niobium Carbide (NbC) Coating. J. Coat. 2018, 8, 216. [Google Scholar] [CrossRef] [Green Version]
- Amaya, A.; Piamba, O.; Olaya Florez, J.J. Vanadium carbide coatings produced on gray cast iron using the thermo-reactive deposition/diffusion technique. J. Ing. Mec. Tecnol. Desarro. 2015, 5, 333–338. [Google Scholar]
- Arai, T.; Harper, S. ASM Handbook: Steel Heat Treating Fundamentals and Processes; ASM International: Materials Park, OH, USA, 2017; Volume 4A. [Google Scholar]
- Arai, T.; Fujita, H.; Sugimoto, Y.; Ohta, Y. Heat Treatment and Surface Engineering, 1st ed.; ASM International: Metal Park, OH, USA, 1988. [Google Scholar]
- Cheng, W.; Wang, C. Microstructural evolution of intermetallic layer in hot-dipped aluminide mild steel with silicon addition. Surf. Coat. Technol. 2011, 205, 4726–4731. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Tsaur, C.C.; Rock, J.C. Microstructure studies of an aluminide coating on 9Cr-1Mo steel during high temperature oxidation. Surf. Coat. Technol. 2006, 200, 6588. [Google Scholar] [CrossRef]
- Richards, R.W.; Jones, R.D.; Clements, P.D.; Clarke, H. Metallurgy of continuous hot dip aluminizing. Int. Mater. Rev. 1994, 39, 191. [Google Scholar] [CrossRef]
- Wang, C.J.; Chen, S.M. The high-temperature oxidation behavior of hot-dipping Al–Si coating on low carbon steel. Surf. Coat. Technol. 2006, 200, 6601. [Google Scholar] [CrossRef]
- Bosselet, F.; Pontevichi, D.; Sacerdote-Peronnet, M.; Viala, J.C. Affinement expérimental de l’isotherme Al-Fe-Si à 1000 K. J. Phys. IV Fr. 2004, 122, 41. [Google Scholar] [CrossRef] [Green Version]
- Deqing, W.; Ziyuan, S.; Longjiang, Z. A liquid aluminum corrosion resistance surface on steel substrate. Appl. Surf. Sci. 2003, 214, 304. [Google Scholar] [CrossRef]
- Mevrel, R.; Duret, C.; Pichoir, R. Pack cementation processes. Mater. Sci. Technol. 1986, 2, 201–206. [Google Scholar] [CrossRef]
- Xiang, Z.D.; Datta, P.K. Relationship between pack chemistry and aluminide coating formation for low-temperature aluminisation of alloy steels. Acta Mater. 2006, 54, 4453–4463. [Google Scholar] [CrossRef]
- Rohr, V. Development of Novel Protective High Temperature Coatings on Heat Exchanger. Ph.D. Thesis, Karl-Winnacker Institute, Frankfurt, Germany, 2005. [Google Scholar]
- Lou, D.C.; Akselsen, O.M.; Onsqien, M.I.; Solberg, J.K.; Berget, J. Surface modification of steel and cast iron to improve corrosion resistance in molten aluminium. J. Surf. Coat. Technol. 2006, 200, 5282–5288. [Google Scholar] [CrossRef]
- Ramadan, M.; Alghamdi, A.S.; Subhani, T.; Halim, K.S.A. Fabrication and characterization of Sn-based babbitt alloy nanocomposite reinforced with Al2O3 nanoparticles/carbon steel bimetallic material. Materials 2020, 13, 2759. [Google Scholar] [CrossRef]
- Ramadan, M.; Ayadi, B.; Rajhi, W.; Alghamdi, A.S. Influence of Tinning Material on Interfacial Microstructures and Mechanical Properties of Al12Sn4Si1Cu /Carbon Steel Bimetallic Castings for Bearing Applications. Key Eng. Mater. 2020, 835, 108–114. [Google Scholar] [CrossRef]
- Ramadan, M.; Hafez, K.M.; Alghamdi, A.S.; Ayadi, B.; Halim, K.S.A. Novel Approach for Using Ductile Iron as Substrate in Bimetallic Materials for Higher Interfacial Bonding Bearings. Inter. Met. Cast 2021, 11, 145. [Google Scholar] [CrossRef]
- Ramadan, M.; Fathy, N.; Halim, K.S.A.; Alghamdi, A.S. New trends and advances in bi-metal casting technologies. Int. J. Adv. Appl. Sci. 2019, 6, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, M.; Subhani, T.; Rajhi, W.; Ayadi, B.; Al-Ghamdi, A.S. A Novel Technique to Prepare Cast Al-bearing alloy/Wrought Steel Bimetallic Specimen for Interfacial Shear Strength. Int. J. Eng. Adv. Technol. 2020, 9, 2020. [Google Scholar] [CrossRef]
- Ramadan, M.; Alghamdi, A.S.; Hafez, K.M.; Subhani, T.; Halim, K.S.A. Development and Optimization of Tin/Flux Mixture for Direct Tinning and Interfacial Bonding in Aluminum/Steel Bimetallic Compound Casting. Materials 2020, 13, 5642. [Google Scholar] [CrossRef]
- El-Meligi, A.A. Corrosion Preventive Strategies as a Crucial Need for Decreasing Environmental Pollution and Saving Economics. Recent Pat. Corros. Sci. 2010, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Heusler, K.E. Present state and future problems of corrosion science and engineering. Corros. Sci 1990, 31, 753. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, Y.; Zhou, J.; Zhu, W.D. Theoretical and experimental research on the micro interface lubrication regime of water lubricated bearing. Mech. Syst. Signal. Process. 2021, 151, 107422. [Google Scholar] [CrossRef]
- Li, J.S.; Qu, Y.; Hua, H. Numerical analysis of added mass and damping of elastic hydrofoils. J. Hydrodyn. 2020, 32, 1009–1023. [Google Scholar] [CrossRef]
- Xie, Z.; Zhu, W.D. An investigation on the lubrication characteristics of floating ring bearing with consideration of multi-coupling factors. Mech. Syst. Signal. Process. 2022, 162, 108086. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, W.; Ren, L. Controlling the number of vortices and torque in Taylor-Couette flow. J. Fluid Mech. 2020, 901, A30. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Hu, Z.; Zhang, X.; Wu, S.; Wang, R.; Zhu, Y. A novel electrodeposition route for fabrication of the superhydrophobic surface with unique self-cleaning, mechanical abrasion and corrosion resistance properties. Chem. Eng. J. 2016, 303, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Lou, C.; Xu, D.; Lu, X.; Xin, Z.; Zhou, C. Cardanol-based polybenzoxazine superhydrophobic coating with improved corrosion resistance on mild steel. Prog. Org. Coat. 2019, 136, 105191. [Google Scholar] [CrossRef]
- Tikale, S.; Prabhu, K.N. Development of low-silver content SAC0307 solder alloy with Al2O3 nanoparticles. Mater. Sci. Eng. A 2020, 787, 139439. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, N.; Zhang, L.; Yao, W.J. The effects of the minority phase on phase separation in Fe–Sn hypermonotectic alloy. J. Alloys Compd. 2013, 555, 88–94. [Google Scholar] [CrossRef]
- Horvath, B.; Illes, B.; Shinohara, T. Growth of intermetallics between Sn/Ni/Cu, Sn/Ag/Cu and Sn/Cu layered structures. Thin Solid Film. 2014, 556, 345–353. [Google Scholar] [CrossRef]
- Zhong, X.L.; Gupta, M. Development of lead-free Sn–0.7Cu/Al2O3 nanocomposite solders with superior strength. J. Phys. D Appl. Phys. 2008, 41, 095403. [Google Scholar] [CrossRef]
- Haseeb, A.S.M.A.; Arafat, M.M.; Tay, S.L.; Leong, Y.M. Effects of Metallic Nanoparticles on Interfacial Intermetallic Compounds in Tin-Based Solders for Microelectronic Packaging. J. Electron. Mater. 2017, 46, 10. [Google Scholar] [CrossRef]
- Zhou, M. Exceptional Properties by Design. Science 2013, 339, 1161–1162. [Google Scholar] [CrossRef]
- Tjong, S.C. Novel nanoparticle-reinforced metal matrix composites with enhanced mechanical properties. Adv. Eng. Mater. 2007, 9, 639–652. [Google Scholar] [CrossRef]
- Ferguson, J.B.; Sheykh-Jaberi, F.; Kim, C.S.; Rohatgi, P.K.; Cho, K. On the strength and strain to failure in particle-reinforced magnesium metal-matrix nanocomposites (Mg MMNCs). Mater. Sci. Eng. A 2012, 558, 193–204. [Google Scholar] [CrossRef]
- Flitt, H.J.; Schweinsberg, D.P. A guide to polarization curve interpretation: Deconstruction of experimental curves typical of the Fe/H2O/H+/O2 corrosion system. Corros. Sci. 2005, 47, 2125. [Google Scholar] [CrossRef]
- Flitt, H.J.; Schweinsberg, D.P. Evaluation of corrosion rate from polarization curves not exhibiting a Tafel region. Corros. Sci. 2005, 47, 3034. [Google Scholar] [CrossRef]
- Mansfeld, F. Tafel slopes and corrosion rates obtained in the pre-Tafel region of polarization curves. Corros. Sci. 2005, 47, 3178. [Google Scholar] [CrossRef]
- Rosborg, B.; Pan, J.; Leygraf, C. Tafel slopes used in the monitoring of copper corrosion in a bentonite/groundwater environment. Corros. Sci. 2005, 47, 3267. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K.F.; Fadl-Allah, S.A. Testing validity of the Tafel extrapolation method for monitoring corrosion of cold-rolled steel in HCl solutions–Experimental and theoretical studies. Corros. Sci. 2010, 52, 140. [Google Scholar] [CrossRef]
- Metikos-Hukovic, M.; Grubac, Z.; Stupnisek-Lisac, E. Organic corrosion inhibitors for aluminum in perchloric acid. Corrosion 1994, 50, 146. [Google Scholar] [CrossRef]
- Li, W.; Cochell, T.; Manthiram, A. Activation of aluminum as an effective reducing agent by pitting corrosion for wet-chemical analysis. Sci. Rep. 2013, 3, 145. [Google Scholar]
- Vigh, A.K. Oxide Films: Influence of Solid-State Properties on Electrochemical Behavior. In Oxide and Oxide Films; Marcel Dekker, Inc.: New York, NY, USA, 1972; pp. 1–92. [Google Scholar]
- Mansfeld, F. Recording and analysis of AC impedance data for corrosion studies. Corrosion 1981, 37, 301. [Google Scholar] [CrossRef]
- ASTM. Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements (G 102-89). In ASTM Book of Standards; ASTM: West Conshohocken, PA, USA, 1994. [Google Scholar]
- Stern, M.; Geary, A.L. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Hafez, K.M. The effect of welding atmosphere on the pitting corrosion of AISI 304L resistance spot welds. Int. J. Adv. Manuf. Technol. 2020, 97, 243–251. [Google Scholar] [CrossRef]
- Ramadan, M.; Hafez, K.M.; Halim, K.S.A.; Fathy, N.; Chiba, T.; Sato, H.; Watanabe, Y. Influence of Heat Treatment on Interface Structure of Stainless Steel /Gray Iron Bimetallic layered Castings. Appl. Mech. Mater. 2017, 873, 3–8. [Google Scholar] [CrossRef]
- Fathy, N.; Ramadan, M.; Hafez, K.M.; Alghamdi, A.S.; Halim, K.S.A. Microstructure and Induced Defects of 6061 Al Alloy after Short Times Cyclic Semi-solid Heat Treatment. MATEC Web Conf. 2016, 67, 05012. [Google Scholar] [CrossRef] [Green Version]






| Chemical Composition | C | Si | Mn | Cu | Cr | Ni | Fe |
|---|---|---|---|---|---|---|---|
| Steel substrate | 0.14 | 0.30 | 0.41 | 0.20 | 0.14 | 0.09 | Bal. |
| Tested Sample | Tafel Extrapolation Method | LPR Method | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ecorr/ mV (SCE) | jcorr/ mA cm−2 | βc/ mV dec−1 | βa/ mV dec−1 | υ/ mm/yr | I (%) | jcorr/ mA cm−2 | Rp/ Ω cm2 | υ/ mm/yr | I (%) | |
| S0 | −1004 (11) | 1.51 (0.03) | −330 (7) | 282 (5.2) | 18.1 (0.22) | - | 1.44 (0.02) | 40.97 (1.1) | 17.3 (0.2) | - |
| S1 | −1008 (9) | 1.072 (0.02) | −366 (8) | 727 (10) | 12.9 (0.15) | 29.0 (0.35) | 0.97 (0.018) | 109.1 (2.1) | 11.6 (0.2) | 32.6 (0.4) |
| S2 | −1050 (13) | 0.29 (0.005) | −207 (4) | 173 (3.2) | 3.5 (0.06) | 80.8 (1.15) | 0.25 (0.004) | 162.8 (3) | 3.0 (0.05) | 82.6 (1.2) |
| S3 | −1060 (12) | 0.16 (0.003) | −190 (3.6) | 260 (5) | 1.9 (0.03) | 89.4 (1.3) | 0.14 (0.002) | 340.7 (6.4) | 1.7 (0.04) | 90.3 (1.5) |
| S4 | −1020 (11) | 0.076 (0.002) | −243 (4.2) | 284 (4.7) | 0.9 (0.02) | 95.0 (1.5) | 0.068 (0.0022) | 831.5 (11) | 0.8 (0.01) | 95.3 (1.3) |
| S5 | −960 (8) | 0.45 (0.01) | −250 (4.8) | 281 (5.3) | 5.4 (0.08) | 70.2 (1.1) | 0.40 (0.009) | 143.4 (2.8) | 4.8 (0.07) | 72.2 (0.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, A.S.; Abdel Halim, K.S.; Amin, M.A.; Alshammari, A.S.; Fathy, N.; Ramadan, M. Interfacial Microstructure and Corrosion Behaviour of Mild Steel Coated with Alumina Nanoparticles Doped Tin Composite via Direct Tinning Route. Coatings 2021, 11, 1318. https://doi.org/10.3390/coatings11111318
Alghamdi AS, Abdel Halim KS, Amin MA, Alshammari AS, Fathy N, Ramadan M. Interfacial Microstructure and Corrosion Behaviour of Mild Steel Coated with Alumina Nanoparticles Doped Tin Composite via Direct Tinning Route. Coatings. 2021; 11(11):1318. https://doi.org/10.3390/coatings11111318
Chicago/Turabian StyleAlghamdi, Abdulaziz S., K. S. Abdel Halim, Mohammed A. Amin, Abdullah S. Alshammari, Naglaa Fathy, and Mohamed Ramadan. 2021. "Interfacial Microstructure and Corrosion Behaviour of Mild Steel Coated with Alumina Nanoparticles Doped Tin Composite via Direct Tinning Route" Coatings 11, no. 11: 1318. https://doi.org/10.3390/coatings11111318
APA StyleAlghamdi, A. S., Abdel Halim, K. S., Amin, M. A., Alshammari, A. S., Fathy, N., & Ramadan, M. (2021). Interfacial Microstructure and Corrosion Behaviour of Mild Steel Coated with Alumina Nanoparticles Doped Tin Composite via Direct Tinning Route. Coatings, 11(11), 1318. https://doi.org/10.3390/coatings11111318






