Influence of Carbon Nanowalls Interlayer on Copper Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental
2.2. Carbon Nanowalls (CNW) Deposition
2.3. Copper Coatings
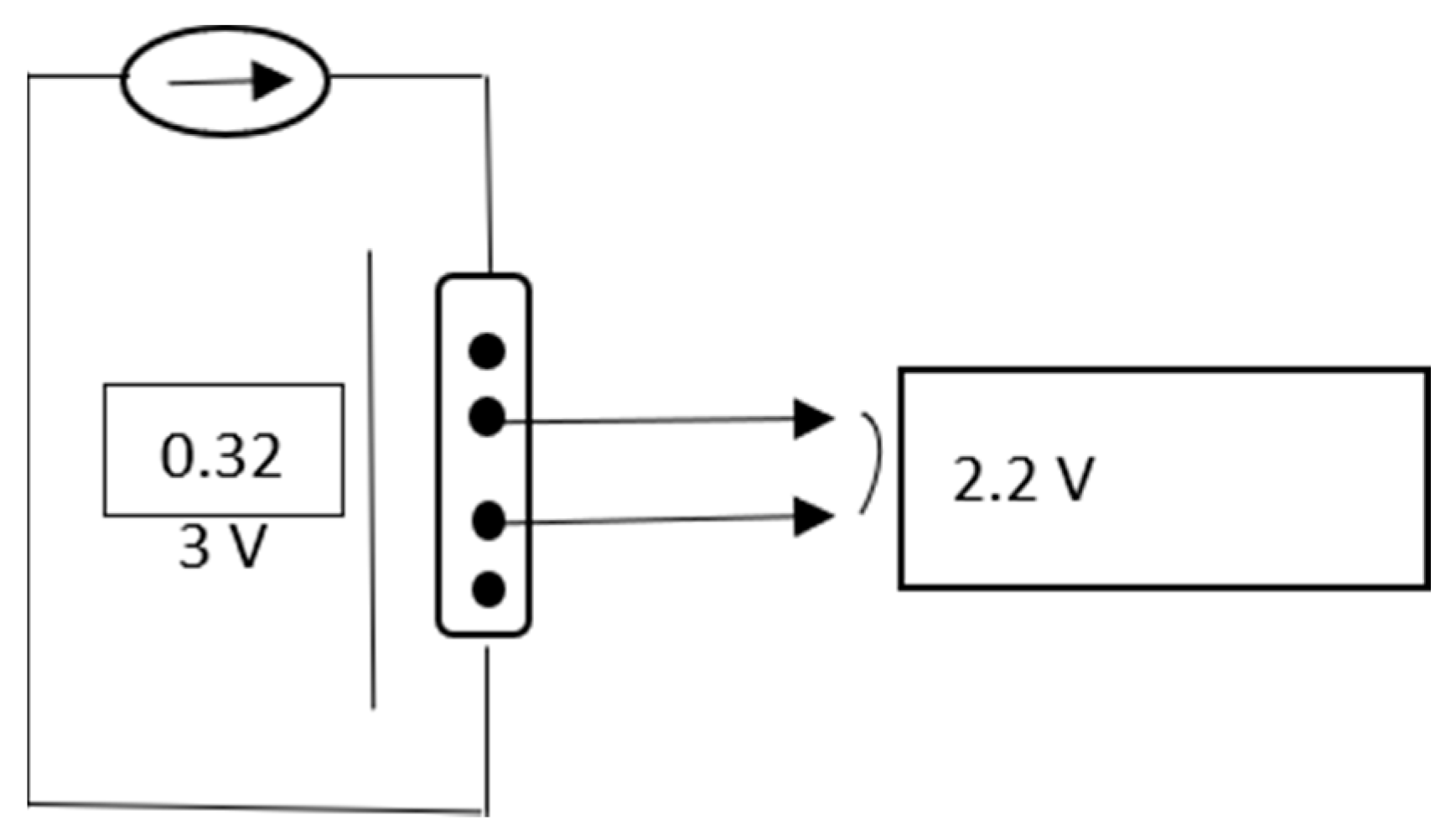
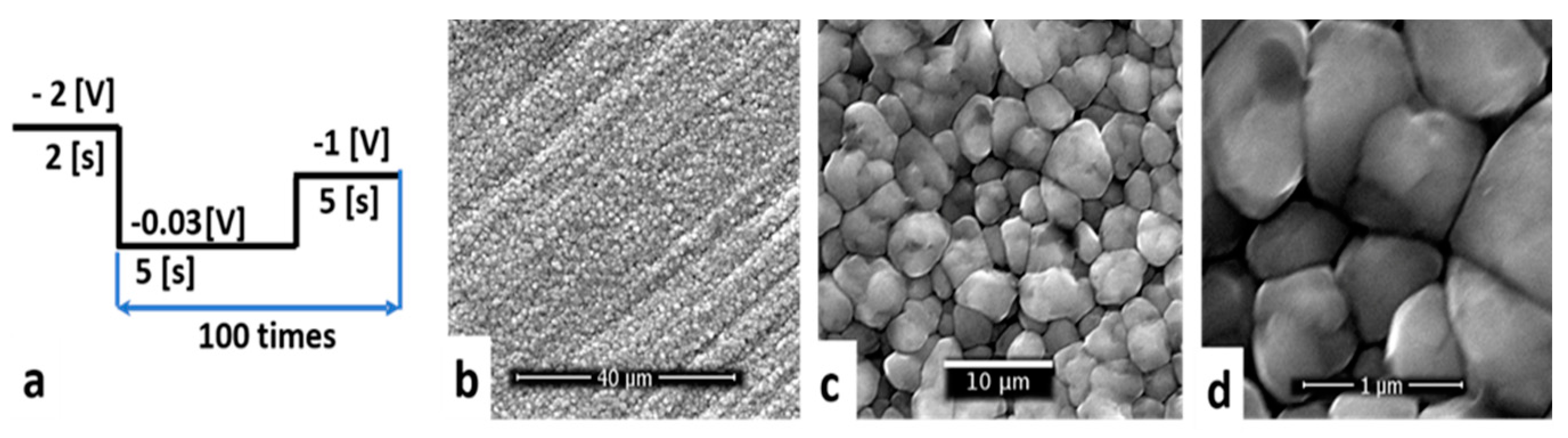
- E1 was −2 V and t1 2 s, to initiate the copper nucleation;
- E2 was the open circuit potential;
- E3 was −1 V and t3 5 s.
2.4. Samples Characterizations
- 5B—the edges of the cuts are completely smooth; none of the squares of the lattice is detached;
- 4B—small flakes of the coating are detached at intersections; less than 5% of the area is affected;
- 3B—small flakes of the coating are detached along the edges and at the intersections of the cuts. The area affected is 5 to 15% of the lattice;
- 2B—the coating has flaked along the edges and on parts of the squares. The area affected is 15 to 35% of the lattice;
- 1B—the coating has flaked along the edges of cuts in large ribbons and whole squares have detached. The area affected is 35 to 65% of the lattice;
- 0B—flaking and detachment is worse than Grade 1.
3. Results and Discussion
3.1. CNW Deposition on K455 Steel
3.2. Copper Electrodeposition
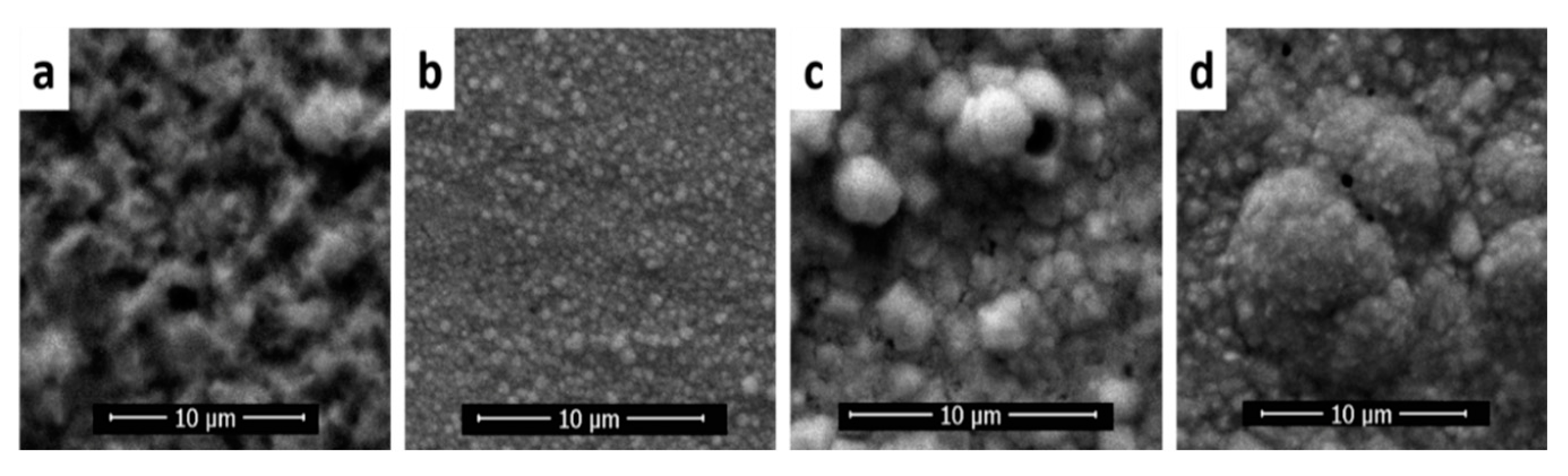
3.2.1. Optimization of Constant Potential Deposition
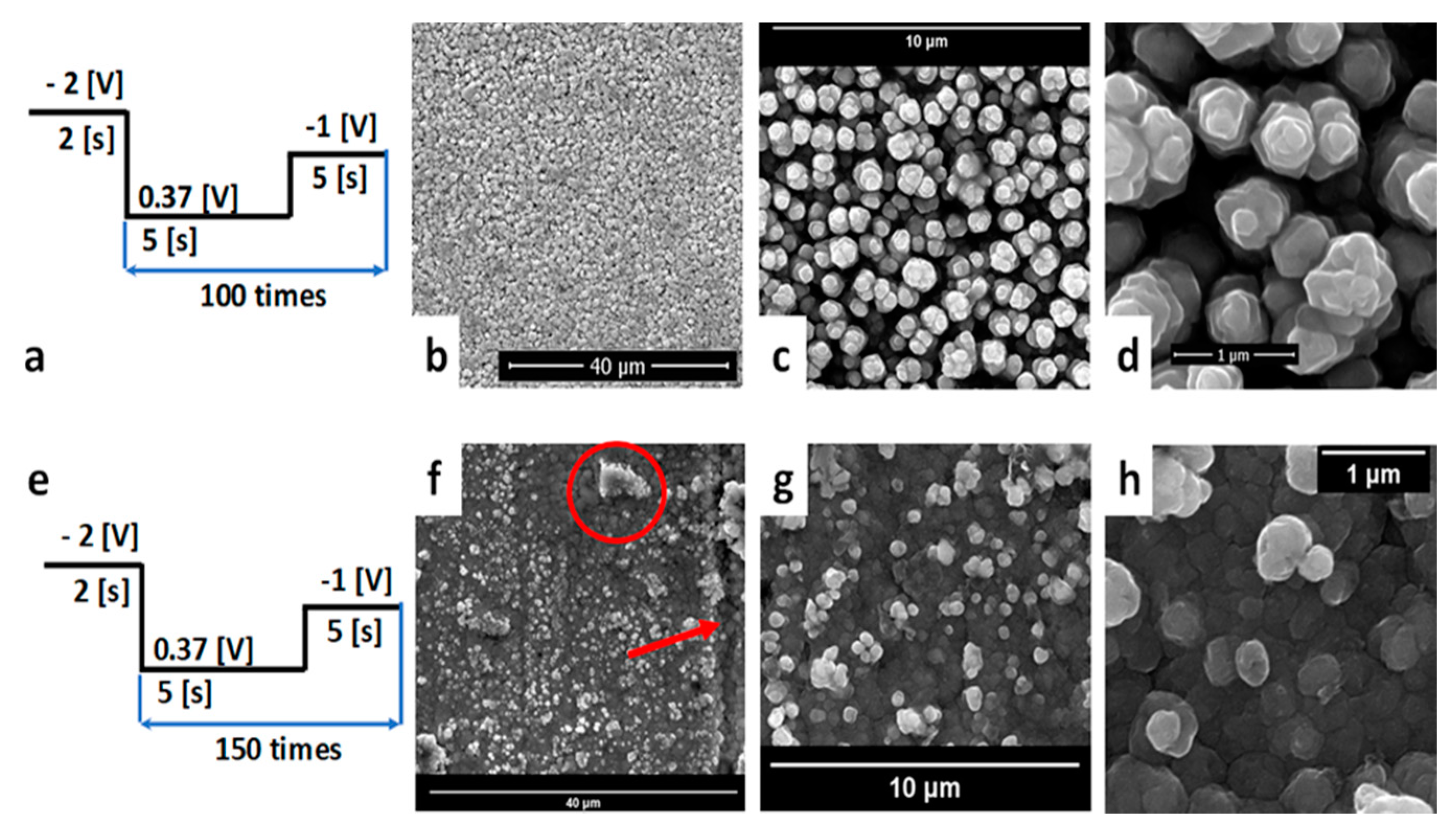
3.2.2. Optimization of Pulsed Electrodeposition Parameters
3.2.3. Copper Electrodeposition on OL/CNW
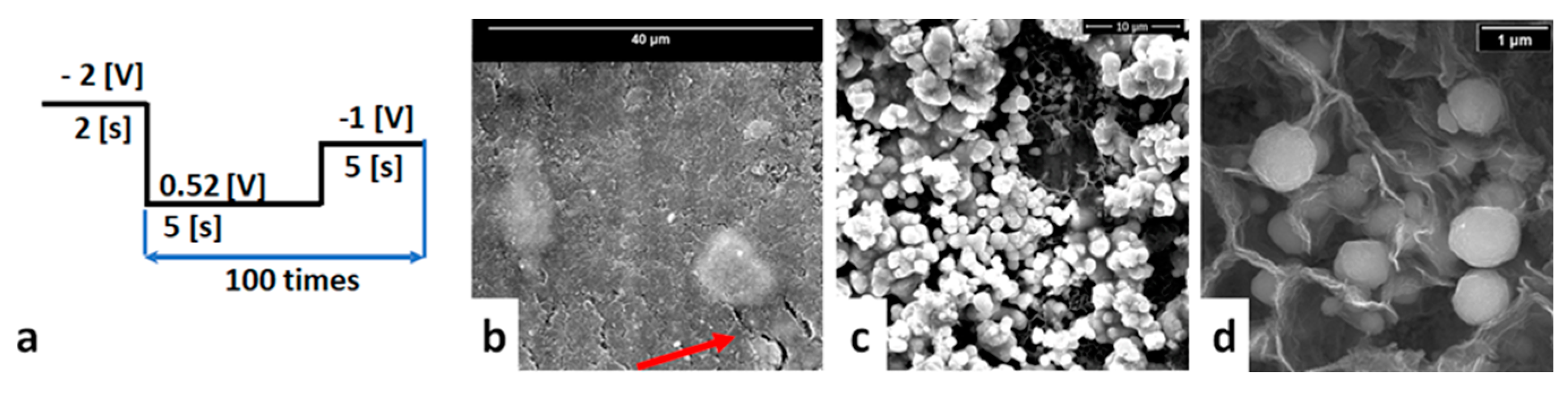
3.3. Copper Deposition by Magnetron Sputtering
3.3.1. Copper Deposition by Magnetron Sputtering on OL
3.3.2. Copper Deposition by Magnetron Sputtering on OL/CNW
3.4. Mechanical Tests—Vickers, Adherence
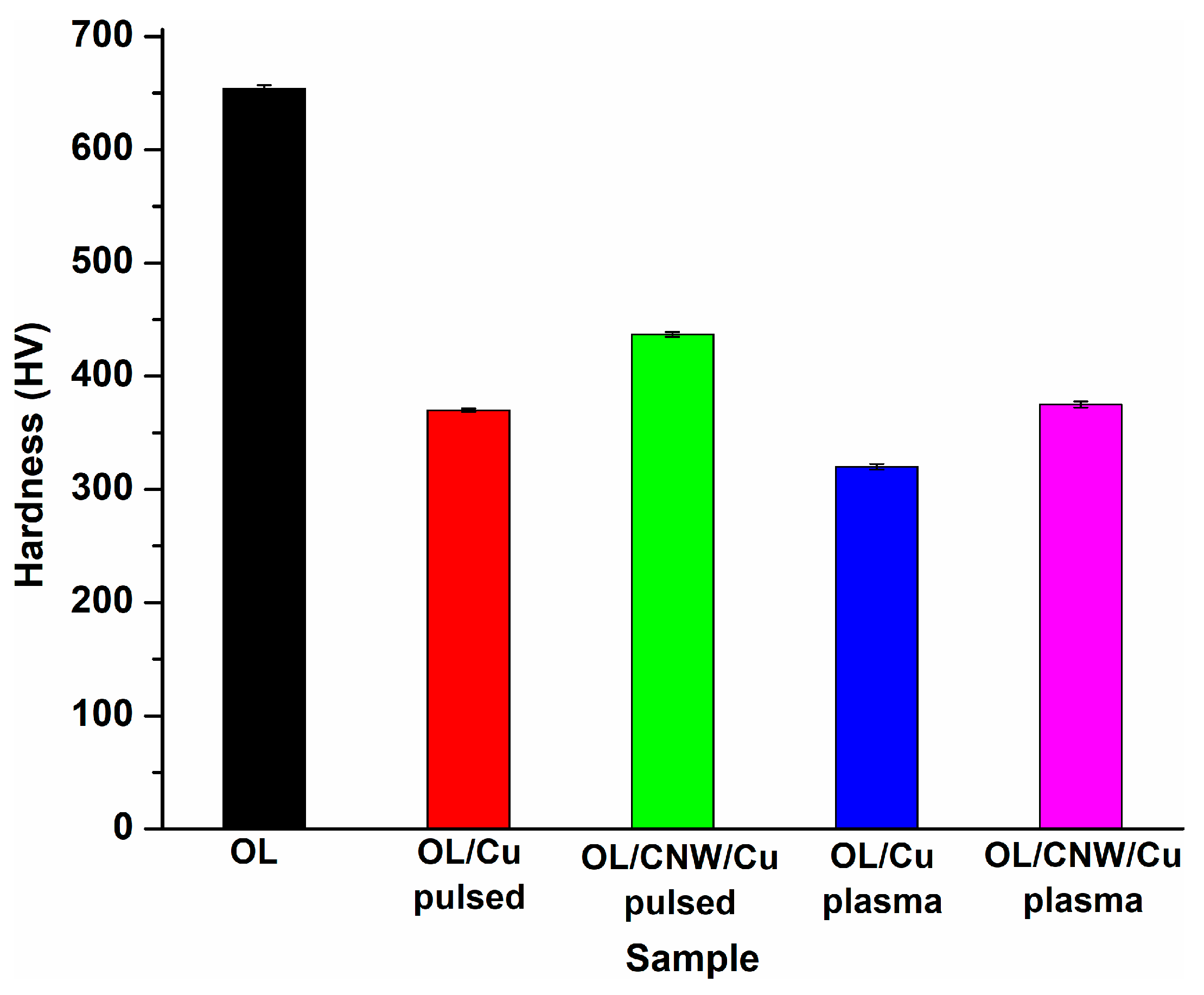
3.4.1. Vickers Hardness Test
3.4.2. Adhesion Test
3.4.3. Electrical Resistivity Evaluation
3.5. Electrochemical Tests
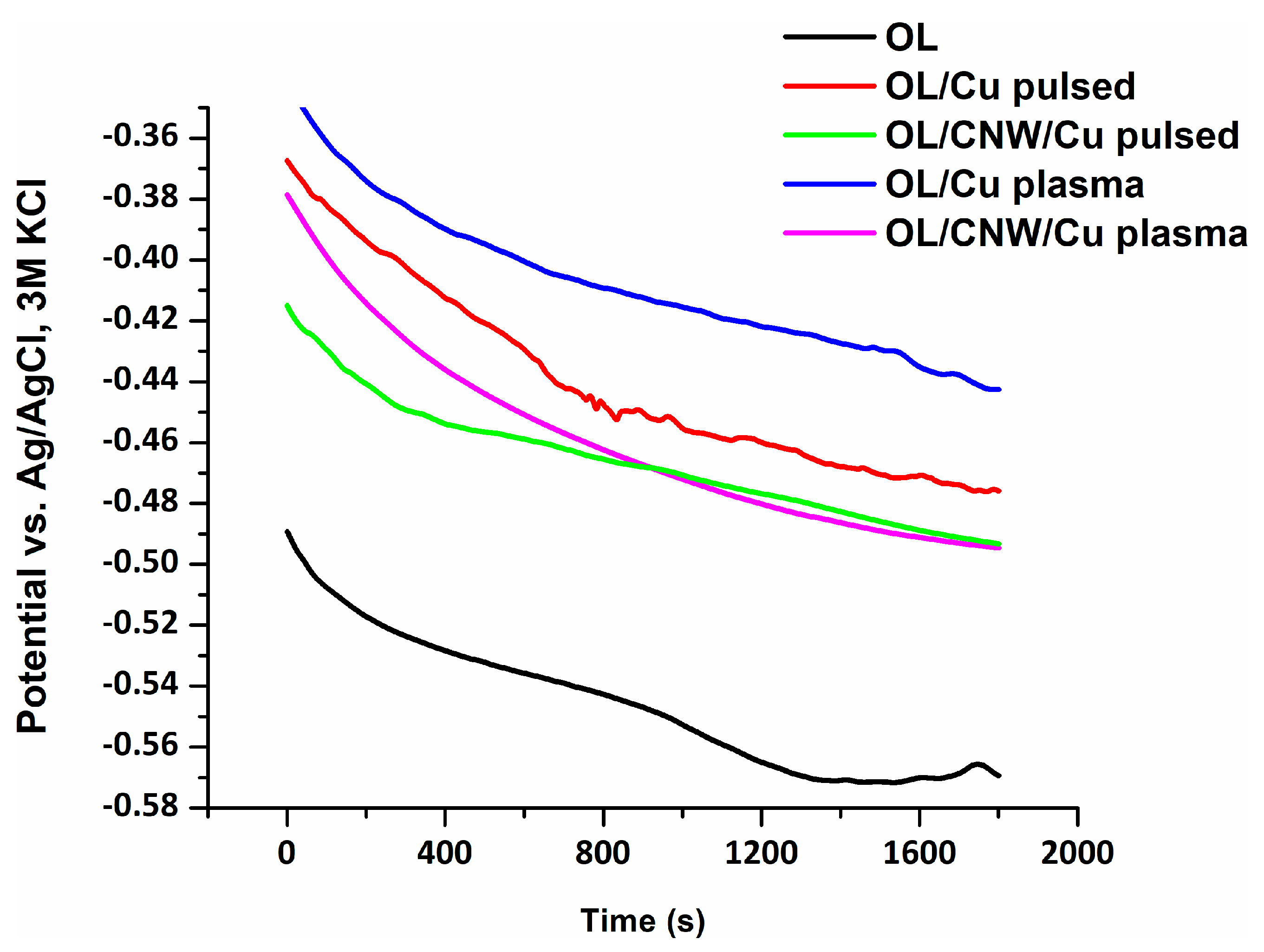
3.5.1. Open Circuit Potential
3.5.2. Tafel Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Çakmakcı Ünver, İ.; Bereket, G.; Duran, B. Corrosion Protection of Stainless Steel by Poly(Carbazole-co-pyrrole) Films Deposited on TiO2 Sol–Gel Film. Polym.-Plast. Technol. Eng. 2018, 57, 242–250. [Google Scholar] [CrossRef]
- Isa, N.N.C.; Mohd, Y.; Mohamad, S.A.S.; Zaki, M.H.M. Antibacterial activity of copper coating electrodeposited on 304 stainless steel substrate. AIP Conf. Proc. 2017, 1901, 020009. [Google Scholar] [CrossRef]
- Yavuz, A.; Kaplan, K.; Bedir, M. Copper oxide coated stainless steel mesh for flexible electrodes. J. Phys. Chem. Solids 2021, 150, 109824. [Google Scholar] [CrossRef]
- Jaramillo-Gutiérrez, M.I.; Sierra-González, S.M.; Ramírez-González, C.A.; Pedraza-Rosas, J.E.; Pedraza-Avella, J.A. Effect of electrodeposition parameters and surface pretreatment on the electrochemical hydrogen production using nickel-plated stainless steel electrodes. Int. J. Hydrog. Energy 2021, 46, 7667–7675. [Google Scholar] [CrossRef]
- Attarzadeh, N.; Molaei, M.; Babaei, K.; Fattah-alhosseini, A. New Promising Ceramic Coatings for Corrosion and Wear Protection of Steels: A Review. Surf. Interfaces 2021, 23, 100997. [Google Scholar] [CrossRef]
- Soufeiani, L.; Foliente, G.; Nguyen, K.T.Q.; San Nicolas, R. Corrosion protection of steel elements in façade systems—A review. J. Build. Eng. 2020, 32, 101759. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface modification of stainless steel for biomedical applications: Revisiting a century-old material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Joy, N.; Sai Venkatesh, B.; Dilip Kumar, S. Microstructure and corrosion behavior of Cr-Cu and Cr-Ag composite coatings for industrial applications. Mater. Today Proc. 2021, 44, 3697–3700. [Google Scholar] [CrossRef]
- Asano, K.; Tsukamoto, M.; Sechi, Y.K.; Sato, Y.; Masuno, S.; Higashino, R.; Hara, T.; Sengoku, M.; Yoshida, M. Laser metal deposition of pure copper on stainless steel with blue and IR diode lasers. Opt. Laser Technol. 2018, 107, 291–296. [Google Scholar] [CrossRef]
- Gupta, D.; Sharma, A.K. Copper coating on austenitic stainless steel using microwave hybrid heating. Proc. Inst. Mech. Eng. Part E J. Process. Mech. Eng. 2011, 226, 132–141. [Google Scholar] [CrossRef]
- Flores, V.; Almanza, R. Behavior of the compound wall copper–steel receiver with stratified two-phase flow regimen in transient states when solar irradiance is arriving on one side of receiver. Sol. Energ. 2004, 76, 195–198. [Google Scholar] [CrossRef]
- Standish, T.; Chen, J.; Jacklin, R.; Jakupi, P.; Ramamurthy, S.; Zagidulin, D.; Keech, P.; Shoesmith, D. Corrosion of Copper-Coated Steel High Level Nuclear Waste Containers Under Permanent Disposal Conditions. Electrochim. Acta 2016, 211, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Standish, T.E.; Zagidulin, D.; Ramamurthy, S.; Keech, P.G.; Noël, J.J.; Shoesmith, D.W. Galvanic corrosion of copper-coated carbon steel for used nuclear fuel containers. Corros. Eng. Sci. Technol. 2017, 52, 65–69. [Google Scholar] [CrossRef]
- Weinstein, M.; Falconer, C.; Doniger, W.; Bailly-Salins, L.; David, R.; Sridharan, K.; Couet, A. Environmental degradation of electroplated nickel and copper coated SS316H in molten FLiNaK salt. Corros. Sci. 2021, 191, 109735. [Google Scholar] [CrossRef]
- Molleda, F.; Mora, J.; Molleda, J.R.; Carrillo, E.; Mora, E.; Mellor, B.G. Copper coating of carbon steel by a furnace brazing process using brass as the braze. Mater. Charact. 2008, 59, 613–617. [Google Scholar] [CrossRef] [Green Version]
- Rivera, L.R.; Cochis, A.; Biser, S.; Canciani, E.; Ferraris, S.; Rimondini, L.; Boccaccini, A.R. Antibacterial, pro-angiogenic and pro-osteointegrative zein-bioactive glass/copper based coatings for implantable stainless steel aimed at bone healing. Bioact. Mater. 2021, 6, 1479–1490. [Google Scholar] [CrossRef]
- Aravindan, N.; Sangaranarayanan, M.V. Influence of solvent composition on the anti-corrosion performance of copper–polypyrrole (Cu–PPy) coated 304 stainless steel. Prog. Org. Coat. 2016, 95, 38–45. [Google Scholar] [CrossRef]
- Lü, X.; Lin, H. Facile fabrication of robust superhydrophobic/superoleophlic Cu coated stainless steel mesh for highly efficient oil/water separation. Sep. Purif. Technol. 2021, 256, 117512. [Google Scholar] [CrossRef]
- Rostami, R.; Moussavi, G.; Darbari, S.; Jafari, A.J. Non-thermal plasma by positive corona glow discharge using nano-structured Cu/CuO coated electrodes for benzene removal from air flow; removal enhancement and energy efficiency improvement. Sep. Purif. Technol. 2021, 275, 119156. [Google Scholar] [CrossRef]
- Zhao, Y.-C.; Tang, J.-C.; Ye, N.; Zhou, W.-W.; Wei, C.-L.; Liu, D.-J. Influence of additives and concentration of WC nanoparticles on properties of WC−Cu composite prepared by electroplating. Trans. Nonferrous Met. Soc. China 2020, 30, 1594–1604. [Google Scholar] [CrossRef]
- Ibrahim, M.; Al-Athel, K.; Arif, A.F.M. Strength and Hardness Assessment of Copper and Copper Alloy Coatings on Stainless Steel Substrates. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Phoenix, AZ, USA, 11–17 November 2016; p. V014T011A019. [Google Scholar]
- Winnicki, M.; Małachowska, A.; Baszczuk, A.; Rutkowska-Gorczyca, M.; Kukla, D.; Lachowicz, M.; Ambroziak, A. Corrosion protection and electrical conductivity of copper coatings deposited by low-pressure cold spraying. Surf. Coat. Technol. 2017, 318, 90–98. [Google Scholar] [CrossRef]
- Deepak, J.R.; Bupesh Raja, V.K.; Kaliaraj, G.S. Mechanical and corrosion behavior of Cu, Cr, Ni and Zn electroplating on corten A588 steel for scope for betterment in ambient construction applications. Results Phys. 2019, 14, 102437. [Google Scholar] [CrossRef]
- Fihri, A.; Bovero, E.; Al-Shahrani, A.; Al-Ghamdi, A.; Alabedi, G. Recent progress in superhydrophobic coatings used for steel protection: A review. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 378–390. [Google Scholar] [CrossRef]
- Ijaola, A.O.; Farayibi, P.K.; Asmatulu, E. Superhydrophobic coatings for steel pipeline protection in oil and gas industries: A comprehensive review. J. Nat. Gas Sci. Eng. 2020, 83, 103544. [Google Scholar] [CrossRef]
- Maizelis, A.A.; Bairachnyi, B.I.; Tul’skii, G.G. Contact Displacement of Copper at Copper Plating of Carbon Steel Parts. Surf. Eng. Appl. Electrochem. 2018, 54, 12–19. [Google Scholar] [CrossRef]
- Lin, C.; Hu, J.; Zhang, J.; Yang, P.; Kong, X.; Han, G.; Li, Q.; An, M. A comparative investigation of the effects of some alcohols on copper electrodeposition from pyrophosphate bath. Surf. Interfaces 2021, 22, 100804. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, R.; Zhan, K.; Bao, L.; Zhang, Y.; Yang, Z.; Yan, Y.; Zhao, B.; Yang, J. Preparation of electro-reduced graphene oxide/copper composite foils with simultaneously enhanced thermal and mechanical properties by DC electro-deposition method. Mater. Sci. Eng. A 2021, 805, 140574. [Google Scholar] [CrossRef]
- Hannula, P.-M.; Peltonen, A.; Aromaa, J.; Janas, D.; Lundström, M.; Wilson, B.P.; Koziol, K.; Forsén, O. Carbon nanotube-copper composites by electrodeposition on carbon nanotube fibers. Carbon 2016, 107, 281–287. [Google Scholar] [CrossRef]
- Zhou, M.; Mai, Y.; Ling, H.; Chen, F.; Lian, W.; Jie, X. Electrodeposition of CNTs/copper composite coatings with enhanced tribological performance from a low concentration CNTs colloidal solution. Mater. Res. Bull. 2018, 97, 537–543. [Google Scholar] [CrossRef]
- Tamayo-Ariztondo, J.; Córdoba, J.M.; Odén, M.; Molina-Aldareguía, J.M.; Elizalde, M.R. Effect of heat treatment of carbon nanofibres on electroless copper deposition. Compos. Sci. Technol. 2010, 70, 2269–2275. [Google Scholar] [CrossRef] [Green Version]
- Barcena, J.; Maudes, J.; Coleto, J.; Baldonedo, J.L.; Gomez de Salazar, J.M. Microstructural study of vapour grown carbon nanofibre/copper composites. Compos. Sci. Technol. 2008, 68, 1384–1391. [Google Scholar] [CrossRef] [Green Version]
- Daoush, W.M.; Lim, B.K.; Mo, C.B.; Nam, D.H.; Hong, S.H. Electrical and mechanical properties of carbon nanotube reinforced copper nanocomposites fabricated by electroless deposition process. Mater. Sci. Eng. A 2009, 513–514, 247–253. [Google Scholar] [CrossRef]
- Hannula, P.-M.; Aromaa, J.; Wilson, B.P.; Janas, D.; Koziol, K.; Forsén, O.; Lundström, M. Observations of copper deposition on functionalized carbon nanotube films. Electrochim. Acta 2017, 232, 495–504. [Google Scholar] [CrossRef]
- Daoush, W.M.; Alkhuraiji, T.S.; Khamis, M.A.; Albogmy, T.S. Microstructure and electrical properties of carbon short fiber reinforced copper composites fabricated by electroless deposition followed by powder metallurgy process. Carbon Lett. 2020, 30, 247–258. [Google Scholar] [CrossRef]
- Bita, B.; Vizireanu, S.; Stoica, D.; Ion, V.; Yehia, S.; Radu, A.; Iftimie, S.; Dinescu, G. On the Structural, Morphological, and Electrical Properties of Carbon Nanowalls Obtained by Plasma-Enhanced Chemical Vapor Deposition. J. Nanomater. 2020, 2020, 8814459. [Google Scholar] [CrossRef]
- Stancu, E.C.; Stanciuc, A.-M.; Vizireanu, S.; Luculescu, C.; Moldovan, L.; Achour, A.; Dinescu, G. Plasma functionalization of carbon nanowalls and its effect on attachment of fibroblast-like cells. J. Phys. D Appl. Phys. 2014, 47, 265203. [Google Scholar] [CrossRef]
- Ion, R.; Vizireanu, S.; Stancu, C.E.; Luculescu, C.; Cimpean, A.; Dinescu, G. Surface plasma functionalization influences macrophage behavior on carbon nanowalls. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 118–125. [Google Scholar] [CrossRef] [PubMed]
- MĂRĂSCU, V.; Vizireanu, S.; Stoica, S.; Barna, V.; Lazeastoyanova, A.; Dinescu, G. FTIR investigation of the ageing process of carbon nanowalls. Rom. Rep. Phys. 2016, 68, 1108–1114. [Google Scholar]
- Davami, K.; Jiang, Y.; Cortes, J.; Lin, C.; Shaygan, M.; Turner, K.T.; Bargatin, I. Tuning the mechanical properties of vertical graphene sheets through atomic layer deposition. Nanotechnology 2016, 27, 155701. [Google Scholar] [CrossRef]
- Cursaru, D.; Giagkas, N.; Vizireanu, S.; Mihai, S.; Matei, D.; BIŢĂ, B.; Stancu, C.; Manta, A.; Ramadan, I. Improvement of Antiwear Properties by Coating the Steel Surfaces and by Lubricant Additivation. Dig. J. Nanomater. Biostruct. 2019, 14, 907–915. [Google Scholar]
- Vizireanu, S.I.; Mitu, B.; Dinescu, G. Nanostructured carbon growth by expanding RF plasma assisted CVD on Ni-coated silicon substrate. Surf. Coat. Technol. 2005, 200, 1132–1136. [Google Scholar] [CrossRef]
- Surdu, L.; Visileanu, E.; Ardeleanu, A.; Mitran, C.; Rădulescu, I.R.; Stancu, C.; Sandulache, I.; Mitu, B. Research regarding the cover factor of magnetron sputtering plasma coated fabrics. Ind. Text. 2019, 70, 154–159. [Google Scholar]
- Imran, M.K.; Masood, S.H.; Brandt, M.; Bhattacharya, S.; Gulizia, S.; Jahedi, M.; Mazumder, J. Thermal fatigue behavior of direct metal deposited H13 tool steel coating on copper alloy substrate. Surf. Coat. Tech. 2012, 206, 2572–2580. [Google Scholar] [CrossRef]
- Krivchenko, V.A.; Itkis, D.M.; Evlashin, S.A.; Semenenko, D.A.; Goodilin, E.A.; Rakhimov, A.T.; Stepanov, A.S.; Suetin, N.V.; Pilevsky, A.A.; Voronin, P.V. Carbon nanowalls decorated with silicon for lithium-ion batteries. Carbon 2012, 50, 1438–1442. [Google Scholar] [CrossRef]
- Stoica, S.D.; Vizireanu, S.; Luculescu, C.R.; Mitu, B.; Dinescu, G. Metastable growth regime for carbon nanowalls and carbon nanofibers in an Ar/H2/C2H2 radiofrequency plasma jet. Plasma Sources Sci. Technol. 2020, 29, 105007. [Google Scholar] [CrossRef]
- Bao, S.; Wei, Q.; Cao, J.; Li, H.; Ma, L.; An, J.; Lin, C.-T.; Luo, J.; Zhou, K. Hydrophilic modification of carbon nanotube to prepare a novel porous copper network-carbon nanotube/erythritol composite phase change material. Compos. Interfaces 2021, 28, 175–189. [Google Scholar] [CrossRef]
- Anijdan, S.H.M.; Sabzi, M.; Zadeh, M.R.; Farzam, M. The effect of electroless bath parameters and heat treatment on the properties of Ni-P and Ni-P-Cu composite coatings. Mater. Res. (Sao Carlos Online) 2018, 21, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hakamada, M.; Nakamoto, Y.; Matsumoto, H.; Iwasaki, H.; Chen, Y.; Kusuda, H.; Mabuchi, M. Relationship between hardness and grain size in electrodeposited copper films. Mater. Sci. Eng. A 2007, 457, 120–126. [Google Scholar] [CrossRef]
- Bigl, S.; Schöberl, T.; Wurster, S.; Cordill, M.J.; Kiener, D. Correlative microstructure and topography informed nanoindentation of copper films. Surf. Coat. Technol. 2016, 308, 404–413. [Google Scholar] [CrossRef]
- Velicu, I.-L.; Ianoş, G.-T.; Porosnicu, C.; Mihăilă, I.; Burducea, I.; Velea, A.; Cristea, D.; Munteanu, D.; Tiron, V. Energy-enhanced deposition of copper thin films by bipolar high power impulse magnetron sputtering. Surf. Coat. Technol. 2019, 359, 97–107. [Google Scholar] [CrossRef]
- Boruah, D.; Robinson, B.; London, T.; Wu, H.; de Villiers-Lovelock, H.; McNutt, P.; Doré, M.; Zhang, X. Experimental evaluation of interfacial adhesion strength of cold sprayed Ti-6Al-4V thick coatings using an adhesive-free test method. Surf. Coat. Technol. 2020, 381, 125130. [Google Scholar] [CrossRef]
- Baiocco, G.; Rubino, G.; Ucciardello, N. Pretreatments effects on mechanical and morphological features of copper coatings. Surf. Interfaces 2020, 20, 100625. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, K.K.; Cui, Y.A.N.; Lim, S.C.; Cho, Y.W.; Kim, S.M.; Lee, Y.H. Adhesion Test of Carbon Nanotube Film Coated Onto Transparent Conducting Substrates. Nano 2010, 5, 133–138. [Google Scholar] [CrossRef]
- Nelyub, V. Methods for Developing Carbon Composites with Functional Properties. In Proceedings of the IOP Conference Series: Materials Science and Engineering Sevastopol, Sevastopol, Crimea, 7–11 September 2020; p. 022037. [Google Scholar]
- Kim, J.; Park, C.; Jung, H.; Kim, H.; Kwon, S.; Choi, H.; Kang, H. Study of a Carbon Nanowall Synthesized on an MWCNT-Based Buffer Layer for Improvement of Electrical Properties. Appl. Sci. 2020, 10, 192. [Google Scholar] [CrossRef] [Green Version]
- Zeng, N.; Ma, J.; Zhang, Y.; Yang, G.; Zhang, S.; Zhang, P. Silver nanosheet-coated copper nanowire/epoxy resin nanocomposites with enhanced electrical conductivity and wear resistance. J. Nanopart. Res. 2017, 19, 91. [Google Scholar] [CrossRef]
- Chakraborty, A.; Singh, J.K.; Sen, D.; Pityana, S.; Manna, I.; Krishna, S.; Dutta Majumdar, J. Microstructures, wear and corrosion resistance of laser composite surfaced austenitic stainless steel (AISI 304 SS) with tungsten carbide. Opt. Laser Technol. 2021, 134, 106585. [Google Scholar] [CrossRef]





| Sample | Adhesion Results |
|---|---|
| OL/CNW | 3B |
| OL/Cu pulsed | 5B |
| OL/CNW/Cu pulsed | 5B |
| OL/Cu plasma | 5B |
| OL/CNW/Cu plasma | 5B |
| Sample | Resistivity (Ω⋅cm) |
|---|---|
| OL/Cu pulsed | 0.91 |
| OL/CNW/Cu pulsed | 1.50 |
| OL/Cu plasma | 0.90 |
| OL/CNW/Cu plasma | 1.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danes, C.-A.; Dumitriu, C.; Vizireanu, S.; Bita, B.; Nicola, I.-M.; Dinescu, G.; Pirvu, C. Influence of Carbon Nanowalls Interlayer on Copper Deposition. Coatings 2021, 11, 1395. https://doi.org/10.3390/coatings11111395
Danes C-A, Dumitriu C, Vizireanu S, Bita B, Nicola I-M, Dinescu G, Pirvu C. Influence of Carbon Nanowalls Interlayer on Copper Deposition. Coatings. 2021; 11(11):1395. https://doi.org/10.3390/coatings11111395
Chicago/Turabian StyleDanes, Cristiana-Alexandra, Cristina Dumitriu, Sorin Vizireanu, Bogdan Bita, Ioana-Maria Nicola, Gheorghe Dinescu, and Cristian Pirvu. 2021. "Influence of Carbon Nanowalls Interlayer on Copper Deposition" Coatings 11, no. 11: 1395. https://doi.org/10.3390/coatings11111395
APA StyleDanes, C.-A., Dumitriu, C., Vizireanu, S., Bita, B., Nicola, I.-M., Dinescu, G., & Pirvu, C. (2021). Influence of Carbon Nanowalls Interlayer on Copper Deposition. Coatings, 11(11), 1395. https://doi.org/10.3390/coatings11111395









