Structural and Optical Properties of CuO Thin Films Synthesized Using Spray Pyrolysis Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of CuO Thin Films
2.3. Measurements
3. Results and Discussion
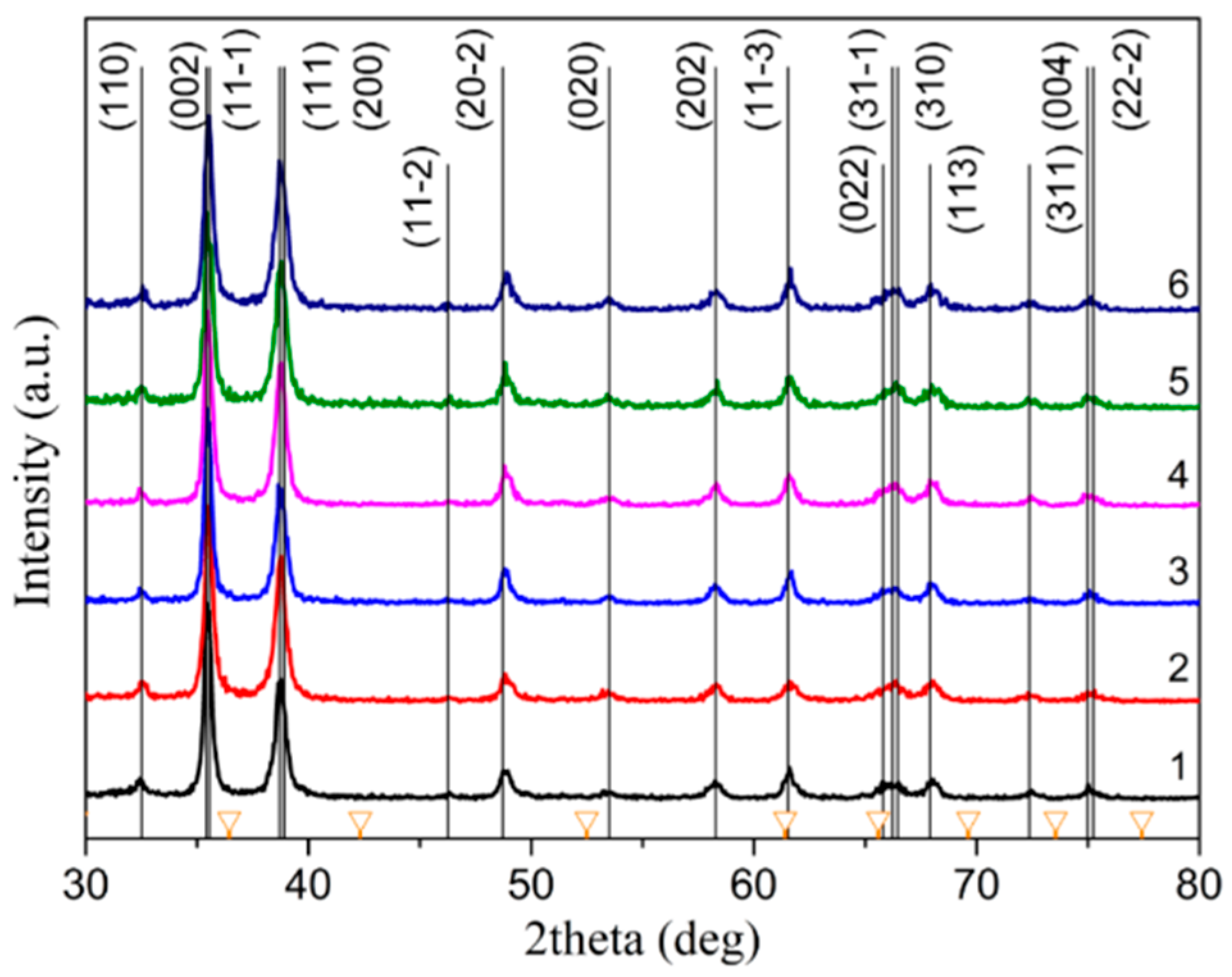
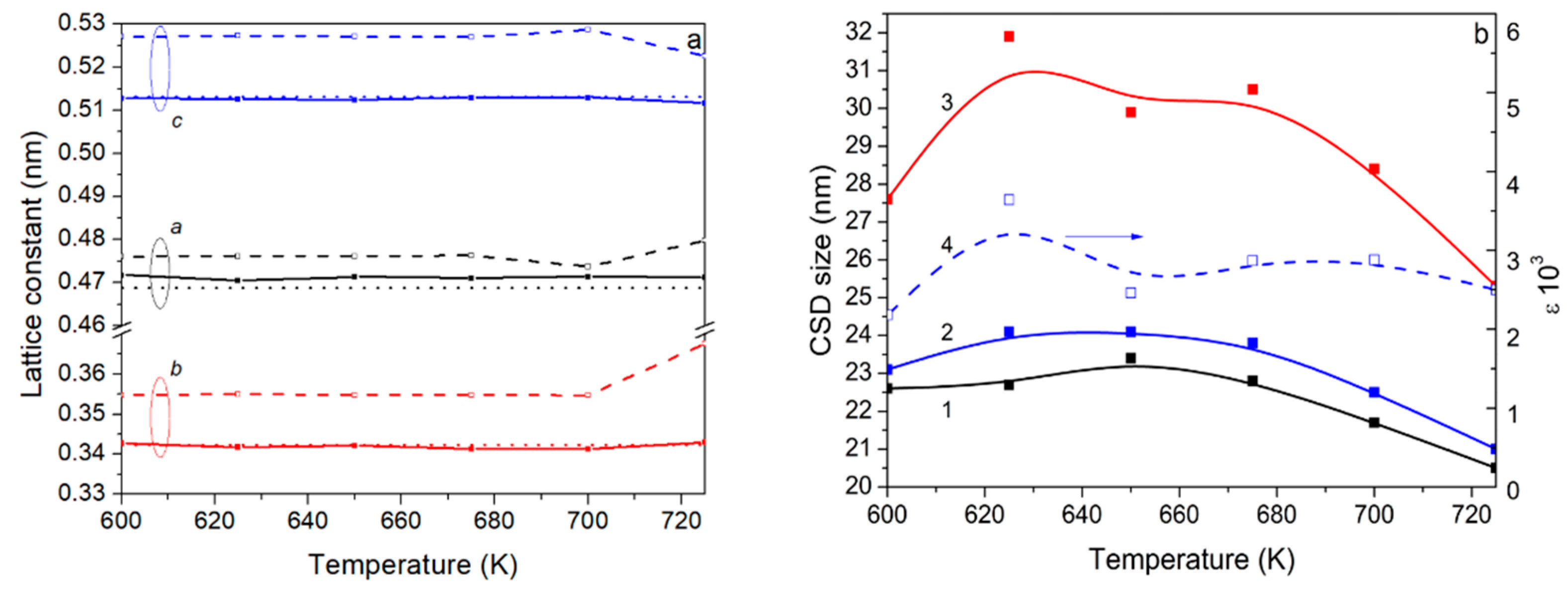
3.1. XRD Analysis
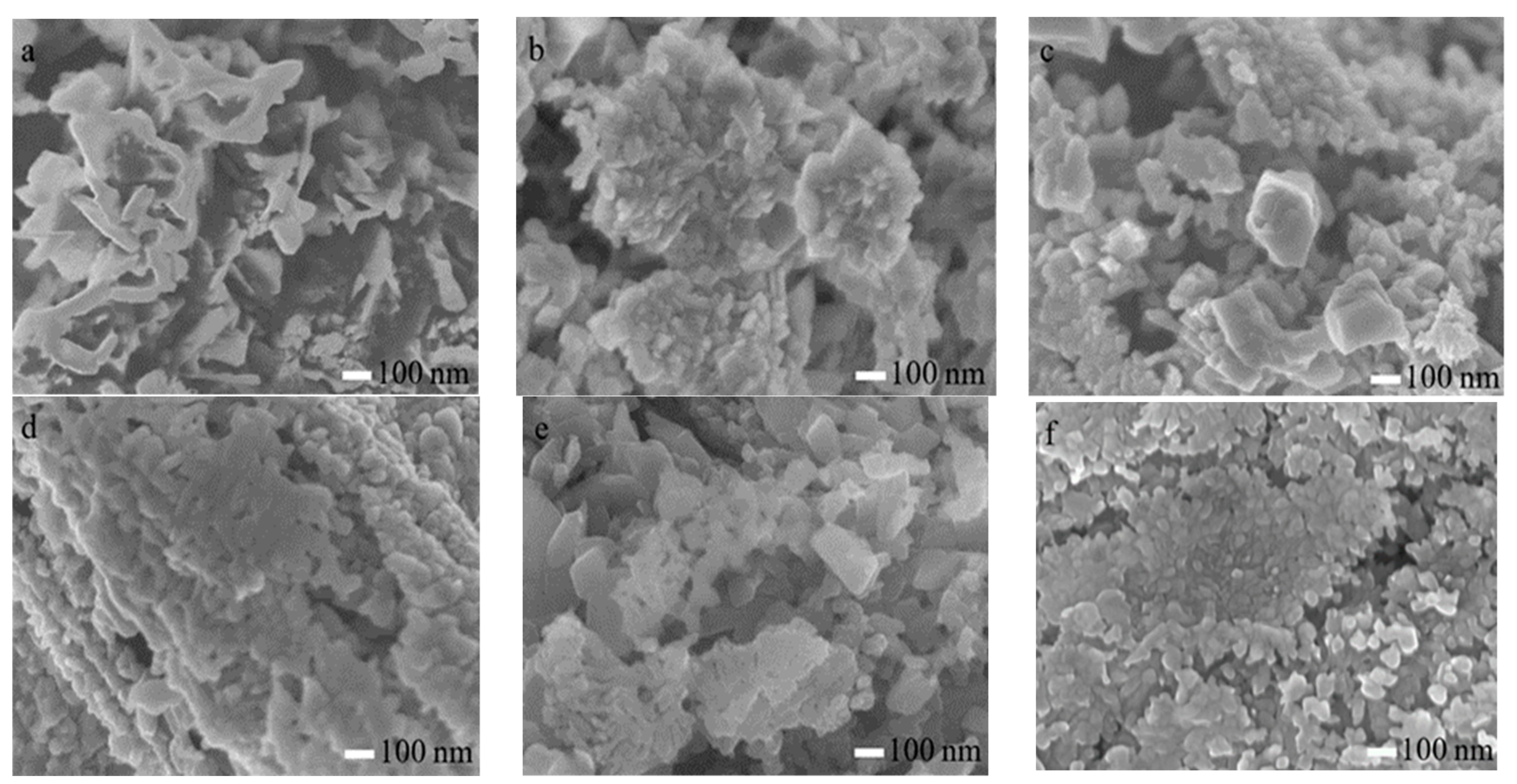
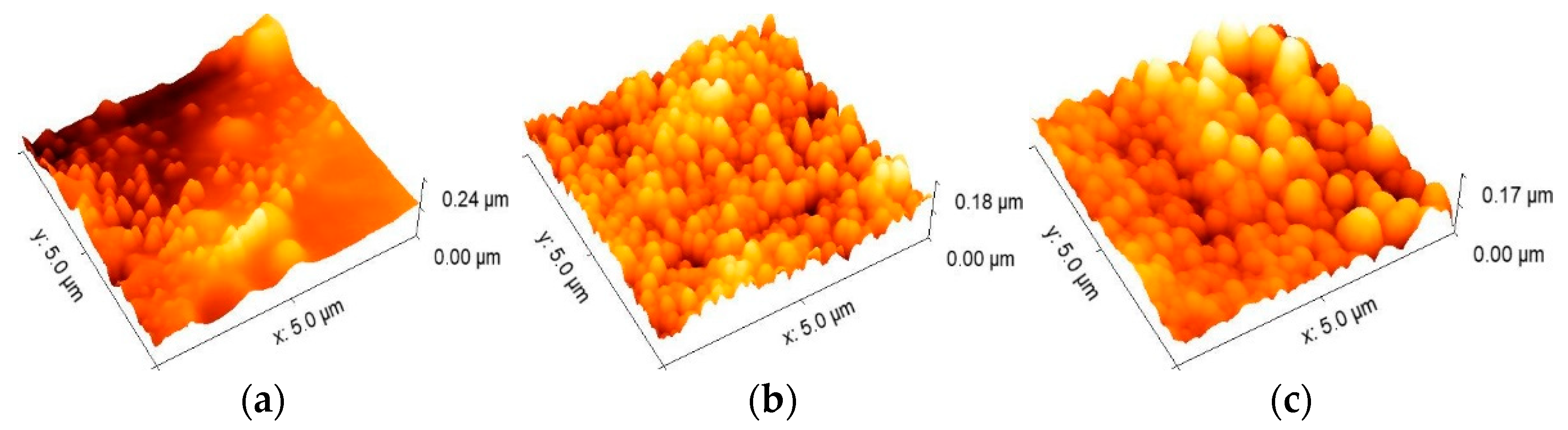
3.2. Surface Morphology
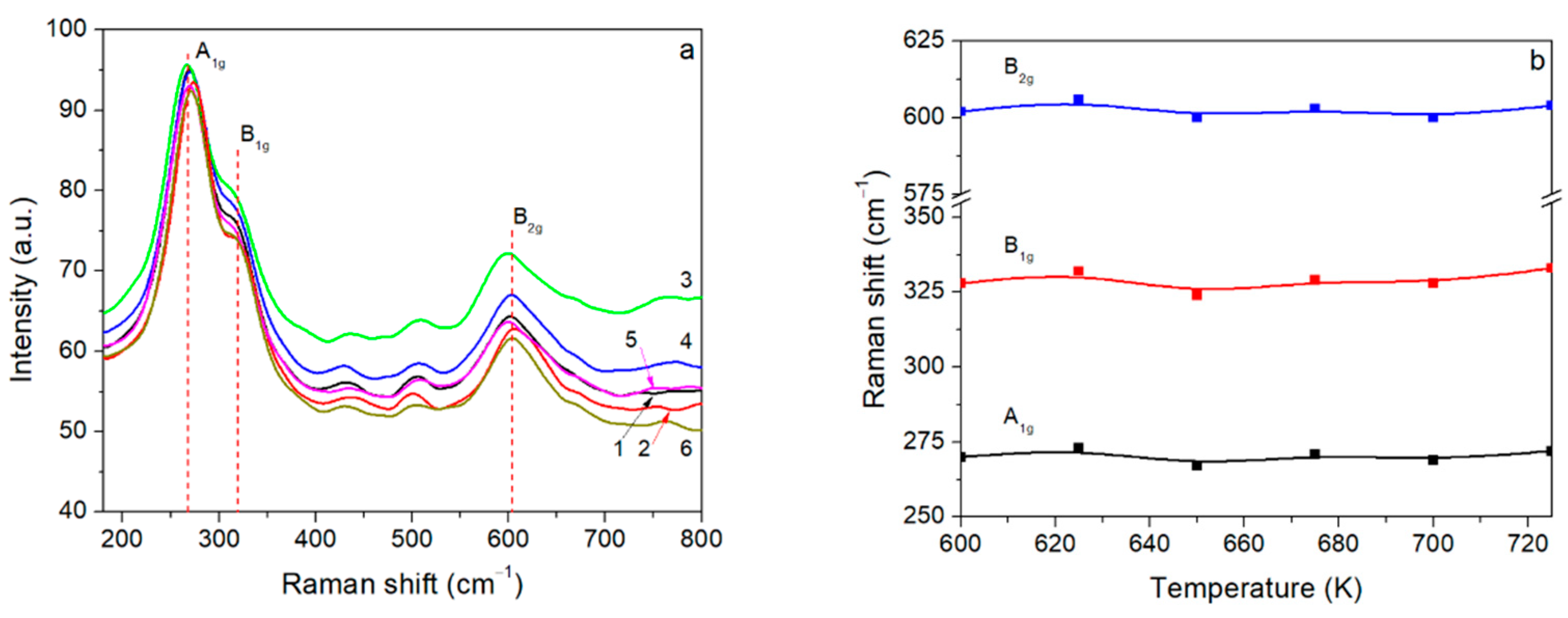
3.3. Raman Measurements
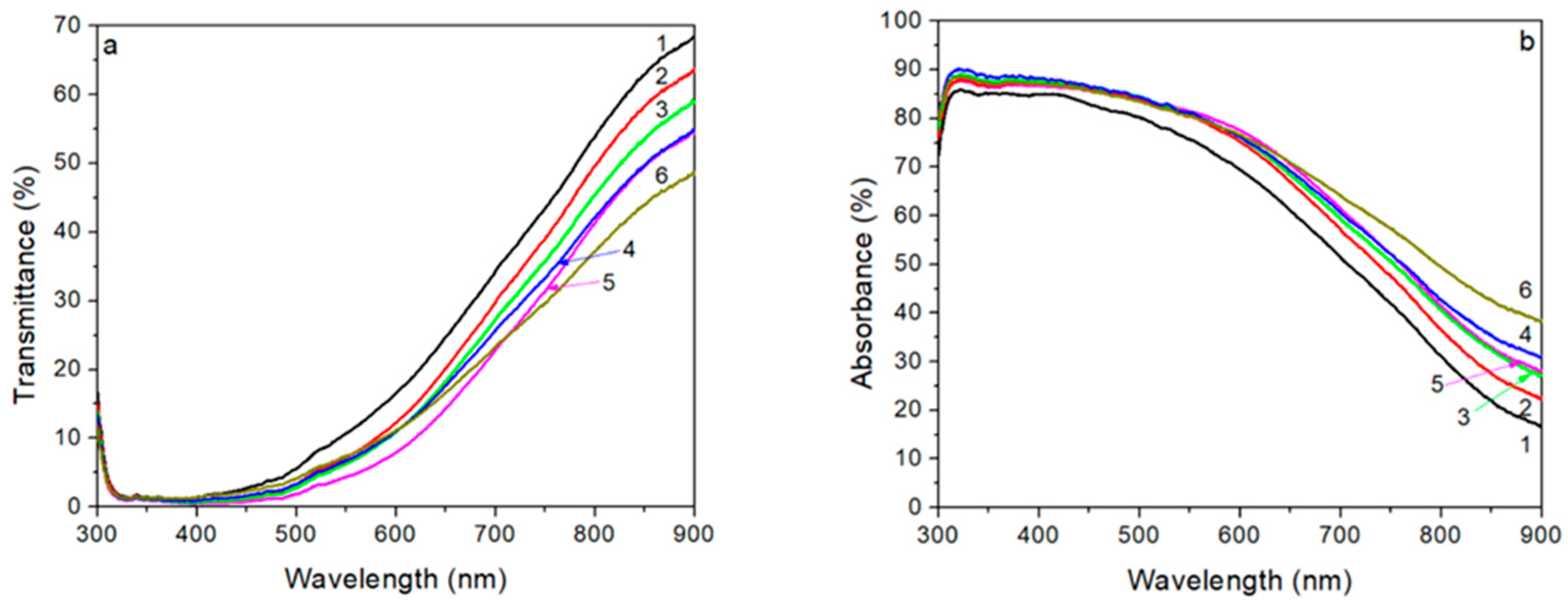
3.4. Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, M.S.; Yim, K.G.; Leem, J.Y.; Kim, S.; Nam, G.; Lee, D.Y.; Kim, J.S. Thickness dependence of properties of ZnO thin films on porous silicon grown by plasma-assisted molecular beam epitaxy. J. Korean Phys. Soc. 2011, 59, 2354–2361. [Google Scholar] [CrossRef]
- Sawicka-Chudy, P.; Sibiński, M.; Rybak-Wilusz, E.; Cholewa, M.; Wisz, G.; Yavorskyi, R. Review of the development of copper oxides with titanium dioxide thin-film solar cells. AIP Adv. 2020, 10, 010701. [Google Scholar] [CrossRef]
- Pavan, M.; Rühle, S.; Ginsburg, A.; Keller, D.A.; Barad, H.N.; Sberna, P.M.; Fortunato, E. TiO2/Cu2O all-oxide heterojunction solar cells produced by spray pyrolysis. Sol. Energy Mater. Sol. Cells 2015, 132, 549–556. [Google Scholar] [CrossRef]
- Morasch, J.; Li, S.; Brötz, J.; Jaegermann, W.; Klein, A. Reactively magnetron sputtered Bi2O3 thin films: Analysis of structure, optoelectronic, interface, and photovoltaic properties. Phys. Status Solidi A 2014, 211, 93–100. [Google Scholar] [CrossRef]
- Moumen, A.; Hartiti, B.; Comini, E.; Arachchige, H.M.M.; Fadili, S.; Thevenin, P. Preparation and characterization of nanostructured CuO thin films using spray pyrolysis technique. Superlattices Microstruct. 2019, 127, 2–10. [Google Scholar] [CrossRef]
- Naveena, D.; Logu, T.; Dhanabal, R.; Sethuraman, K.; Bose, A.C. Comparative study of effective photoabsorber CuO thin films prepared via different precursors using chemical spray pyrolysis for solar cell application. J. Mater. Sci. Mater. Electron. 2019, 30, 561–572. [Google Scholar] [CrossRef]
- Ooi, P.K.; Ng, S.S.; Abdullah, M.J.; Hassan, H.A.; Hassan, Z. Effects of oxygen percentage on the growth of copper oxide thin films by reactive radio frequency sputtering. Mater. Chem. Phys. 2013, 140, 243–248. [Google Scholar] [CrossRef]
- Shabu, R.; Raj, A.M.E.; Sanjeeviraja, C.; Ravidhas, C. Assessment of CuO thin films for its suitablity as window absorbing layer in solar cell fabrications. Mater. Res. Bull. 2015, 68, 1–8. [Google Scholar] [CrossRef]
- Valladares, L.D.L.S.; Salinas, D.H.; Dominguez, A.B.; Najarro, D.A.; Khondaker, S.I.; Mitrelias, T.; Majima, Y. Crystallization and electrical resistivity of Cu2O and CuO obtained by thermal oxidation of Cu thin films on SiO2/Si substrates. Thin Solid Films 2012, 520, 6368–6374. [Google Scholar] [CrossRef]
- Li, Y.; Tung, S.; Schneider, E.; Xi, S. A review on development of nanofluid preparation and characterization. Powder Technol. 2009, 196, 89–101. [Google Scholar] [CrossRef]
- Zoolfakar, A.S.; Rani, R.A.; Morfa, A.J.; O’Mullane, A.P.; Kalantar-Zadeh, K. Nanostructured copper oxide semiconductors: A perspective on materials, synthesis methods and applications. J. Mater. Chem. C 2014, 2, 5247–5270. [Google Scholar] [CrossRef]
- Deng, Z.; Ma, Z.; Li, Y.; Li, Y.; Chen, L.; Yang, X.; Su, B.L. Boosting lithium-ion storage capability in CuO nanosheets via synergistic engineering of defects and pores. Front. Chem. 2018, 6, 428. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, M.; Chaichi, M.J.; Hosseini, S.N. Comparison of CuO nanoparticle and CuO/MWCNT nanocomposite for amplification of chemiluminescence immunoassay for detection of the hepatitis B surface antigen in biological samples. Sens. Actuators B Chem. 2017, 247, 319–328. [Google Scholar] [CrossRef]
- Dolai, S.; Dey, R.; Das, S.; Hussain, S.; Bhar, R.; Pal, A.K. Cupric oxide (CuO) thin films prepared by reactive dc magnetron sputtering technique for photovoltaic application. J. Alloys Compd. 2017, 724, 456–464. [Google Scholar] [CrossRef]
- Hilman, J.; Yost, A.J.; Tang, J.; Leonard, B.; Chien, T. Low temperature growth of CuO nanowires through direct oxidation. Nano-Struct. Nano-Objects 2017, 11, 124–128. [Google Scholar] [CrossRef]
- Wang, S.B.; Hsiao, C.H.; Chang, S.J.; Lam, K.T.; Wen, K.H.; Hung, S.C.; Huang, B.R. A CuO nanowire infrared photodetector. Sens. Actuators A 2011, 171, 207–211. [Google Scholar] [CrossRef]
- Zhou, K.; Li, Y. Catalysis based on nanocrystals with well-defined facets. Angew. Chem. Int. Ed. 2012, 51, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Diachenko, O.V.; Dobrozhan, O.A.; Opanasyuk, A.S.; Ivashchenko, M.M.; Protasova, T.O.; Kurbatov, D.I.; Čerškus, A. The influence of optical and recombination losses on the efficiency of thin-film solar cells with a copper oxide absorber layer. Superlattices Microstruct. 2018, 122, 476–485. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 1–30. [Google Scholar] [CrossRef]
- Dhas, C.R.; Alexander, D.; Christy, A.J.; Jeyadheepan, K.; Raj, A.M.E.; Raja, C.S. Preparation and characterization of CuO thin films prepared by spray pyrolysis technique for ethanol gas sensing application. Asian J. Appl. Sci. 2014, 7, 671–684. [Google Scholar] [CrossRef][Green Version]
- Gopalakrishna, D.; Vijayalakshmi, K.; Ravidhas, C. Effect of pyrolytic temperature on the properties of nano-structured CuO optimized for ethanol sensing applications. J. Mater. Sci. Mater. Electron. 2013, 24, 1004–1011. [Google Scholar] [CrossRef]
- Yang, K.G.; Hu, P.; Wu, S.X.; Ren, L.Z.; Yang, M.; Zhou, W.Q.; Li, S.W. Room-temperature ferromagnetic CuO thin film grown by plasma-assisted molecular beam epitaxy. Mater. Lett. 2016, 166, 23–25. [Google Scholar] [CrossRef]
- Sahu, K.; Choudhary, S.; Khan, S.A.; Pandey, A.; Mohapatra, S. Thermal evolution of morphological, structural, optical and photocatalytic properties of CuO thin films. Nano-Struct. Nano-Objects 2019, 17, 92–102. [Google Scholar] [CrossRef]
- Sagadevan, S.; Pal, K.; Chowdhury, Z.Z. Fabrication of CuO nanoparticles for structural, optical and dielectric analysis using chemical precipitation method. J. Mater. Sci. Mater. Electron. 2017, 28, 12591–12597. [Google Scholar] [CrossRef]
- Dobrozhan, O.; Vorobiov, S.; Kurbatov, D.; Baláž, M.; Kolesnyk, M.; Opanasyuk, A. Structural properties and chemical composition of ZnO films deposited onto flexible substrates by spraying polyol mediated nanoinks. Superlattices Microstruct. 2020, 140, 106455. [Google Scholar] [CrossRef]
- Lim, Y.F.; Chua, C.S.; Lee, C.J.J.; Chi, D. Sol–gel deposited Cu 2 O and CuO thin films for photocatalytic water splitting. Phys. Chem. Chem. Phys. 2014, 16, 25928–25934. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Li, J.; Ni, Y. Urchin-like CuO microspheres: Synthesis, characterization, and properties. J. Alloys Compd. 2009, 481, 610–615. [Google Scholar] [CrossRef]
- Ozga, M.; Kaszewski, J.; Seweryn, A.; Sybilski, P.; Godlewski, M.; Witkowski, B.S. Ultra-fast growth of copper oxide (II) thin films using hydrothermal method. Mater. Sci. Semicond. Process. 2020, 120, 105279. [Google Scholar] [CrossRef]
- Diachenko, O.V.; Opanasiuk, A.S.; Kurbatov, D.I.; Cheong, H.; Kuznetsov, V.M. Effect of substrate temperature on structural and substructural properties of MgO thin films. Funct. Mater. 2015, 22, 487–493. [Google Scholar] [CrossRef]
- Umanskij, J.S.; Skakov, J.A.; Ivanov, A.N.; Rastorgujev, L.N. Crystallogaphy, X-ray Graph and Electronmicroscopy; Metallurgy: Moskow, Russia, 1982. [Google Scholar]
- Holland, T.J.B.; Redfern, S.A.T. Unitcell: A nonlinear least-squares program for cell-parameter refinement and implementing regression and deletion diagnostics. J. Appl. Crystallogr. 1997, 30, 84. [Google Scholar] [CrossRef]
- Dobrozhan, O.; Kurbatov, D.; Opanasyuk, A.; Cheong, H.; Cabot, A. Influence of substrate temperature on the structural and optical properties of crystalline ZnO films obtained by pulsed spray pyrolysis. Surf. Interface Anal. 2015, 47, 601–606. [Google Scholar] [CrossRef]
- Akgul, F.A.; Akgul, G.; Yildirim, N.; Unalan, H.E.; Turan, R. Influence of thermal annealing on microstructural, morphological, optical properties and surface electronic structure of copper oxide thin films. Mater. Chem. Phys. 2014, 147, 987–995. [Google Scholar] [CrossRef]
- Opanasyuk, A.S.; Kurbatov, D.I.; Kosyak, V.V. Characteristics of structure formation in zinc and cadmium chalcogenide films deposited on nonorienting substrates. Crystallogr. Rep. 2012, 57, 927–933. [Google Scholar] [CrossRef]
- Åsbrink, S.; Norrby, L.J. A refinement of the crystal structure of copper (II) oxide with a discussion of some exceptional esd’s. Acta crystallogr. B Struct. Crystallogr. Cryst. Chem. 1970, 26, 8–15. [Google Scholar] [CrossRef]
- Mageshwari, K.; Sathyamoorthy, R. Flower-shaped CuO nanostructures: Synthesis, characterization and antimicrobial activity. J. Mater. Sci. Technol. 2013, 29, 909–914. [Google Scholar] [CrossRef]
- Maji, S.K.; Mukherjee, N.; Mondal, A.; Adhikary, B.; Karmakar, B. Chemical synthesis of mesoporous CuO from a single precursor: Structural, optical and electrical properties. J. Solid State Chem. 2010, 183, 1900–1904. [Google Scholar] [CrossRef]
- Wang, X.; Xi, G.; Xiong, S.; Liu, Y.; Xi, B.; Yu, W.; Qian, Y. Solution-phase synthesis of single-crystal CuO nanoribbons and nanorings. Cryst. Growth Des. 2007, 7, 930–934. [Google Scholar] [CrossRef]
- Murthy, P.S.; Venugopalan, V.P.; Arunya, D.D.; Dhara, S.; Pandiyan, R.; Tyagi, A.K. Antibiofilm activity of nano sized CuO. In Proceedings of the IEEE International Conference on Nanoscience, Engineering and Technology, Chennai, India, 28–30 November 2011; pp. 580–583. [Google Scholar]
- Hinna, M.; Hartiti, B.; Batan, A.; Reniers, F.; Buess-Herman, C.; Segato, T.; Thévenin, P. Elaboration and Characterization of CuO Thin Films by Spray Pyrolysis Method for Gas Sensors Applications. Multidiscip. Digit. Publ. Inst. Proc. 2019, 14, 55. [Google Scholar] [CrossRef]
- Arguello, C.A.; Rousseau, D.L.; Porto, S.D.S. First-order Raman effect in wurtzite-type crystals. Phys. Rev. 1969, 181, 1351. [Google Scholar] [CrossRef]
- Murali, D.S.; Kumar, S.; Choudhary, R.J.; Wadikar, A.D.; Jain, M.K.; Subrahmanyam, A. Synthesis of Cu2O from CuO thin films: Optical and electrical properties. AIP Adv. 2015, 5, 047143. [Google Scholar] [CrossRef]
- Sengupta, J.; Sahoo, R.K.; Bardhan, K.K.; Mukherjee, C.D. Influence of annealing temperature on the structural, topographical and optical properties of sol–gel derived ZnO thin films. Mater. Lett. 2011, 65, 2572–2574. [Google Scholar] [CrossRef]
- Hong, R.; Huang, J.; He, H.; Fan, Z.; Shao, J. Influence of different post-treatments on the structure and optical properties of zinc oxide thin films. Appl. Surf. Sci. 2005, 242, 346–352. [Google Scholar] [CrossRef]
- Koffyberg, F.P.; Benko, F.A. A photoelectrochemical determination of the position of the conduction and valence band edges of p-type CuO. J. Appl. Phys. 1982, 53, 1173–1177. [Google Scholar] [CrossRef]
- Dhineshbabu, N.R.; Rajendran, V.; Nithyavathy, N.; Vetumperumal, R. Study of structural and optical properties of cupric oxide nanoparticles. Appl. Nanosci. 2016, 6, 933–939. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diachenko, O.; Kováč, J., Jr.; Dobrozhan, O.; Novák, P.; Kováč, J.; Skriniarova, J.; Opanasyuk, A. Structural and Optical Properties of CuO Thin Films Synthesized Using Spray Pyrolysis Method. Coatings 2021, 11, 1392. https://doi.org/10.3390/coatings11111392
Diachenko O, Kováč J Jr., Dobrozhan O, Novák P, Kováč J, Skriniarova J, Opanasyuk A. Structural and Optical Properties of CuO Thin Films Synthesized Using Spray Pyrolysis Method. Coatings. 2021; 11(11):1392. https://doi.org/10.3390/coatings11111392
Chicago/Turabian StyleDiachenko, Oleksii, Jaroslav Kováč, Jr., Oleksandr Dobrozhan, Patrik Novák, Jaroslav Kováč, Jaroslava Skriniarova, and Anatoliy Opanasyuk. 2021. "Structural and Optical Properties of CuO Thin Films Synthesized Using Spray Pyrolysis Method" Coatings 11, no. 11: 1392. https://doi.org/10.3390/coatings11111392
APA StyleDiachenko, O., Kováč, J., Jr., Dobrozhan, O., Novák, P., Kováč, J., Skriniarova, J., & Opanasyuk, A. (2021). Structural and Optical Properties of CuO Thin Films Synthesized Using Spray Pyrolysis Method. Coatings, 11(11), 1392. https://doi.org/10.3390/coatings11111392






