New Strategy for Creating TiO2 Thin Films with Embedded Au Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of AuCNP Ink
2.2. Printing Methods
2.3. Characterization Methods
3. Results and Discussion
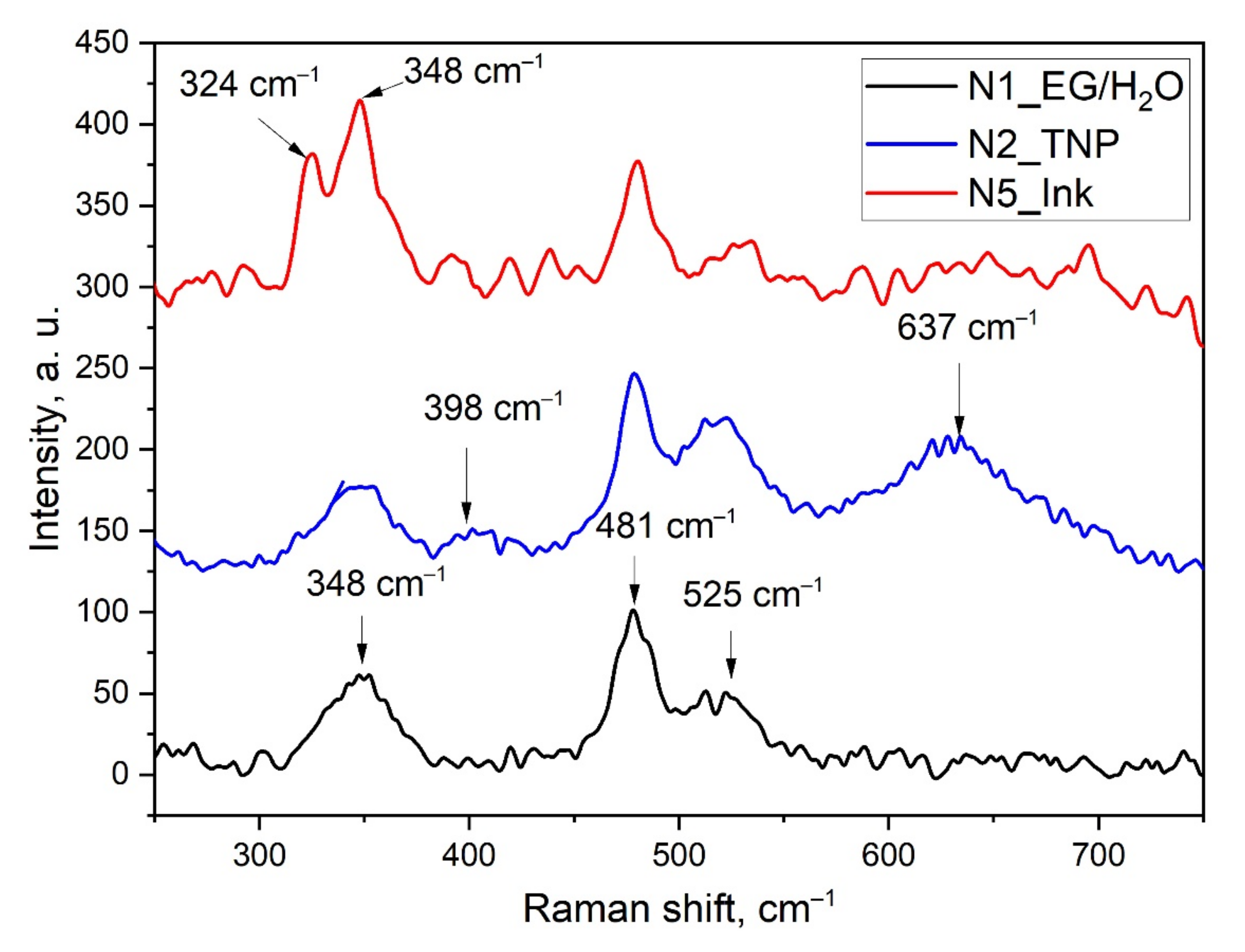
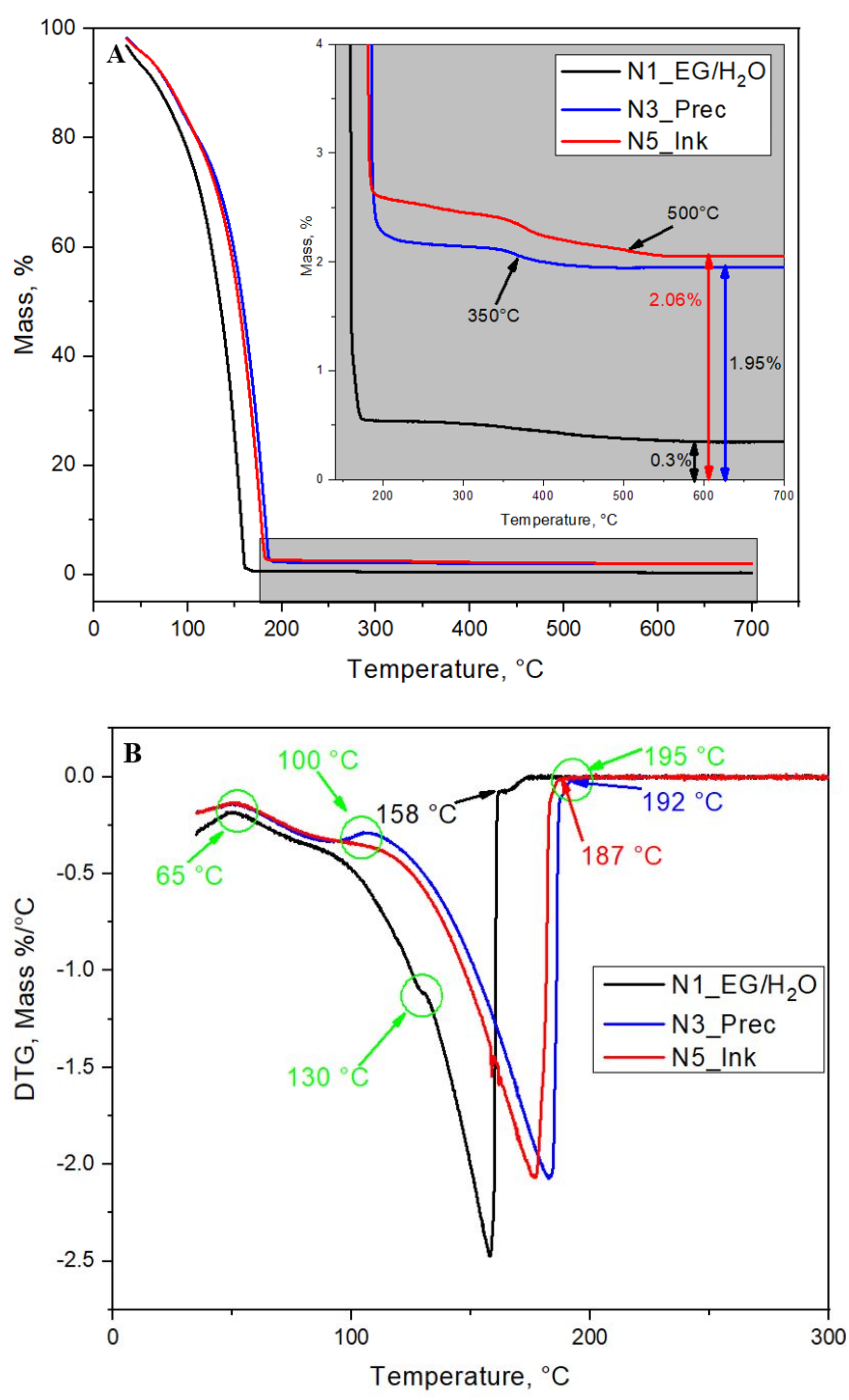
3.1. Characterization of the AuCNP Ink
- N1—The solution of EG/H2O 3:2 by volume, that is the ink’s base liquid components.
- N2—The colloid solution of TNP in EG/H2O 3:2 without adding HAuCl4.
- N3—The AuCNP precursor, which is the mixture of all ink components without heating and boiling, with stirring only.
- N4—The water AuCNP ink without EG.
- N5—The AuCNP ink.
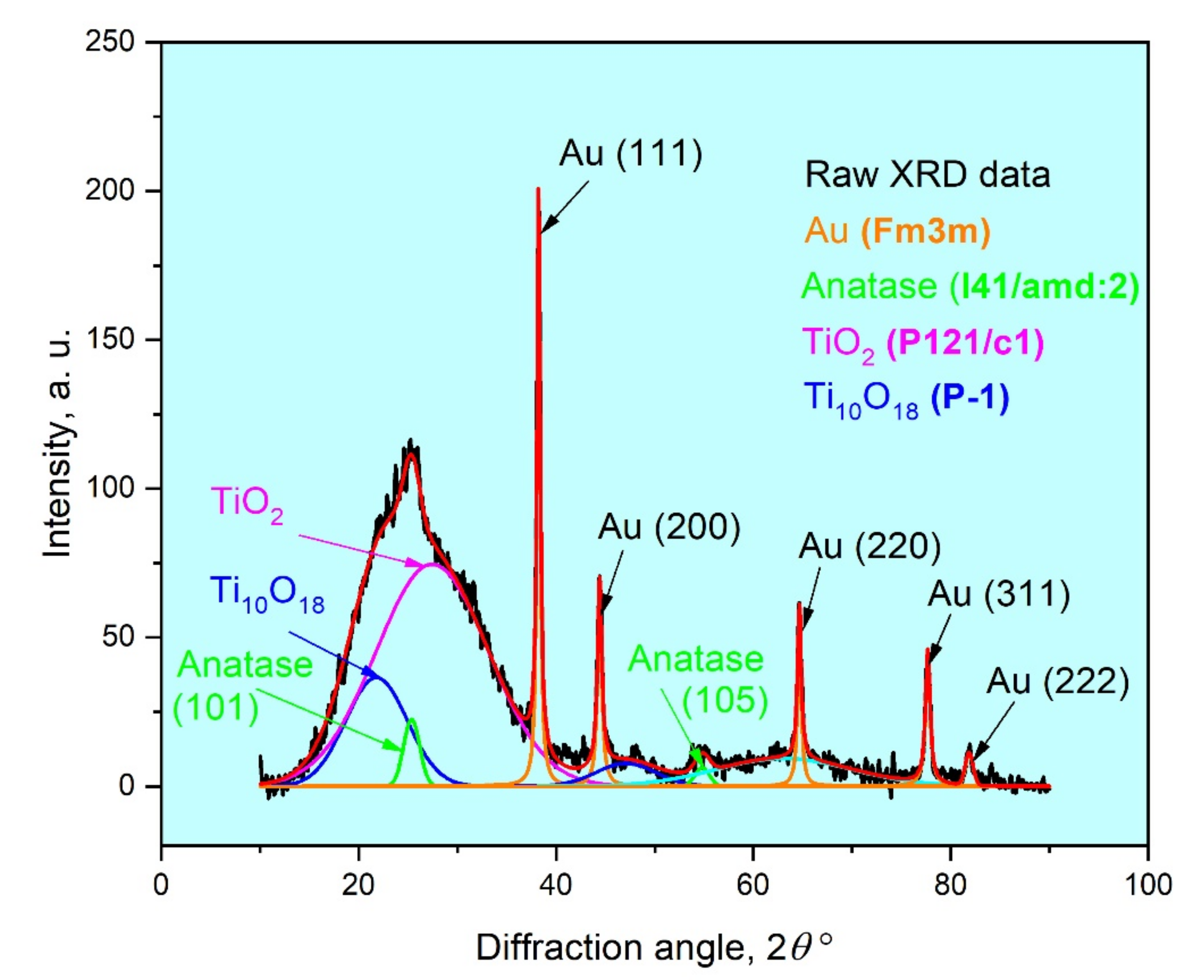
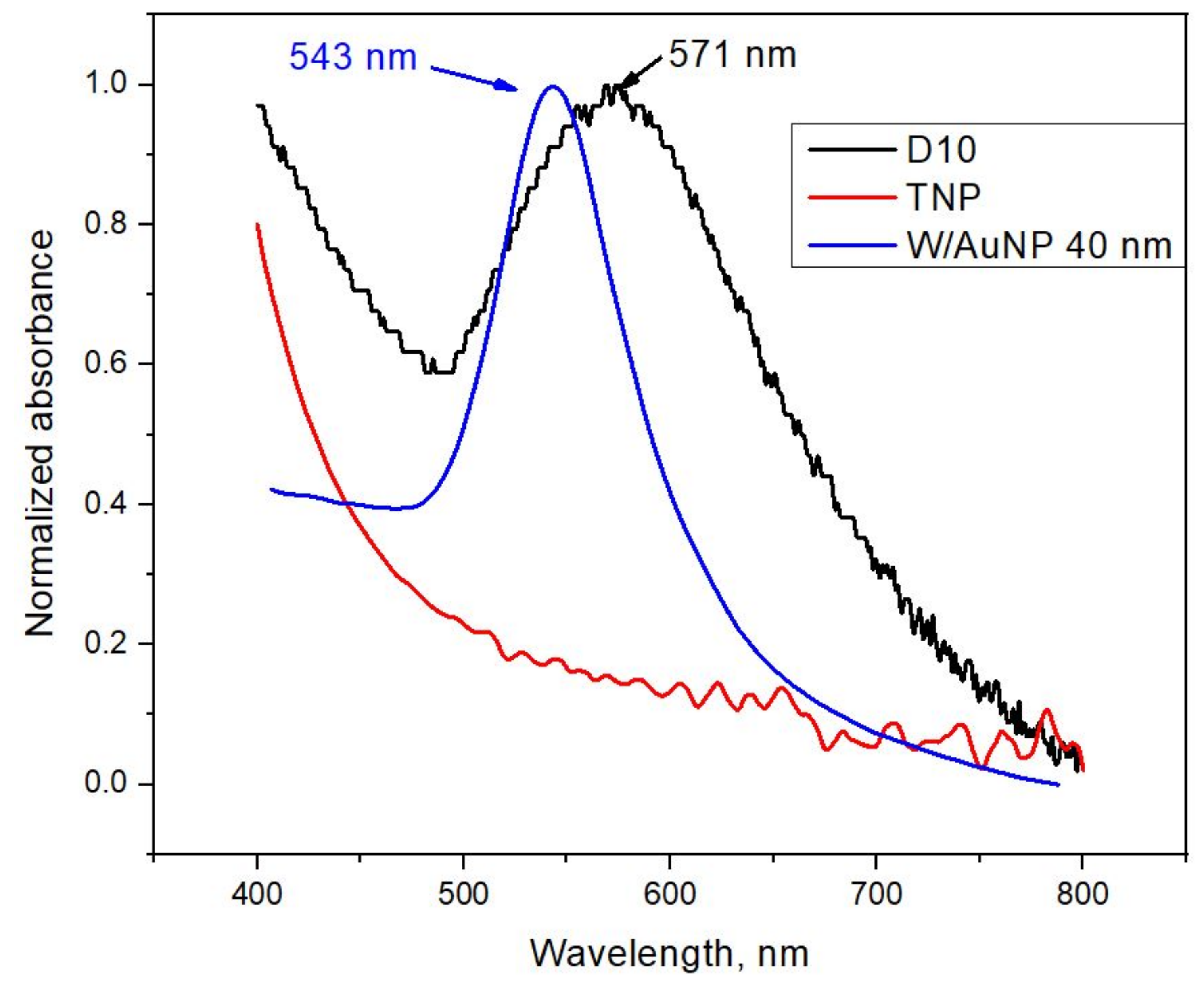
3.2. Characterization of TiO2/AuNP Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suchomel, P.; Kvitek, L.; Prucek, R.; Panacek, A.; Halder, A.; Vajda, S.; Zboril, R. Simple size-controlled synthesis of Au nanoparticles and their size-dependent catalytic activity. Sci. Rep. 2018, 8, 4589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; He, X.; Liu, X.; Li, X.; Kang, L. One-step implementation of plasmon enhancement and solvent annealing effects for air-processed high-efficiency perovskite solar cells. J. Mater. Chem. A 2018, 6, 24036. [Google Scholar] [CrossRef]
- Li, C.; Xu, C.; Cahen, D.; Jin, Y. Unprecedented efficient electron transport across Au nanoparticles with up to 25-nm insulating SiO2-shells. Sci. Rep. 2019, 9, 18336. [Google Scholar] [CrossRef] [Green Version]
- Yao, K. Plasmonic Metal Nanoparticles with Core-Bishell Structure for High-Performance Organic and Perovskite Solar Cells. ACS Nano 2019, 13, 5397–5409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Luo, Q.; Shi, J.; Yue, L.; Wang, Z.; Chena, X.; Huang, S. Efficient perovskite solar cells by combination use of Au nanoparticles and insulating metal oxide. Nanoscale 2017, 9, 2852–2864. [Google Scholar] [CrossRef] [Green Version]
- Tran, Q.N.; Lee, H.K.; Kim, J.H.; Park, S.J. Influence of Gold-Silver Rough-Surface Nanoparticles on Plasmonic Light Scattering in Organic Solar Cells. J. Nanosci. Nanotechnol. 2020, 20, 304–311. [Google Scholar] [CrossRef]
- Brown, M.D.; Suteewong, T.; Kumar, R.S.S.; D’Innocenzo, V.; Petrozza, A.; Lee, M.M.; Wiesner, U.; Snaith, H.J. Plasmonic Dye-Sensitized Solar Cells Using Core-Shell Metal-Insulator Nanoparticles. Nano Lett. 2011, 11, 438–445. [Google Scholar] [CrossRef]
- Moakhar, R.S. Recent Advances in Plasmonic Perovskite Solar Cells. Adv. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Wang, B. Enhancing the Photovoltaic Performance of Perovskite Solar Cells Using Plasmonic Au@Pt@Au Core-Shell Nanoparticles. Nanomaterials 2019, 9, 1263. [Google Scholar] [CrossRef] [Green Version]
- Sreedharan, R.S.; Kavitha, V.S.; Suresh, S.; Krishnan, R.R.; Bose, R.J.; Pillai, V.P.M. Tailoring the properties of zinc oxide films by incorporating gold nanoparticles using RF magnetron sputtering. Appl. Phys. A 2018, 124, 815. [Google Scholar] [CrossRef]
- Li, J.; Zhou, H.; Qian, S.; Liu, Z.; Feng, J.; Jin, P.; Liu, X. Plasmonic gold nanoparticles modified titania nanotubes for antibacterial application. Appl. Phys. Lett. 2014, 104, 261110. [Google Scholar] [CrossRef]
- Tan, B.J.Y.; Sow, C.H.; Koh, T.S.; Chin, K.C.; Wee, A.T.S.; Ong, C.K. Fabrication of Size-Tunable Gold Nanoparticles Array with Nanosphere Lithography, Reactive Ion Etching, and Thermal Annealing. J. Phys. Chem. B 2005, 109, 11100–11109. [Google Scholar] [CrossRef]
- Bi, K. Direct electron-beam patterning of transferrable plasmonic gold nanoparticles using a HAuCl4/PVP composite resist. Nanoscale 2019, 11, 1245. [Google Scholar] [CrossRef]
- Keller, K.; Khramenkova, E.V.; Slabov, V.; Musin, A.; Kalashnikov, A.; Vinogradov, A.V.; Pidko, E.A. Inkjet Printing of Sc-Doped TiO2 with Enhanced Photoactivity. Coatings 2019, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Turkevich, J.; Stevenson, P.C.; Hiller, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of Thiol-derivatised Gold Nanoparticles in a Two-phase Liquid-Liquid System. J. Chem. Soc. Chem. Commun. 1994, 1994, 801–802. [Google Scholar] [CrossRef]
- Deraedt, C.; Salmon, L.; Gatard, S.; Ciganda, R.; Hernandez, R.; Ruiza, J.; Astruc, D. Sodium borohydride stabilizes very active gold nanoparticle catalysts. Chem. Commun. 2014, 50, 14194–14196. [Google Scholar] [CrossRef]
- Fiévet, F. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef]
- Silvert, P.Y.; Tekaia-Elhsissen, K. Synthesis of monodisperse submicronic gold particles by the polyol process. Solid State Ionics 1995, 82, 53–60. [Google Scholar] [CrossRef]
- Sankar, M. Role of the Support in Gold-Containing Nanoparticles as Heterogeneous Catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonerba, A.; Grassi, A. Trends in Sustainable Synthesis of Organics by Gold Nanoparticles Embedded in Polymer Matrices. Catalysts 2021, 11, 714. [Google Scholar] [CrossRef]
- Haruta, M.; Tsubota, S.; Kobayashi, T.; Kageyama, H.; Genet, M.J.; Delmon, B. Low-Temperature Oxidation of CO Over Gold Supported on TiO2, α-Fe2O3, and Co3O4. J. Catal. 1993, 144, 175–192. [Google Scholar] [CrossRef]
- Soejima, T.; Tada, H.; Kawahara, T.; Ito, S. Formation of Au Nanoclusters on TiO2 Surfaces by a Two-Step Method Consisting of Au(III)-Complex Chemisorption and Its Photoreduction. Langmuir 2002, 18, 4191–4194. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Perelstein, G.; Feldhoff, A.; Condó, A.; Tolley, A.J.; Grela, M.A. The spontaneous room temperature reduction of HAuCl4 in ethylene glycol in the presence of ZnO: A simple strategy to obtain stable Au/ZnO nanostructures exhibiting strong surface plasmon resonance and efficient electron storage properties. New J. Chem. 2015, 39, 909. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.M.d.S.; Ciotti, L.; Vaz, J.M.; Spinacé, E.V. Preparation of Au/TiO2 Catalyst by a Liquid-Phase Reduction Method for Preferential Oxidation of Carbon Monoxide in a Hydrogen Rich-Stream (CO-PROX reaction). Mater. Res. 2018, 21. [Google Scholar] [CrossRef] [Green Version]
- Haruta, M. Size- and support-dependency in the catalysts of gold. Catal. Today 1997, 36, 153–166. [Google Scholar] [CrossRef]
- Haruta, M. Role of perimeter interfaces in catalysis by gold nanoparticles. Faraday Discuss. 2011, 152, 11–32. [Google Scholar] [CrossRef]
- Brar, K.S.; Verma, M. Measurement of nanoparticles by light-scattering techniques. Trends Anal. Chem. 2011, 30, 4–17. [Google Scholar] [CrossRef]
- Xu, R. Progress in nanoparticles characterization: Sizing and zeta potential measurement. Particuology 2008, 6, 112–115. [Google Scholar] [CrossRef]
- Murphy, P.J.; LaGrange, M.S. Raman spectroscopy of gold chloro-hydroxy speciation in fluids at ambient temperature and pressure: A re-evaluation of the effects of pH and chloride concentration. Geochim. Cosmochim. Acta 1998, 62, 3515–3526. [Google Scholar] [CrossRef]
- Ivanova, S.; Petit, C.; Pitchon, V. A new preparation method for the formation of gold nanoparticles on an oxide support. Appl. Catal. A Gen. 2004, 267, 191–201. [Google Scholar] [CrossRef]
- Zanella, R.; Giorgio, S.; Henry, C.R.; Louis, C. Alternative Methods for the Preparation of Gold Nanoparticles Supported on TiO2. J. Phys. Chem. B 2002, 106, 7634–7642. [Google Scholar] [CrossRef]
- Mazza, T.; Barborini, E.; Piseri, P.; Milani, P.; Cattaneo, D.; Li Bassi, A.; Ducati, C. Raman spectroscopy characterization ofTiO2 rutile nanocrystals. Phys. Rev. B 2007, 75, 045416. [Google Scholar] [CrossRef]
- Krishnan, K.; Krishnan, R.S. Raman and infrared spectra of ethylene glycol. Proc. Indian Acad. Sci. Sect. A 1966, 64, 111–123. [Google Scholar] [CrossRef]
- Hoath, S.D. Fundamentals of Inkjet Printing. In The Science of Inkjet and Droplets; Wiley: Weinheim, Germany, 2016; p. 98. [Google Scholar] [CrossRef]
- Reis, N.; Derby, B. Ink jet deposition of ceramic suspensions: Modelling and experiments of droplet formation. Mater. Res. Soc. Symp. Proc. 2000, 625, 117–122. [Google Scholar] [CrossRef]
- Jang, D.; Kim, D.; Moon, J. Influence of fluid properties on ink-jet printability. Langmuir 2009, 25, 2629–2635. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-K.; Hwang, Y.J.; Yoon, C.; Yoon, H.-O.; Chang, K.S.; Lee, G.; Lee, S.; Yi, G.-R. Experimental approach to the fundamental limit of the extinction coefficients of ultra-smooth and highly spherical gold nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 20786–20794. [Google Scholar] [CrossRef]
- Lavie, A.; Yadgarov, L.; Houben, L.; Popovitz-Biro, R.; Shaul, T.E.; Nagler, A.; Suchowski, H.; Tenne, R. Synthesis of core–shell single-layer MoS2 sheathing gold nanoparticles, AuNP@ 1L-MoS2. Nanotechnology 2017, 28, 24LT03. [Google Scholar] [CrossRef]
- Aiboushev, A.; Gostev, F.; Shelaev, I.; Kostrov, A.; Kanaev, A.; Museur, L.; Traore, M.; Sarkisova, O.; Nadtochenko, V. Spectral properties of the surface plasmon resonance and electron injection from gold nanoparticles to TiO2 mesoporous film: Femtosecond study. Photochem. Photobiol. Sci. 2012, 12, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Bonkerud, J.; Zimmermann, C.; Weiser, P.M.; Vines, L.; Monakhov, E.V. On the permittivity of titanium dioxide. Sci. Rep. 2021, 11, 12443. [Google Scholar] [CrossRef] [PubMed]
- Behnajady, M.A.; Eskandarloo, H.; Modirshahla, N.; Shokri, M. Sol-gel low-temperature synthesis of stable anatase-type TiO2 nanoparticles under different conditions and its photocatalytic activity. Photochem. Photobiol. 2011, 87, 1002–1008. [Google Scholar] [CrossRef] [PubMed]





| Sample | Composition | pH, ±0.01% | Zeta Potential, mV |
|---|---|---|---|
| N2 | TiO2 colloid | 0.45 | 30.0 ± 0.3 |
| N3 | Ink precursor | 1.04 | 37.4 ± 1.0 |
| N4 | AuCNP without EG | 1.94 | 5.8 ± 0.3 |
| N5 | AuCNP ink | 0.62 | 31.4 ± 0.3 |
| Sample | Composition | Viscosity, cP, ±0.2 | Surface Tension, N/m, ±0.5 | Density, g/cm3, ±0.05 | Z, ±0.1 | Contact Angles, °, ±0.5 |
|---|---|---|---|---|---|---|
| N1 | EG/H2O 3:2 | 4.9 | 54.2 | 1.10 | 7.30 | 13.9 |
| N2 | TNP | 5.2 | 53.4 | 1.11 | 6.87 | 16.7 |
| N5 | AuCNP ink | 9.7 | 52.8 | 1.11 | 3.60 | 18.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubtsov, S.; Musin, A.; Zinigrad, M.; Kalashnikov, A.; Danchuk, V. New Strategy for Creating TiO2 Thin Films with Embedded Au Nanoparticles. Coatings 2021, 11, 1525. https://doi.org/10.3390/coatings11121525
Rubtsov S, Musin A, Zinigrad M, Kalashnikov A, Danchuk V. New Strategy for Creating TiO2 Thin Films with Embedded Au Nanoparticles. Coatings. 2021; 11(12):1525. https://doi.org/10.3390/coatings11121525
Chicago/Turabian StyleRubtsov, Sofia, Albina Musin, Michael Zinigrad, Alexander Kalashnikov, and Viktor Danchuk. 2021. "New Strategy for Creating TiO2 Thin Films with Embedded Au Nanoparticles" Coatings 11, no. 12: 1525. https://doi.org/10.3390/coatings11121525
APA StyleRubtsov, S., Musin, A., Zinigrad, M., Kalashnikov, A., & Danchuk, V. (2021). New Strategy for Creating TiO2 Thin Films with Embedded Au Nanoparticles. Coatings, 11(12), 1525. https://doi.org/10.3390/coatings11121525







