Plasma Enhanced Atomic Layer Deposition of Ruthenium Films Using Ru(EtCp)2 Precursor
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
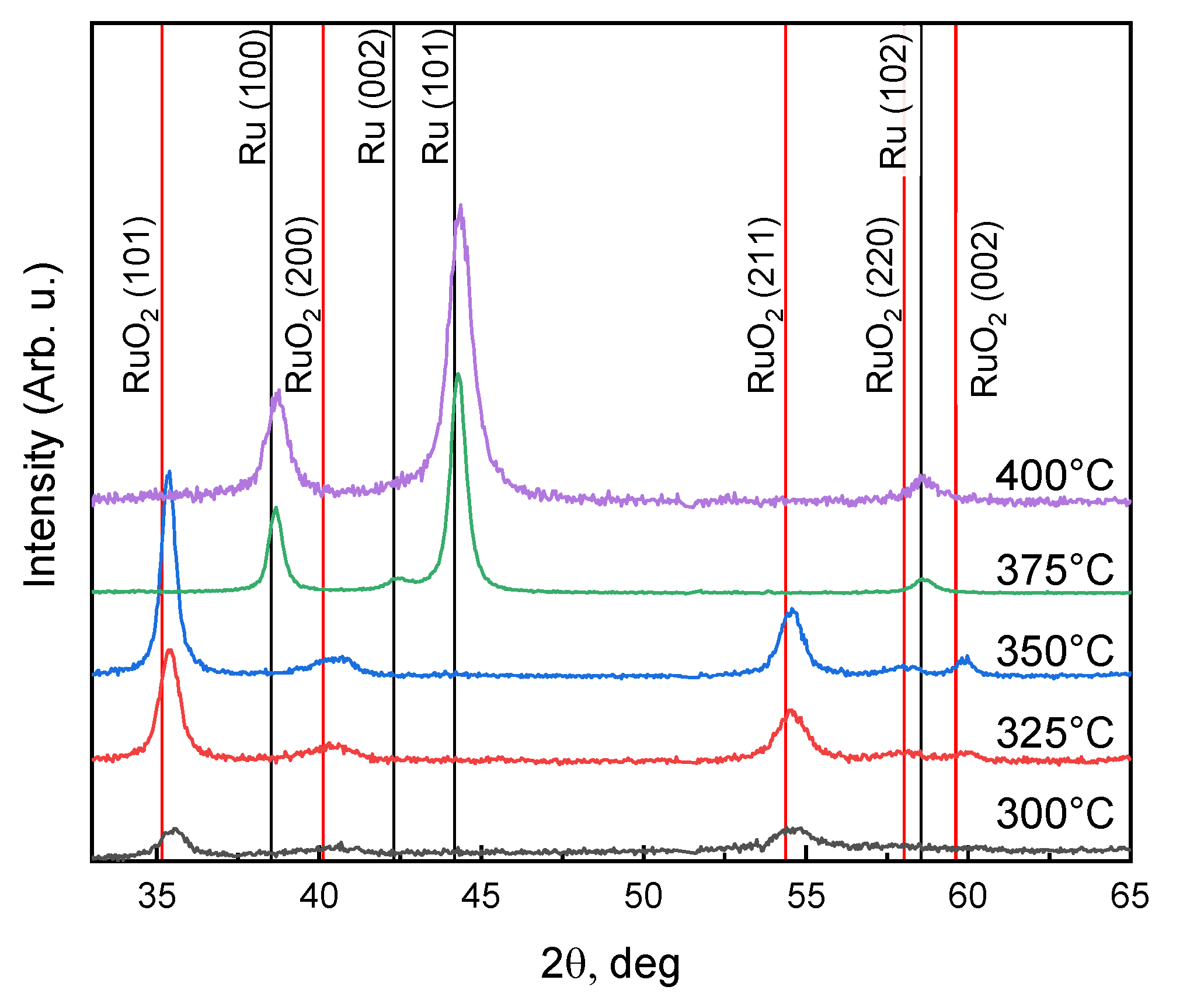
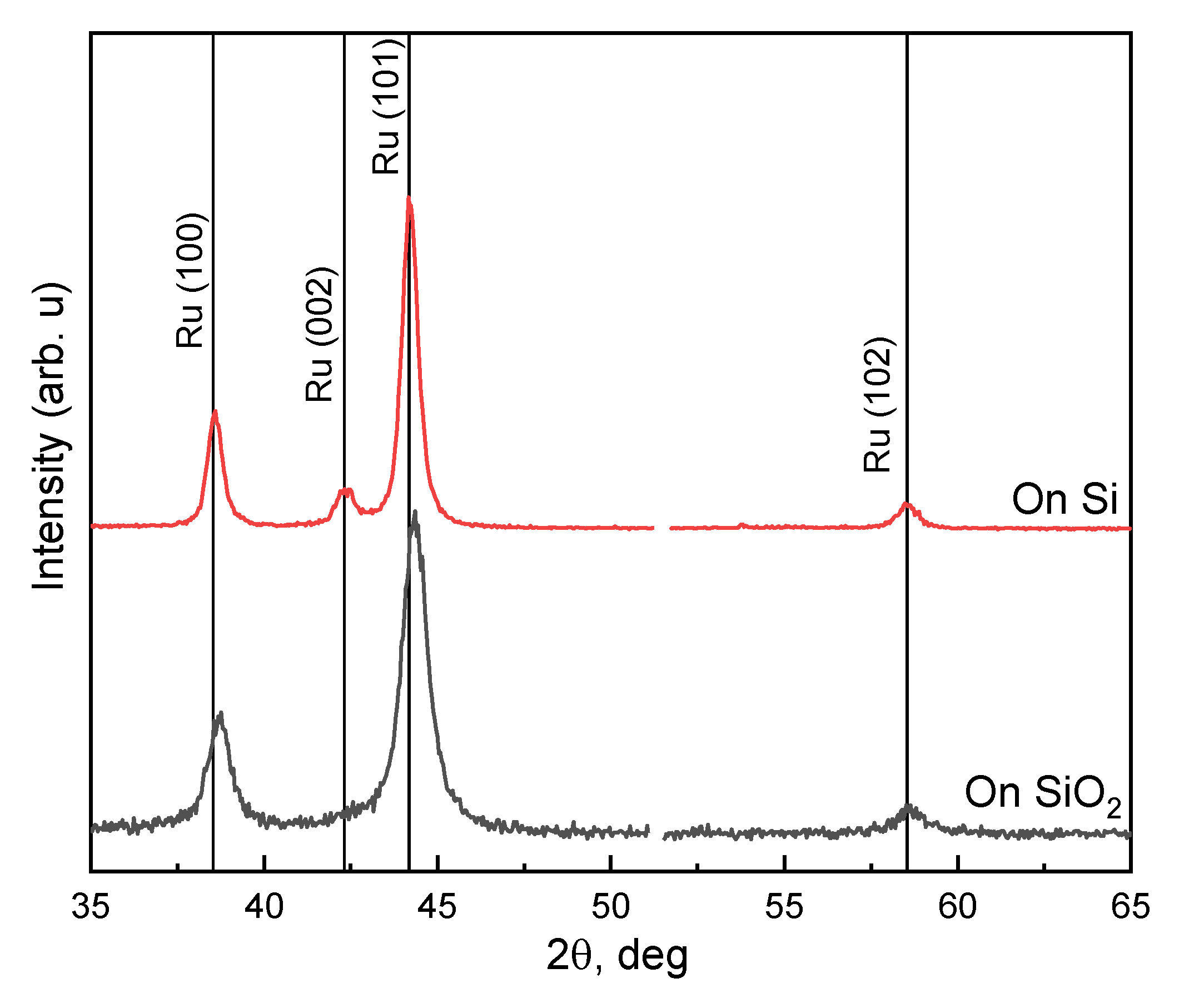
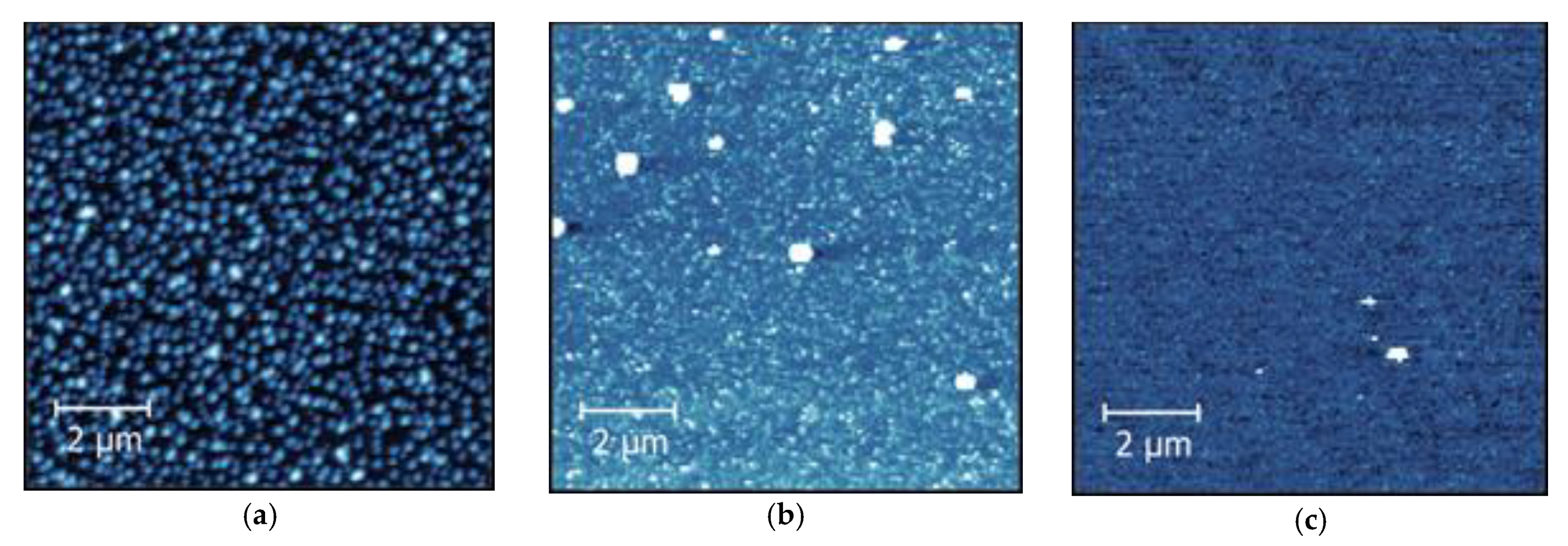
3.1. Influence of Substrate Temperature on ALD
3.2. Mechanical Stress
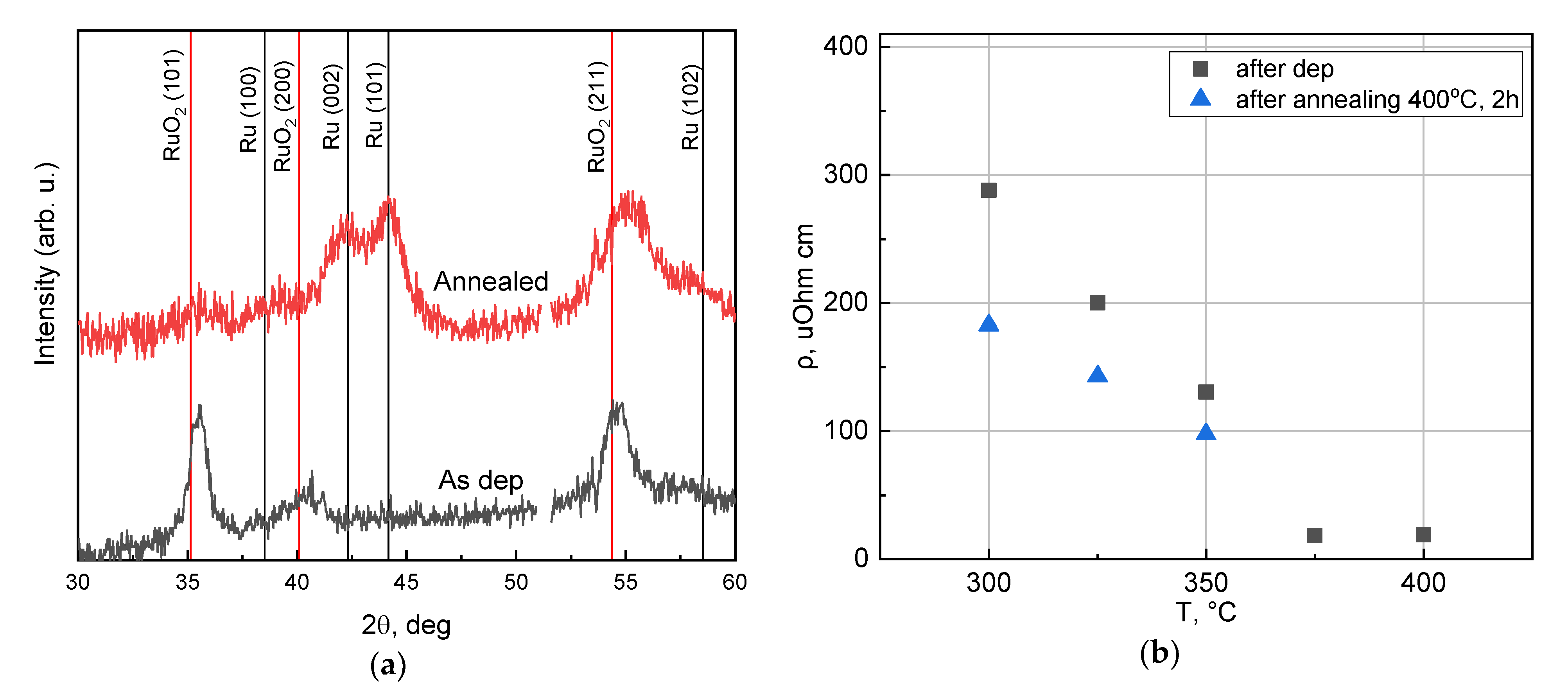
3.3. Post Annealing of Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dutta, S.; Kundu, S.; Gupta, A.; Jamieson, G.; Granados, J.F.G.; Bömmels, J.; Wilson, C.J.; Tőkei, Z.; Adelmann, C. Highly Scaled Ruthenium Interconnects. IEEE Electron. Device Lett. 2017, 38, 949–951. [Google Scholar] [CrossRef]
- Van der Veen, M.H.; Heyler, N.; Pedreira, O.V.; Ciofi, I.; Decoster, S.; Gonzalez, V.V.; Jourdan, N.; Struyf, H.; Croes, K.; Wilson, C.J.; et al. Damascene Benchmark of Ru, Co and Cu in Scaled Dimensions. In Proceedings of the 2018 IEEE International Interconnect Technology Conference (IITC), Santa Clara, CA, USA, 4–7 June 2018; pp. 172–174. [Google Scholar] [CrossRef]
- Croes, K.; Adelmann, C.; Wilson, C.J.; Zahedmanesh, H.; Pedreira, O.V.; Wu, C.; Leśniewska, A.; Oprins, H.; Beyne, S.; Ciofi, I.; et al. Interconnect Metals beyond Copper: Reliability Challenges and Opportunities. In Proceedings of the 2018 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 1–5 December 2018; pp. 5.3.1–5.3.4. [Google Scholar] [CrossRef]
- Bernasconi, R.; Magagnin, L. Review—Ruthenium as Diffusion Barrier Layer in Electronic Interconnects: Current Literature with a Focus on Electrochemical Deposition Methods. J. Electrochem. Soc. 2018, 166, D3219. [Google Scholar] [CrossRef]
- Liang, G.W.; Adelmann, C.; Pedreira, O.V.; Dutta, S.; Popovici, M.; Briggs, B.; Heylen, N.; Vanstreels, K.; Wilson, C.J.; Elshocht, S.V.; et al. Ruthenium Metallization for Advanced Interconnects. In Proceedings of the 2016 IEEE International Interconnect Technology Conference / Advanced Metallization Conference (IITC/AMC), San Jose, CA, USA, 23–26 May 2016; pp. 34–36. [Google Scholar] [CrossRef]
- Kwon, O.-K.; Kim, J.-H.; Park, H.-S.; Kang, S.-W. Atomic Layer Deposition of Ruthenium Thin Films for Copper Glue Layer. J. Electrochem. Soc. 2004, 151, 109–112. [Google Scholar] [CrossRef]
- Li, Z.; Tian, Y.; Teng, C.; Cao, H. Recent Advances in Barrier Layer of Cu Interconnects. Materials (Basel) 2020, 13, 5049. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kil, D.-S.; Kim, J.-H.; Kwon, S.-H.; Ahn, J.-H.; Roh, J.-S.; Park, S.-K. Ru Films from Bis(Ethylcyclopentadienyl)Ruthenium Using Ozone as a Reactant by Atomic Layer Deposition for Capacitor Electrodes. J. Electrochem. Soc. 2012, 159, 560–564. [Google Scholar] [CrossRef]
- Misra, V.; Lucovsky, G.; Parsons, G. Issues in High-K Gate Stack Interfaces. MRS Bull. 2002, 27, 212–216. [Google Scholar] [CrossRef]
- Karabanov, S.; Karabanov, A.S.; Suvorov, D.V.; Grappe, B.; Coutier, C.; Sibuet, H.; Sazhin, B.N.; Krutilin, A.A. Nanoscale Ruthenium Coatings of MEMS Switches Contacts. MRS Online Proc. Libr. (OPL) 2010, 1249. [Google Scholar] [CrossRef]
- Basu, A.; Hennessy, R.; Adams, G.; McGruer, N. Reliability in Hot Switched Ruthenium on Ruthenium MEMS Contacts. In Proceedings of the 2013 IEEE 59th Holm Conference on Electrical Contacts (Holm 2013), Newport, RI, USA, 22–25 September 2013; pp. 1–8. [Google Scholar] [CrossRef]
- Axet, M.R.; Philippot, K. Catalysis with Colloidal Ruthenium Nanoparticles. Chem. Rev. 2020, 120, 1085–1145. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; Khodakov, A.Y.; Legras, B.; Lancelot, C. Fischer–Tropsch Synthesis on a Ruthenium Catalyst in Two-Phase Systems: An Excellent Opportunity for the Control of Reaction Rate and Selectivity. Catal. Sci. Technol. 2014, 4, 2896–2899. [Google Scholar] [CrossRef]
- Amano, A.; Taylor, H. The Decomposition of Ammonia on Ruthenium, Rhodium and Palladium Catalysts Supported on Alumina. J. Am. Chem. Soc. 1954, 76, 4201–4204. [Google Scholar] [CrossRef]
- Hayashi, F.; Toda, Y.; Kanie, Y.; Kitano, M.; Inoue, Y.; Yokoyama, T.; Hara, M.; Hosono, H. Ammonia Decomposition by Ruthenium Nanoparticles Loaded on Inorganic Electride C12A7:E−. Chem. Sci. 2013, 4, 3124–3130. [Google Scholar] [CrossRef]
- Sergey, Y. Multilayer Coatings for EUV/Soft X-ray Mirrors. In Optical Interference Coatings; Kaiser, N., Pulker, H.K., Eds.; Springer: Berlin, Germany, 2003; p. 299. [Google Scholar] [CrossRef]
- Scholze, F.; Tümmler, J.; Ulm, G. High-Accuracy Radiometry in the EUV Range at the PTB Soft x-Ray Beamline. Metrologia 2003, 40, 224. [Google Scholar] [CrossRef]
- Müller, R.; Ghazaryan, L.; Schenk, P.; Wolleb, S.; Beladiya, V.; Otto, F.; Kaiser, N.; Tünnermann, A.; Fritz, T.; Szeghalmi, A. Growth of Atomic Layer Deposited Ruthenium and Its Optical Properties at Short Wavelengths Using Ru(EtCp)2 and Oxygen. Coatings 2018, 8, 413. [Google Scholar] [CrossRef]
- Hill, S.B.; Ermanoski, I.; Tarrio, C.; Lucatorto, T.B.; Madey, T.E.; Bajt, S.; Fang, M.; Chandhok, M. Critical Parameters Influencing the EUV-Induced Damage of Ru-Capped Multilayer Mirrors. Proc. SPIE 2007, 6517. [Google Scholar] [CrossRef]
- Smith, K.C.; Sun, Y.-M.; Mettlach, N.R.; Hance, R.L.; White, J.M. Evaluation of Precursors for Chemical Vapor Deposition of Ruthenium. Thin Solid Films 2000, 376, 73–81. [Google Scholar] [CrossRef]
- Nabatame, T.; Hiratani, M.; Kadoshima, M.; Shimamoto, Y.; Matsui, Y.; Ohji, Y.; Asano, I.; Fujiwara, T.; Suzuki, T. Properties of Ruthenium Films Prepared by Liquid Source Metalorganic Chemical Vapor Deposition Using Ru(EtCp)2 with Tetrahydrofuran Solvent. Jpn. J. Appl. Phys. 2000, 39, L1188. [Google Scholar] [CrossRef]
- Sysoev, S.V.; Kuzin, T.M.; Zelenina, L.N.; Zherikova, K.V.; Gelfond, N.V. Thermodynamic Characterization of Ruthenium β-Diketonate Complex Ru(Thd)3 as a Precursor for the CVD Preparation of Coatings. Russ. J. Inorg. Chem. 2020, 65, 747–751. [Google Scholar] [CrossRef]
- Senzaki, Y.; Gladfelter, W.L.; McCormick, F.B. Chemical Vapor Deposition of Ruthenium and Osmium Thin Films Using (Hexafluoro-2-Butyne)Tetracarbonylruthenium and –Osmium. Chem. Mater. 1993, 5, 1715–1721. [Google Scholar] [CrossRef]
- Papadatos, F.; Consiglio, S.; Skordas, S.; Eisenbraun, E.T.; Kaloyeros, A.E.; Peck, J.; Thompson, D.; Hoover, C. Chemical Vapor Deposition of Ruthenium and Ruthenium Oxide Thin Films for Advanced Complementary Metal-Oxide Semiconductor Gate Electrode Applications. J. Mater. Res. 2004, 19, 2947–2955. [Google Scholar] [CrossRef]
- Aaltonen, T.; Alén, P.; Ritala, M.; Leskelä, M. Ruthenium Thin Films Grown by Atomic Layer Deposition. Chem. Vap. Depos 2003, 9, 45–49. [Google Scholar] [CrossRef]
- Lee, J.; Song, Y.W.; Lee, K.; Lee, Y.; Jang, H.K. Atomic Layer Deposition of Ru by Using a New Ru-Precursor. ECS Trans. 2006, 2, 1–11. [Google Scholar] [CrossRef]
- Kukli, K.; Aarik, J.; Aidla, A.; Jõgi, I.; Arroval, T.; Lu, J.; Sajavaara, T.; Laitinen, M.; Kiisler, A.-A.; Ritala, M. Atomic Layer Deposition of Ru Films from Bis(2,5-Dimethylpyrrolyl)Ruthenium and Oxygen. Thin Solid Films 2012, 520, 2756–2763. [Google Scholar] [CrossRef]
- Yeo, S.; Choi, S.-H.; Park, J.-Y.; Kim, S.-H.; Cheon, T.; Lim, B.-Y.; Kim, S. Atomic Layer Deposition of Ruthenium (Ru) Thin Films Using Ethylbenzen-Cyclohexadiene Ru (0) as a Seed Layer for Copper Metallization. Thin Solid Films 2013, 546, 2–8. [Google Scholar] [CrossRef]
- Minjauw, M.M.; Dendooven, J.; Capon, B.; Schaekers, M.; Detavernier, C. Atomic Layer Deposition of Ruthenium at 100 °C Using the RuO4-Precursor and H2. J. Mater. Chem. C 2015, 3, 132–137. [Google Scholar] [CrossRef]
- Kukli, K.; Kemell, M.; Puukilainen, E.; Aarik, J.; Aidla, A.; Sajavaara, T.; Laitinen, M.; Tallarida, M.; Sundqvist, J.; Ritala, M. Atomic Layer Deposition of Ruthenium Films from (Ethylcyclopentadienyl)(Pyrrolyl)Ruthenium and Oxygen. J. Electrochem. Soc. 2011, 158, 158. [Google Scholar] [CrossRef]
- Leick-Marius, N. Atomic Layer Deposition of Ruthenium Films: Properties and Surface Reactions. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2014. [Google Scholar]
- Hämäläinen, J.; Ritala, M.; Leskelä, M. Atomic Layer Deposition of Noble Metals and Their Oxides. Chem. Mater. 2014, 26, 786–801. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, W.-H.; Lee, H.-B.-R.; Maeng, W.J.; Kim, H. Thermal and Plasma Enhanced Atomic Layer Deposition Ruthenium and Electrical Characterization as a Metal Electrode. Microelectron. Eng. 2008, 85, 39–44. [Google Scholar] [CrossRef]
- Lu, J.; Elam, J.W. Low Temperature ABC-Type Ru Atomic Layer Deposition through Consecutive Dissociative Chemisorption, Combustion, and Reduction Steps. Chem. Mater. 2015, 27, 4950–4956. [Google Scholar] [CrossRef]
- Smirnova, E.A.; Miakonkikh, A.V.; Rogozhin, A.E.; Rudenko, K.V. Atomic Layer Deposition of Ruthenium on Different Interfaces for an Advanced Metallization System of ICs. J. Phys. Conf. Ser. 2020, 1695, 012045. [Google Scholar] [CrossRef]
- Yim, S.-S.; Lee, D.-J.; Kim, K.-S.; Kim, S.-H.; Yoon, T.-S.; Kim, K.-B. Nucleation Kinetics of Ru on Silicon Oxide and Silicon Nitride Surfaces Deposited by Atomic Layer Deposition. J. Appl. Phys. 2008, 103, 113509. [Google Scholar] [CrossRef]
- Somani, S.; Mukhopadhyay, A.; Musgrave, C. Atomic Layer Deposition of Tantalum Nitride Using A Novel Precursor. J. Phys. Chem. C 2011, 115, 11507–11513. [Google Scholar] [CrossRef]
- Rudenko, K.V.; Myakon’kikh, A.V.; Rogozhin, A.E.; Gushchin, O.P.; Gvozdev, V.A. Atomic Layer Deposition in the Production of a Gate HkMG Stack Structure with a Minimum Topological Size of 32 Nm. Russ. Microelectron 2018, 47, 1–10. [Google Scholar] [CrossRef]
- Ardigo, M.R.; Ahmed, M.; Besnard, A. Stoney Formula: Investigation of Curvature Measurements by Optical Profilometer. AMR 2014, 996, 361–366. [Google Scholar] [CrossRef]
- Morozkin, A.V.; Seropegin, Y.D. Sm–Ru–Ge System at 1070 K. J. Alloys Compd. 2004, 365, 168–172. [Google Scholar] [CrossRef]
- Foo, M.L.; Huang, Q.; Lynn, J.W.; Lee, W.L.; Klimczuk, T.; Hagemann, I.S.; Ong, N.P.; Cava, R.J. Synthesis, Structure and Physical Properties of Ru Ferrites: BaMRu5O11 (M=Li and Cu) and BaM′2Ru4O11 (M′=Mn, Fe and Co). J. Solid State Chem. 2006, 179, 563–572. [Google Scholar] [CrossRef]
- Austin, D.Z.; Jenkins, M.A.; Allman, D.; Hose, S.; Price, D.; Dezelah, C.L.; Conley, J.F. Atomic Layer Deposition of Ruthenium and Ruthenium Oxide Using a Zero-Oxidation State Precursor. Chem. Mater. 2017, 29, 1107–1115. [Google Scholar] [CrossRef]


| Sample # | # of TaN Cycles | # of Ru Cycles | Dep Temp, °C | Thickness, nm | Resistivity, µOhm·cm |
|---|---|---|---|---|---|
| 1 | 5 | 750 | 200 | 6 ± 1 * | 652.6 ± 1.5 |
| 2 | 5 | 750 | 250 | 9 ± 1 * | 508.5 ± 1.5 |
| 3 | 5 | 750 | 300 | 17 ± 2 | 288.0 ± 1.0 |
| 4 | 5 | 750 | 325 | 20 ± 2 | 200.2 ± 1.0 |
| 5 | 5 | 750 | 350 | 25 ± 2 | 130.5 ± 1.0 |
| 6 | 5 | 750 | 375 | 27 ± 1 ** | 18.2 ± 0.8 |
| 7 | 5 | 750 | 400 | 33 ± 1 ** | 19.1 ± 0.8 |
| # of Sample | Thickness after Deposition, nm | Thickness after Annealing, nm |
|---|---|---|
| 3 | 17 ± 2 | 9 ± 2 |
| 4 | 20 ± 2 | 10.7 ± 2 |
| 5 | 25 ± 2 | 14.7 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogozhin, A.; Miakonkikh, A.; Smirnova, E.; Lomov, A.; Simakin, S.; Rudenko, K. Plasma Enhanced Atomic Layer Deposition of Ruthenium Films Using Ru(EtCp)2 Precursor. Coatings 2021, 11, 117. https://doi.org/10.3390/coatings11020117
Rogozhin A, Miakonkikh A, Smirnova E, Lomov A, Simakin S, Rudenko K. Plasma Enhanced Atomic Layer Deposition of Ruthenium Films Using Ru(EtCp)2 Precursor. Coatings. 2021; 11(2):117. https://doi.org/10.3390/coatings11020117
Chicago/Turabian StyleRogozhin, Alexander, Andrey Miakonkikh, Elizaveta Smirnova, Andrey Lomov, Sergey Simakin, and Konstantin Rudenko. 2021. "Plasma Enhanced Atomic Layer Deposition of Ruthenium Films Using Ru(EtCp)2 Precursor" Coatings 11, no. 2: 117. https://doi.org/10.3390/coatings11020117
APA StyleRogozhin, A., Miakonkikh, A., Smirnova, E., Lomov, A., Simakin, S., & Rudenko, K. (2021). Plasma Enhanced Atomic Layer Deposition of Ruthenium Films Using Ru(EtCp)2 Precursor. Coatings, 11(2), 117. https://doi.org/10.3390/coatings11020117







