Preparation and Optical Application of SiO2-TiO2 Composite Hardening Coatings with Controllable Refractive Index by Synchronous Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Reagents
2.2. Preparation of Sol-Gel Composite Hard Coating Solution
2.3. Hardening Liquid Curing Process
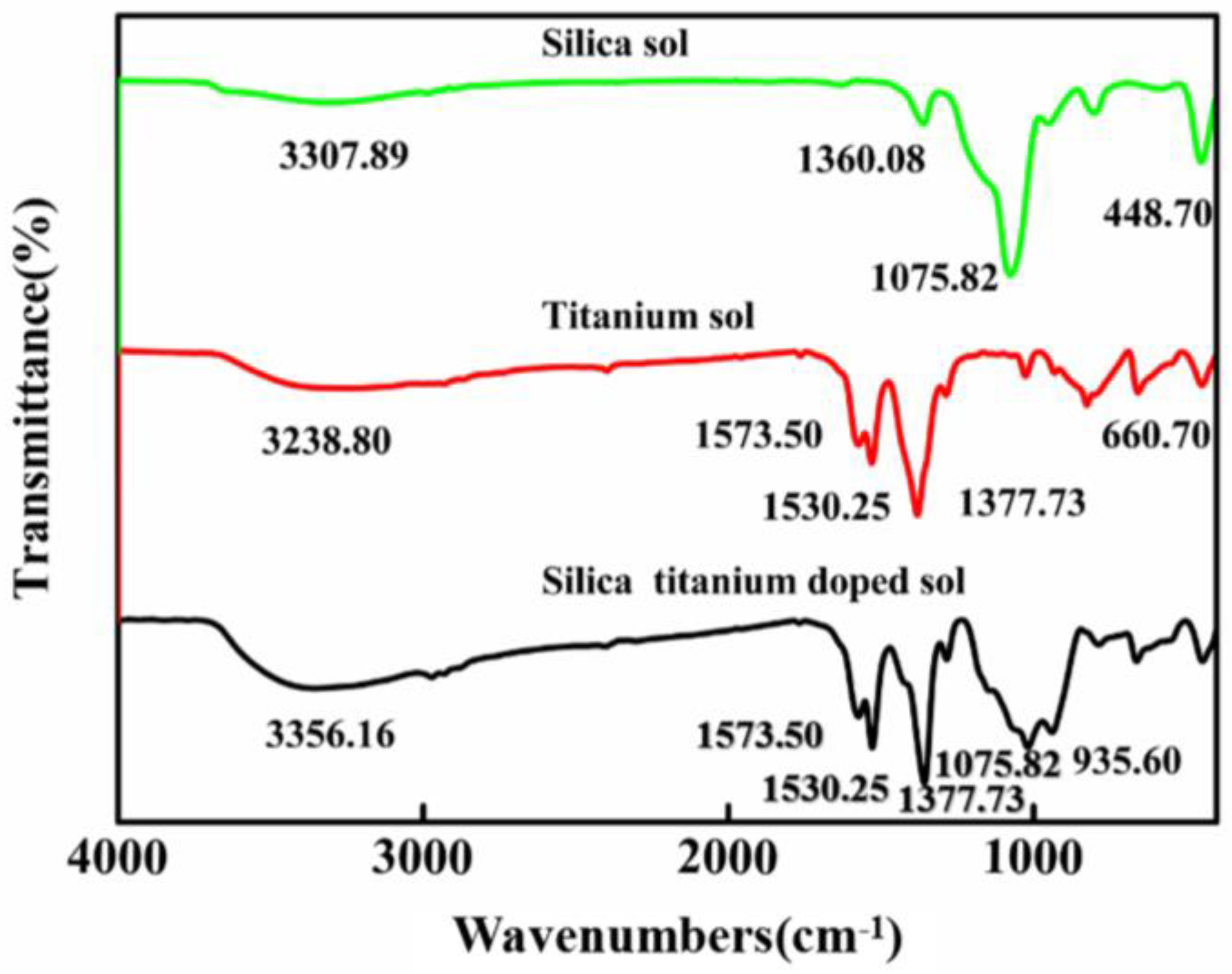
2.4. Characterization of Sol-Gel and Hard Coating
3. Results
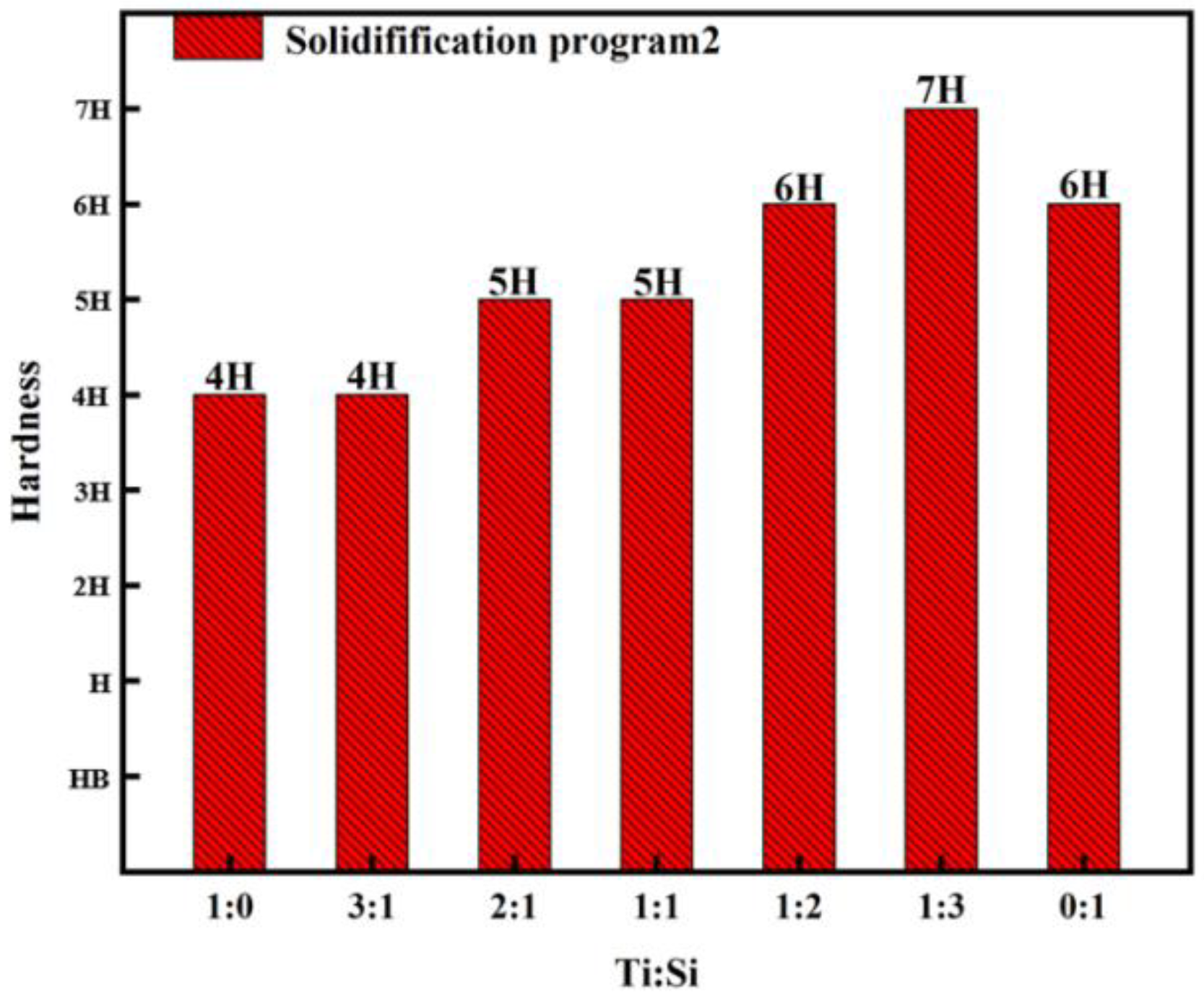
3.1. The Effect of Ti:Si Ratio on the Hardness of Si-Ti Composite Coatings
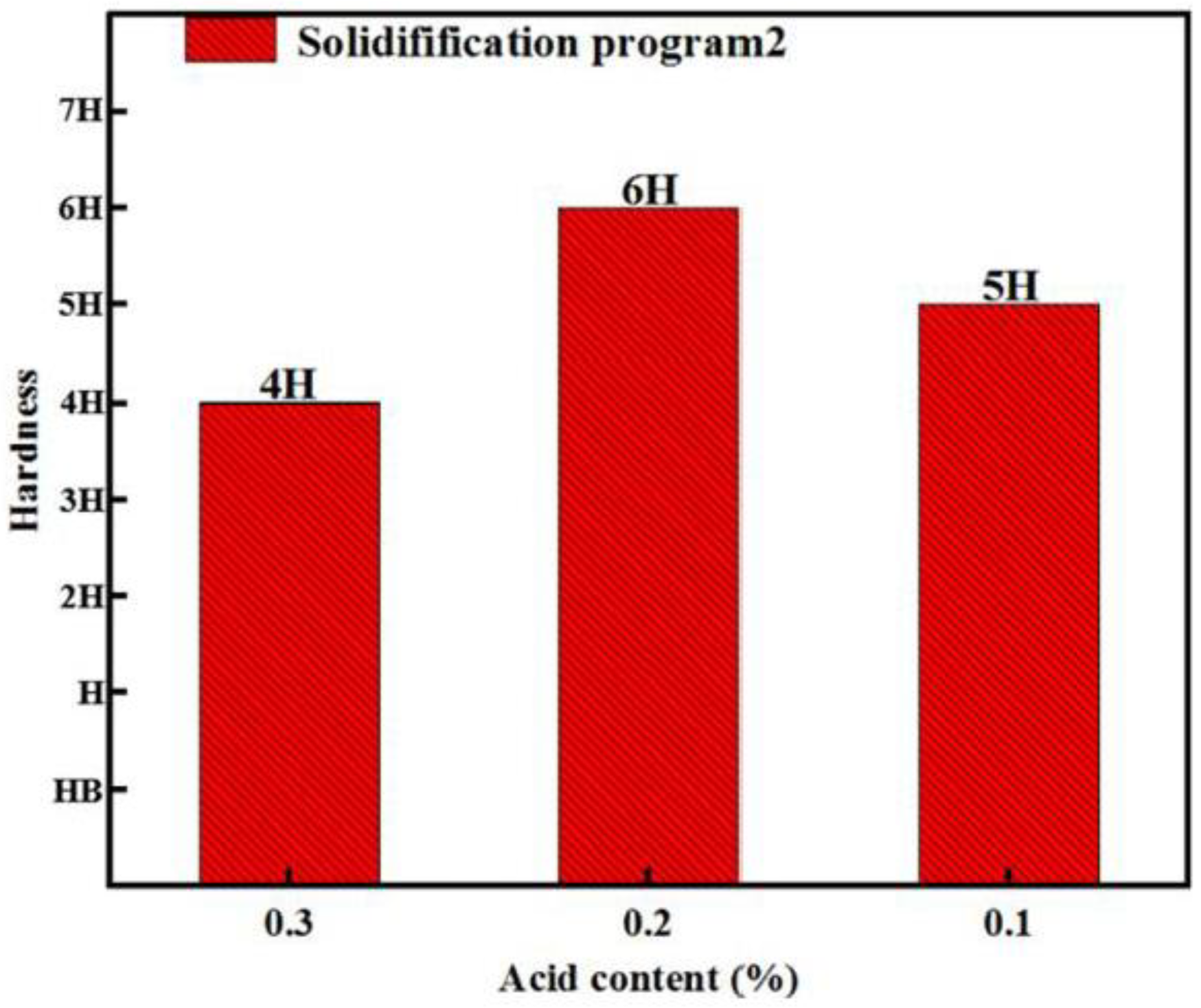
3.2. The Effect of Acid Content on the Hardness of the Coating
3.3. Effect of Solid Content on the Performances of Coatings
3.4. Influence of Crosslinker Content on Coating Properties
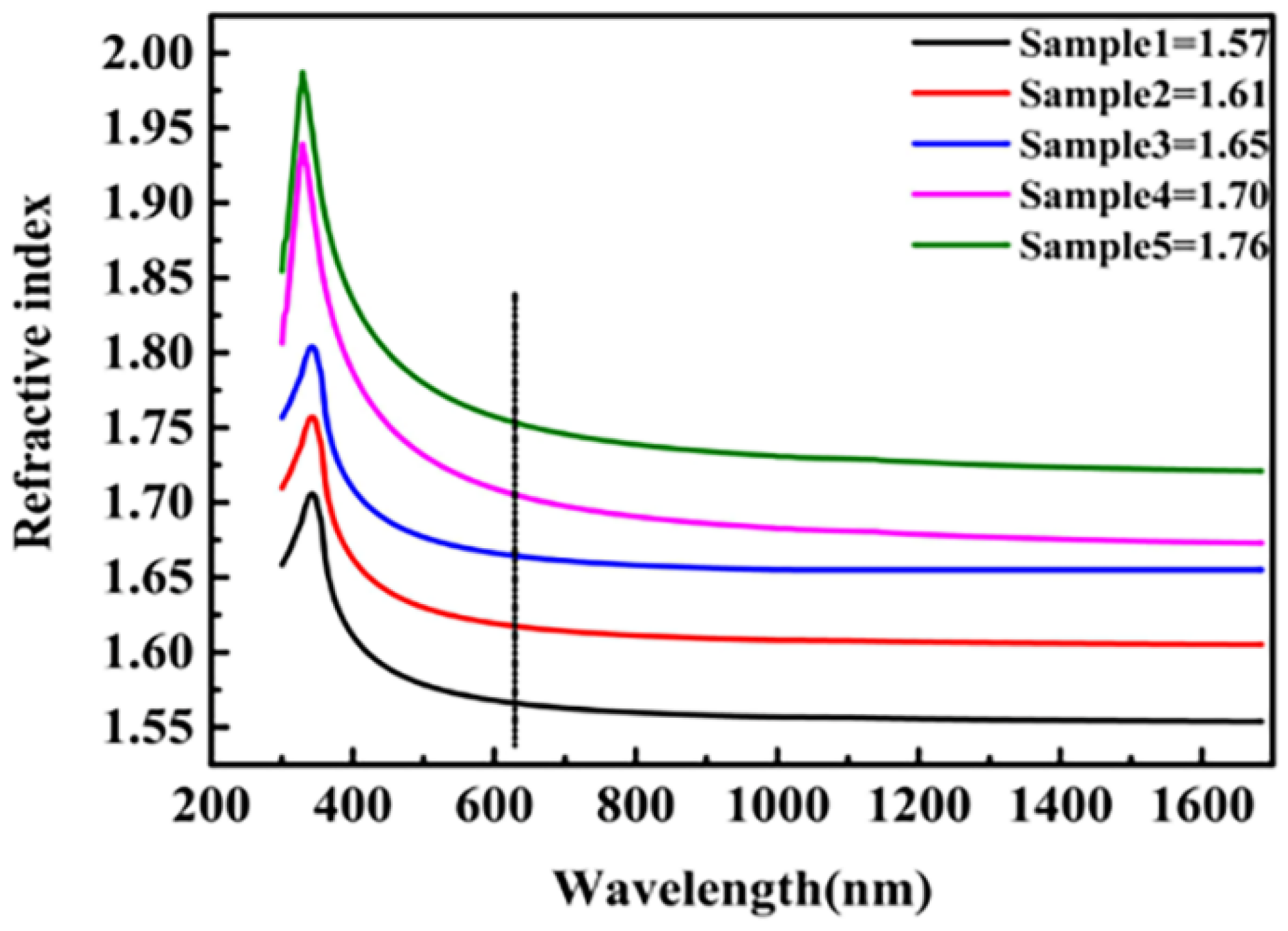
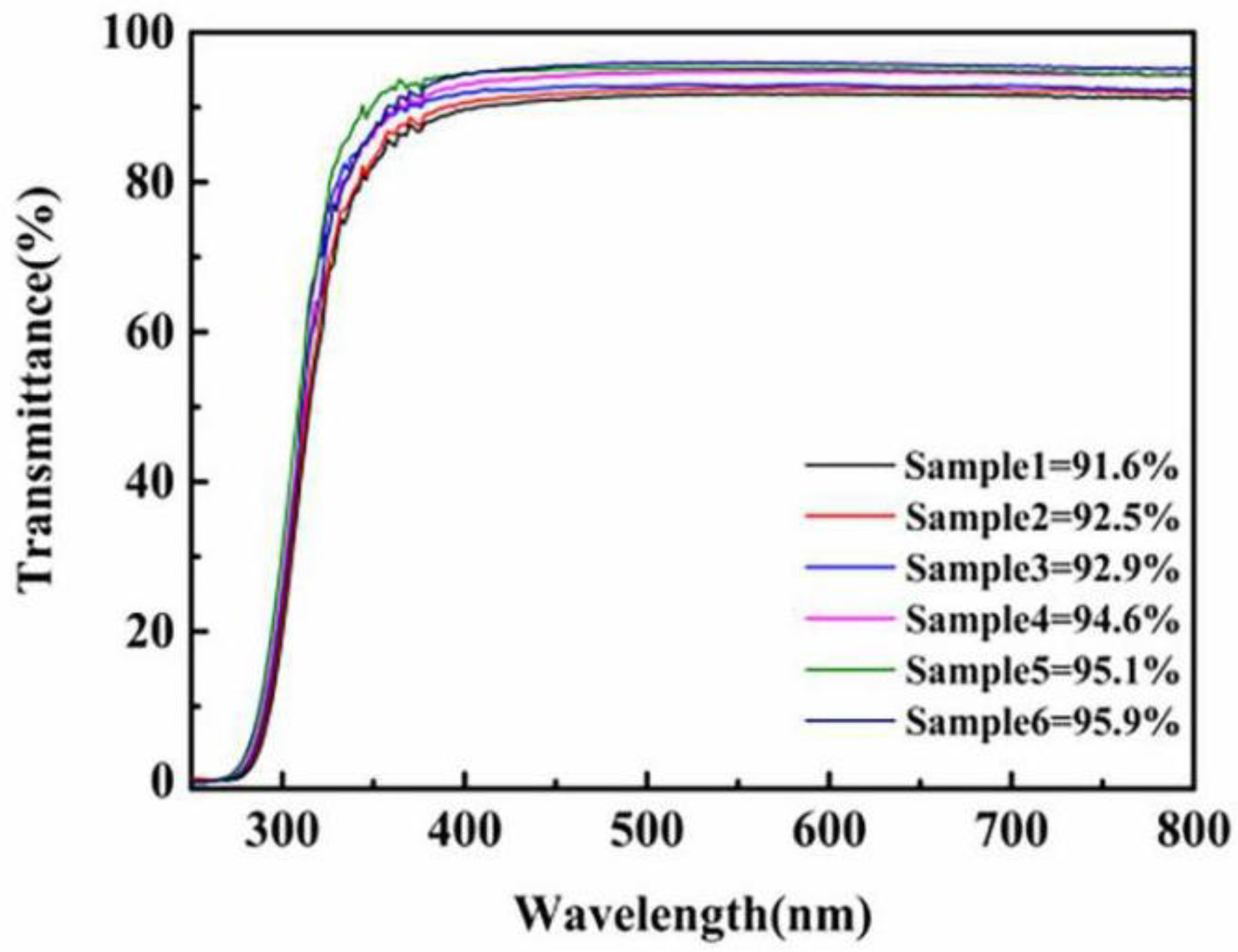
3.5. Optical Properties of Hard Coating Made by Sol-Gel
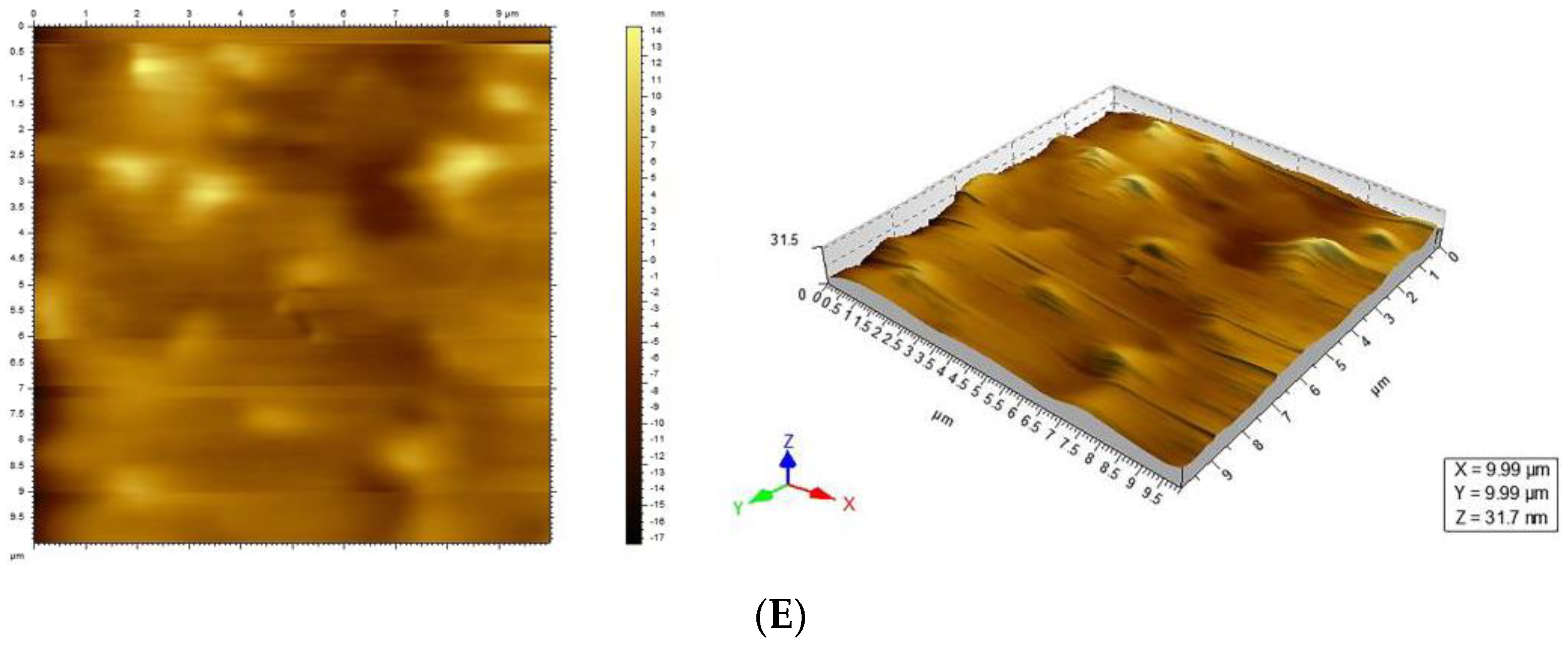
3.6. Microstructure of SiO2/TiO2 Composite Sol Hard Coating
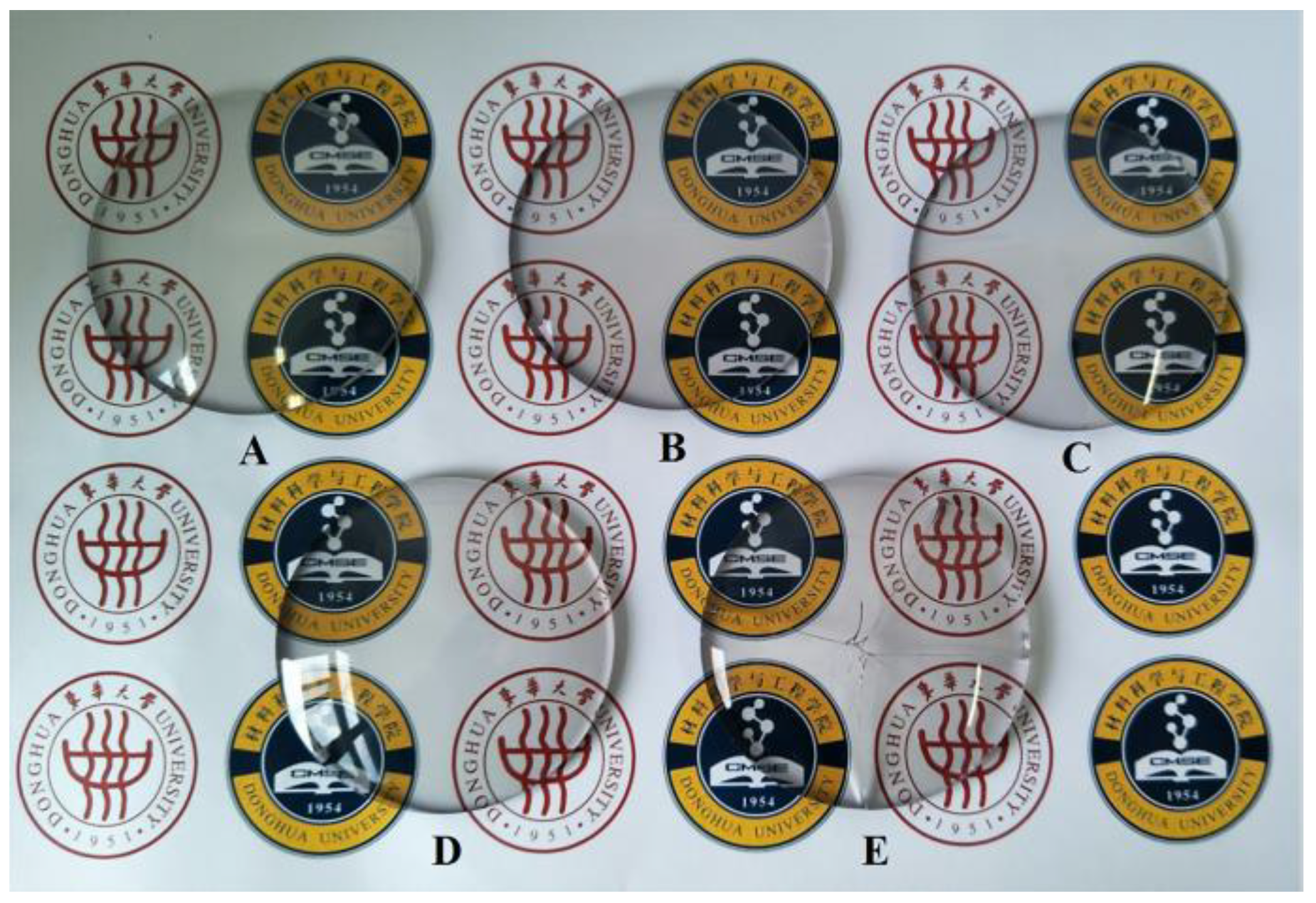
3.7. The Application of SiO2/TiO2 Composite Sol Hard Coating on the Surface of Resin Lens
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, X.; Zhai, T.; Gautam, U.K. ZnS nanostructures: From synthesis to applications. Prog. Mater. Sci. 2011, 56, 175–287. [Google Scholar] [CrossRef]
- Bochev, B.; Yordanov, G. Room temperature synthesis of thioglycolate-coated zinc sulfide (ZnS) nanoparticles in aqueous medium and their physicochemical characterization. Colloids Surf. Physicochem. Eng. Asp. 2014, 441, 84–90. [Google Scholar] [CrossRef]
- Hock, M.; Schäffer, E.; Döll, W.; Kleer, G. Composite coating materials for the moulding of diffractive and refractive optical components of inorganic glasses. Surf. Coat. Technol. 2003, 163. [Google Scholar] [CrossRef]
- Chen, H.-S.; Huang, S.-H.; Perng, T.-P. Highly transparent hard bio-coating synthesized by low temperature sol–gel process. Surf. Coat. Technol. 2013, 233, 140–146. [Google Scholar] [CrossRef]
- Que, W.; Sun, Z.; Zhou, Y. Optical and mechanical properties of TiO2/SiO2/organically modified silane composite films prepared by sol–gel processing. Thin Solid Film. 2000, 359, 177–183. [Google Scholar] [CrossRef]
- Sangermano, M.; Voit, B.; Sordo, F. High refractive index transparent coatings obtained via UV/thermal dual-cure process. Polymer 2000, 49, 2018–2022. [Google Scholar] [CrossRef]
- Hakan Yavas, C.; Duygu Öztürk Selçuk, A.E.; Sinan Özhan, C.D. A parametric study on processing of scratch resistant hybrid sol–gel silica coatings on polycarbonate. Thin Solid Film. 2014, 556, 112–119. [Google Scholar] [CrossRef]
- Takashi, I.; Mackenzie, J.D. Ormosils of High Hardness. MRS Proc. 1994, 346. [Google Scholar] [CrossRef]
- Murahara, M.; Yabe, T.; Uchida, S. Anti-Reflective and Waterproof Hard Coating for High Power Laser Optical Elements. AIP Conf. Proc. 2006, 830, 457–465. [Google Scholar]
- Burgos, M.; Langlet, M. Condensation and Densification Mechanism of Sol-Gel TiO2 Layers at Low Temperature. J. Sol-Gel Sci. Technol. 1999, 16, 267–276. [Google Scholar] [CrossRef]
- Nair, S.; Kumar, P. Molecular characterization of a lipase-producingBacillus pumilusstrain (NMSN-1d) utilizing colloidal water-dispersible polyurethane. World J. Microbiol. Biotechnol. 2007, 23, 1441–1449. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Cordero-Cabrera, M.C.; Fray, D.J. Preparation of high surface area titania (TiO2) films and powders using particulate sol–gel route aided by polymeric fugitive agents. Sens. Actuators B 2006, 120, 86–95. [Google Scholar] [CrossRef]
- Wu, D.; Xu, H.; Qiu, F. Preparation Morphology and Properties of Waterborne-Polyurethane/Silica. J. Macromol. Sci. Part D-Rev. Polym. Process. 2011, 50, 498–508. [Google Scholar] [CrossRef]
- Agrawal, S.; Patidar, D.; Saxena, N.S. Glass transition temperature and thermal stability of ZnS/PMMA nanocomposites. Phase Transit. 2011, 84, 888–900. [Google Scholar] [CrossRef]
- Hassan, M.L.; Fadel, S.M.; Moorefield, C. Dendronized Cellulose Nanocrystals as Templates for Preparation of ZnS and CdS Quantum Dots. J. Macromol. Sci. Part A 2014, 51, 743–749. [Google Scholar] [CrossRef]
- Jena, K.K.; Narayan, R.; Raju, K.V.S.N. Surface functionalized zinc oxide (ZnO) nanoparticle filled organic–inorganic hybrid materials with enhanced thermo-mechanical properties. Prog. Org. Coat. 2015, 89, 82–90. [Google Scholar] [CrossRef]
- Liu, B.T.; Li, P.-S.; Chen, W.-C. Ex situ synthesis of high-refractive-index polyimide hybrid films containing TiO2 chelated by 4-aminobenzoic acid. Eur. Polym. J. 2014, 50, 54–60. [Google Scholar] [CrossRef]
- Li, G.J.; Huang, Y.G.; Zhu, Z.Z. Fluorescence spectra of zinc sulfide quantum dots/polyurethane nano-composite. J. South China Univ. Technol. 2009, 37, 1–5. [Google Scholar]
- Salleh, N.G.N.; Alias, M.S.; Gläsel, H.-J.; Mehnert, R. High performance radiation curable hybrid coatings. Radiat. Phys. Chem. 2013, 84, 70–73. [Google Scholar] [CrossRef]
- Xu, S.; Hong, Y.; Chen, C. A general synthetic strategy for ordered, extra-large mesoporous metal oxides via uniform sol–gel coating. J. Mater. Chem. A 2013, 1, 6191. [Google Scholar] [CrossRef]
- Faustini, M.; Nicole, L. Hydrophobic, Antireflective, Self-Cleaning, and Antifogging SolGel Coatings: An Example of Multifunctional Nanostructured Materials for Photovoltaic Cells. Chem. Mater. 2010, 22, 4406–4413. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Basak, S.; Das, J.K.; Medda, S.K.; Chattopadhyay, K.; De, G. Ag-TiO2 nanoparticle codoped SiO2 films on ZrO2 barrier-coated glass substrates with antibacterial activity in ambient condition. ACS Appl. Mater. Interfaces 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Que, W.; Hu, X. Optical and mechanical properties of sol–gel silica–titania hard optical coatings derived from methyltrimethoxysilane and tetrapropylorthotitanate as precursors. Opt. Mater. 2003, 22, 31–37. [Google Scholar] [CrossRef]
- Lukosz, W.; Tiefenthaler, K. Embossing technique for fabricating integrated optical components in hard inorganic waveguiding materials. Opt. Lett. 1983, 8, 537–539. [Google Scholar] [CrossRef] [Green Version]
- Mallak, M.; Bockmeyer, M.; Löbmann, P. Liquid phase deposition of TiO2 on glass: Systematic comparison to films prepared by sol–gel processing. Thin Solid Film. 2007, 515, 8072–8077. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Deepthi, B.; Rajam, K.S. Growth and characterization of aluminum nitride coatings prepared by pulsed-direct current reactive unbalanced magnetron sputtering. Thin Solid Film. 2008, 516, 4168. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, W.; Liu, W. Organic modification and application of silica sol. Appl. Chem. Ind. 2020, 49, 536–539. [Google Scholar]
- Shen, Y.; Wang, L.; Zhang, H. Preparation and characterization of titania/silicone nanocomposite material. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012021. [Google Scholar] [CrossRef]









| Sample | Solid Content (%) | Gel State | Film Forming Property | Storage Period (d) |
|---|---|---|---|---|
| 1 | 35 | Deep yellow | edge cracking | ≤15 |
| 2 | 30 | Yellow | edge cracking | ≤60 |
| 3 | 25 | Light yellow transparent | Good | ≥120 |
| 4 | 20 | Light yellow transparent | Good | ≥120 |
| 5 | 15 | Light yellow transparent | Good | ≥120 |
| 6 | 10 | Light yellow transparent | Good | ≥120 |
| 7 | 5.0 | Colorless transparent | Good | ≥120 |
| Si Ti ratio | Water Resistance Test | Alkali Resistance Test | Adhesion Grade |
|---|---|---|---|
| 1:2 | No blistering, wrinkling, cracking and falling off | No blistering, pulverization, softening and falling off | 0 |
| 1:3 | No blistering, wrinkling, cracking and falling off | No blistering, pulverization, softening and falling off | 0 |
| 1:5 | No blistering, wrinkling, cracking and falling off | No blistering, pulverization, softening and falling off | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, W.; Cai, S.; Zhang, Y.; Chen, H. Preparation and Optical Application of SiO2-TiO2 Composite Hardening Coatings with Controllable Refractive Index by Synchronous Polymerization. Coatings 2021, 11, 129. https://doi.org/10.3390/coatings11020129
Du W, Cai S, Zhang Y, Chen H. Preparation and Optical Application of SiO2-TiO2 Composite Hardening Coatings with Controllable Refractive Index by Synchronous Polymerization. Coatings. 2021; 11(2):129. https://doi.org/10.3390/coatings11020129
Chicago/Turabian StyleDu, Weiping, Shuting Cai, Yang Zhang, and Huifang Chen. 2021. "Preparation and Optical Application of SiO2-TiO2 Composite Hardening Coatings with Controllable Refractive Index by Synchronous Polymerization" Coatings 11, no. 2: 129. https://doi.org/10.3390/coatings11020129





