Development of Adhesive, Bioactive and Antibacterial Titania Sol-Gel Coating on Titanium Substrate by Dip-Coating Technique
Abstract
:1. Introduction
2. Experimental
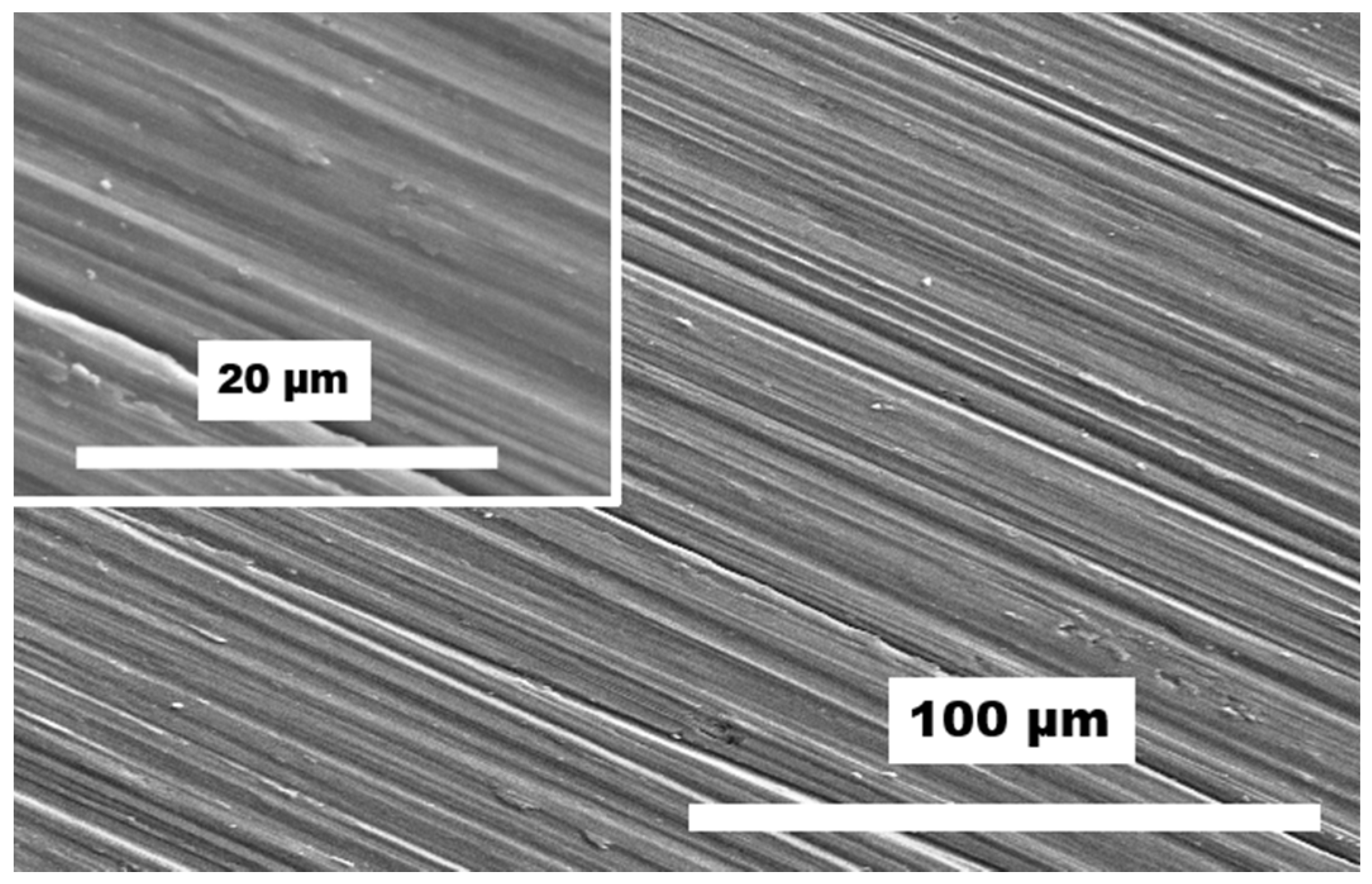
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keane, T.J.; Badylak, S.F. Biomaterials for tissue engineering applications. Sem. Ped. Sur. 2014, 23, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Gerhard, E.; Zhang, C.; Tierney, J.W.; Xie, D.; Liu, Z.; Yang, J. Polymeric biomaterials for biophotonic applications. Bioact. Mat. 2018, 3, 434–445. [Google Scholar] [CrossRef]
- Hussein, M.A.; Mohammed, A.S.; Al-Aqeeli, N. Wear characteristics of metallic biomaterials: A review. Materials 2015, 8, 2749–2768. [Google Scholar] [CrossRef]
- Milella, E.; Cosentino, F.; Licciulli, A.; Massaro, C. Preparation and characterisation of titania/hydroxyapatite composite coatings obtained by sol-gel proces. Biomaterials 2001, 22, 1425–1431. [Google Scholar] [CrossRef]
- Guo, L.; Feng, W.; Liu, X.; Lin, C.; Li, B.; Qiang, Y. Sol-gel synthesis of antibacterial hybrid coatings on titanium. Mat. Let. 2015, 160, 448–451. [Google Scholar] [CrossRef] [Green Version]
- Fu, T.; Shen, Y.; Alajmi, Z.; Wang, Y.; Yang, S.; Li, G. Sol-gel derived Ag-containing TiO2 films on surface roughened biomedical NiTi alloy. Ceram. Int. 2014, 40, 12423–12429. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Methods for Measuring Adhesion by Tape Test; ASTM International: West Conshohocken, PA, USA, 2006. [Google Scholar]
- Cheng, K.; Ren, C.; Weng, W.; Du, P.; Shen, G.; Han, G.; Zhang, S. Bonding strength of fluoridated hydroxyapatite coatings: A comparative study on pull-out and scratch analysis. Thin Solid Films 2009, 517, 5361–5364. [Google Scholar] [CrossRef]
- Zhang, S.; Xianting, Z.; Yongsheng, W.; Kui, C.; Wenjian, W. Adhesion strength of sol-gel derived fluoridated hydroxyapatite coatings. Surf. Coat. Tech. 2006, 200, 6350–6354. [Google Scholar] [CrossRef]
- Wen, C.E.; Xu, W.; Hu, W.Y.; Hodgson, P.D. Hydroxyapatite/titania sol-gel coatings on titanium-zirconium alloy for biomedical applications. Acta Biomater. 2007, 3, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.N.; Teo, E.Y.; Ho, B.; Tay, B.Y.; Thian, E.S. Effect of silver content on the antibacterial and bioactive properties of silver-substituted hydroxyapatite. J. Biomed. Mater. Res. A. 2013, 101, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Hu, W.; Li, M. Sol-gel derived hydroxyapatite/titania biocoatings on titanium substrate. Mater. Lett. 2006, 60, 1575–1578. [Google Scholar] [CrossRef]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ouyang, S.; Umezawa, N.; Cao, J.; Ye, J. Facet effect of single-crystalline Ag3PO4 sub-microcrystals on photocatalytic properties. J. Am. Chem. Soc. 2011, 133, 6490–6492. [Google Scholar] [CrossRef] [PubMed]
- Saravanapavan, P.; Gough, J.E.; Jones, J.R.; Hench, L.L. Antimicrobial macroporous gell-glasses: Disolution and cytotoxicity. key Eng. Mater. 2004, 254, 1087–1090. [Google Scholar] [CrossRef]
- Buckley, J.J.; Lee, A.F.; Olivi, L.; Wilson, K. Hydroxyapatite supported antibacterial Ag3PO4 nanoparticles. J. Mater. Chem. 2010, 20, 8056–8063. [Google Scholar] [CrossRef] [Green Version]
- Viana, M.M.; Mohallem, N.D.S.; Miquita, D.R.; Balzuweit, K.; Silva-Pinto, E. Preparation of amorphous and crystalline Ag/TiO2 nanocomposite thin films. App. Surf. Sci. 2013, 265, 130–136. [Google Scholar] [CrossRef]
- Babapour, A.; Akhavan, O.; Moshfegh, A.Z.; Hosseini, A.A. Size variation and optical absorption of sol-gel Ag nanoparticles doped SiO2 thin film. Thin Solid Films 2006, 515, 771–774. [Google Scholar] [CrossRef]
- Horkavcová, D.; Zmeškal, Z.; Šanda, L.; Cílová, Z.Z.; Jablonská, E.; Helebrant, A. Preparation of titania sol-gel coatings containing silver in various forms and measurement of their bactericidal effects against E. coli. Ceramics-Silikáty 2015, 59, 238–243. [Google Scholar]
- Horkavcová, D.; Novák, P.; Fialová, I.; Černý, M.; Jablonská, E.; Lipov, J.; Ruml, T.; Helebrant, A. Titania sol-gel coatings contain-ing silver on newly developed TiSi alloys and their antibacterial effect. Mater. Sci. Eng. C 2017, 76, 25–30. [Google Scholar] [CrossRef]
- ISO 23317:2014. Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials; International Organization for Standard: Geneva, Switzerland, 2014. [Google Scholar]
- Martí, M.; Frígols, B.; Serrano-Aroca, A. Antimicrobial characterization of advanced materials for bioengineering applications. J. Vis. Exp. 2018, 138, e57710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. A sol-gel based silver nanoparticle/polytetrafluorethylene (AgNP/PTFE) coating with enhanced antibacterial and anti-corrosive properties. App. Surf. Sci. 2021, 535, 147675. [Google Scholar] [CrossRef]




| Type of the Sol (Coating) | Composition of the Sol (Coating) |
|---|---|
| T | Titania basic sol |
| TAN | Titania basic sol + AgNO3 |
| TAP | Titania basic sol + Ag3PO4 |
| THA | Titania basic sol + hydroxyapatite |
| TANHA | Titania basic sol + AgNO3 + hydroxyapatite |
| TAPHA | Titania basic sol + Ag3PO4 + hydroxyapatite |
| Solution | Ionic Concentration (mmol/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | Mg2+ | Cl− | HPO42− | HCO3− | SO42− | |
| MSBF | 142.0 | 5.0 | 2.5 | 1.0 | 131 | 1.0 | 5.0 | 1.0 |
| Type of the Coating | Antibacterial Effect (%) | |
|---|---|---|
| After 4 h | After 24 h | |
| T | 19 | 52 |
| TAN | 7 | 77 |
| TAP | 7 | 60 |
| THA | 0 | 61 |
| TANHA | 31 | 100 |
| TAPHA | 58 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horkavcová, D.; Doubet, Q.; Lecomte-Nana, G.L.; Jablonská, E.; Helebrant, A. Development of Adhesive, Bioactive and Antibacterial Titania Sol-Gel Coating on Titanium Substrate by Dip-Coating Technique. Coatings 2021, 11, 243. https://doi.org/10.3390/coatings11020243
Horkavcová D, Doubet Q, Lecomte-Nana GL, Jablonská E, Helebrant A. Development of Adhesive, Bioactive and Antibacterial Titania Sol-Gel Coating on Titanium Substrate by Dip-Coating Technique. Coatings. 2021; 11(2):243. https://doi.org/10.3390/coatings11020243
Chicago/Turabian StyleHorkavcová, Diana, Quentin Doubet, Gisèle Laure Lecomte-Nana, Eva Jablonská, and Aleš Helebrant. 2021. "Development of Adhesive, Bioactive and Antibacterial Titania Sol-Gel Coating on Titanium Substrate by Dip-Coating Technique" Coatings 11, no. 2: 243. https://doi.org/10.3390/coatings11020243
APA StyleHorkavcová, D., Doubet, Q., Lecomte-Nana, G. L., Jablonská, E., & Helebrant, A. (2021). Development of Adhesive, Bioactive and Antibacterial Titania Sol-Gel Coating on Titanium Substrate by Dip-Coating Technique. Coatings, 11(2), 243. https://doi.org/10.3390/coatings11020243








