Eco-Friendly Fire-Resistant Coatings Containing Dihydrogen Ammonium Phosphate Microcapsules and Tannins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Prepolymer and Preparation of the MAP Microcapsules
2.2. Coating Formulation, Surface Preparation, and Application onto Substrates
2.3. Characterization and Fire Behavior Tests
2.3.1. Chemical, Morphological, and Structural Characterization of Microcapsules
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. Evaluation of the Properties of the Coatings
2.3.4. Fire Behavior of the Coating
3. Results and Discussions
3.1. Chemical, Morphological, and Structural Characterization of Microcapsules
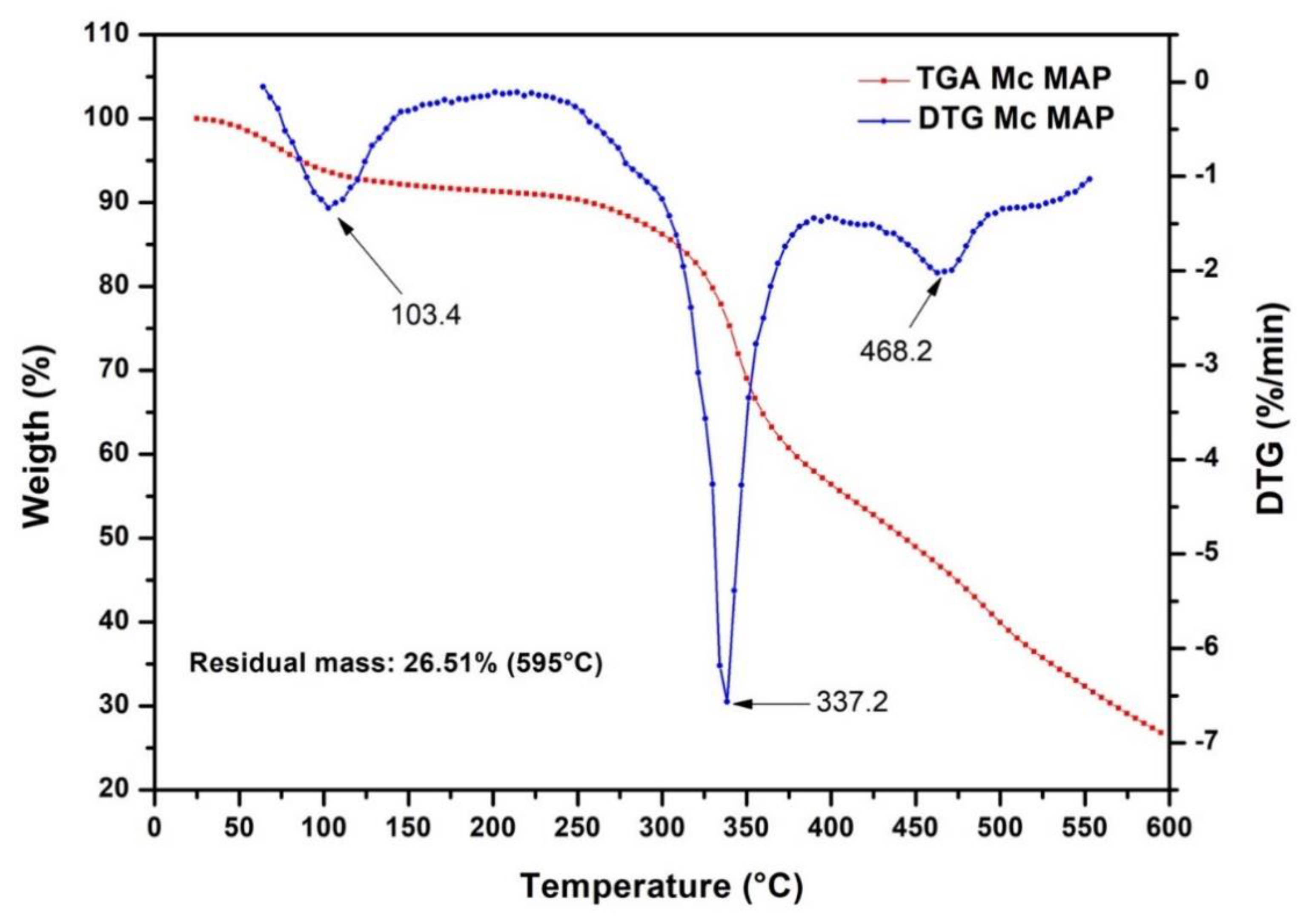
3.2. Thermogravimetric Analysis (TGA)
3.3. Formulation of Coatings with MAP Microcapsules
3.4. Evaluation of the Mechanical Properties of the Coatings
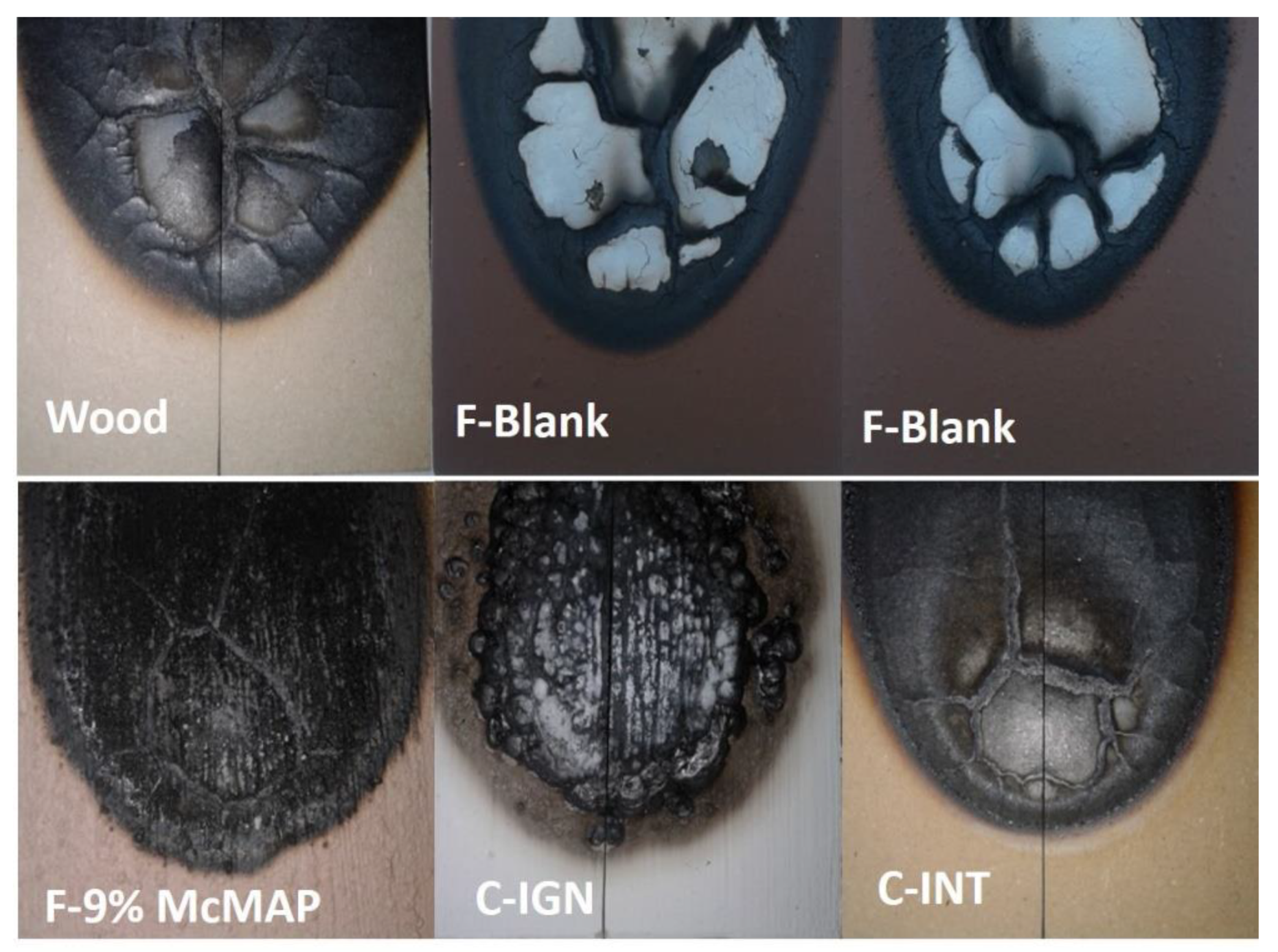
3.5. Fire Behavior of the Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jimenez, M.; Duquesne, S.; Bourbigot, S. Intumescent fire protective coating: Toward a better understanding of their mechanism of action. Thermochim. Acta 2006, 449, 16–26. [Google Scholar] [CrossRef]
- Puri, R.G.; Khanna, A. Effect of cenospheres on the char formation and fire protective performance of water-based intumescent coatings on structural steel. Prog. Org. Coat. 2016, 92, 8–15. [Google Scholar] [CrossRef]
- Han, Y.; Wu, H.; Zhang, W.; Zou, D.; Liu, G.; Qiao, G. Constitutive equation and dynamic recrystallization behavior of as-cast 254SMO super-austenitic stainless steel. Mater. Des. 2015, 69, 230–240. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Han, E.-H.; Ke, W. Fire-resistant effect of nanoclay on intumescent nanocomposite coatings. J. Appl. Polym. Sci. 2006, 103, 1681–1689. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, M.; Zhang, Y.; Gao, X.; Zhang, Q. Preparation and performances of novel waterborne intumescent fire retardant coatings. Prog. Org. Coat. 2016, 95, 100–106. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Deng, C.; Tan, Y.; Chen, M.-J.; Chen, L.; Wang, Y.-Z. Flame retardation of polypropylene via a novel intumescent flame retardant: Ethylenediamine-modified ammonium polyphosphate. Polym. Degrad. Stab. 2014, 106, 88–96. [Google Scholar] [CrossRef]
- Cao, K.; Wu, S.-L.; Wang, K.-L.; Yao, Z. Kinetic study on surface modification of ammonium polyphosphate with melamine. Ind. Eng. Chem. Res. 2011, 50, 8402–8406. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, M.; Hu, Q.; Liu, S.; Gao, X.; Ren, F.; Zhang, Q. Effect of microencapsulated ammonium polyphosphate on the durability and fire resistance of waterborne intumescent fire-retardant coatings. J. Coat. Technol. Res. 2019, 16, 135–145. [Google Scholar] [CrossRef]
- Yew, M.C.; Sulong, N.R. Fire-resistive performance of intumescent flame-retardant coatings for steel. Mater. Des. 2012, 34, 719–724. [Google Scholar] [CrossRef]
- Wu, K.; Song, L.; Wang, Z.; Hu, Y. Microencapsulation of ammonium polyphosphate with PVA-melamine-formaldehyde resin and its flame retardance in polypropylene. Polym. Adv. Technol. 2008, 19, 1914–1921. [Google Scholar] [CrossRef]
- Montoya, L.; Contreras, D.; Jaramillo, A.; Carrasco, C.; Fernández, K.; Schwederski, B.; Rojas, D.; Melendrez, M. Study of anticorrosive coatings based on high and low molecular weight polyphenols extracted from the Pine radiata bark. Prog. Org. Coat. 2019, 127, 100–109. [Google Scholar] [CrossRef]
- Jaramillo, A.; Montoya, L.; Prabhakar, J.M.; Sanhueza, J.; Fernández, K.; Rohwerder, M.; Rojas, D.; Montalba, C.; Melendrez, M. Formulation of a multifunctional coating based on polyphenols extracted from the Pine radiata bark and functionalized zinc oxide nanoparticles: Evaluation of hydrophobic and anticorrosive properties. Prog. Org. Coat. 2019, 135, 191–204. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Stein, N.; Boulanger, C.; Pizzi, A.; Celzard, A. Pore structure and electrochemical performances of tannin-based carbon cryogels. Biomass Bioenergy 2012, 39, 274–282. [Google Scholar] [CrossRef]
- Celzard, A.; Fierro, V.; Amaral-Labat, G.; Pizzi, A.; Torero, J. Flammability assessment of tannin-based cellular materials. Polym. Degrad. Stab. 2011, 96, 477–482. [Google Scholar] [CrossRef]
- ASTM D1360-90a Standard Test Method for Small Scale Evaluation of Fire-Retardant Paints; American Society for Testing and Materials: Philadelphia, PA, USA, 1994.
- Bocalandro, C.; Sanhueza, V.; Gómez-Caravaca, A.M.; González-Álvarez, J.; Fernández, K.; Roeckel, M.; Rodríguez-Estrada, M.T. Comparison of the composition of Pinus radiata bark extracts obtained at bench- and pilot-scales. Ind. Crops Prod. 2012, 38, 21–26. [Google Scholar] [CrossRef]
- Asbeck, W.K.; Van Loo, M. Critical pigment volume relationships. Ind. Eng. Chem. 1949, 41, 1470–1475. [Google Scholar] [CrossRef]
- Rodríguez, M.; Rodrı́guez, M.; Gracenea, J.; Saura, J.; Suay, J. The influence of the critical pigment volume concentration (CPVC) on the properties of an epoxy coating Part II. Anticorrosion and economic properties. Prog. Org. Coat. 2004, 50, 68–74. [Google Scholar] [CrossRef]
- ASTM D562 Standard Test Method for Consistency of Paints Measuring Krebs Unit (KU) Viscosity Using a Stormer-Type Viscometer; American Society for Testing and Materials: West Conshohocken, PA, USA, 2018.
- ASTM D6132-13(2017) Standard Test Method for Nondestructive Measurement of Dry Film Thickness of Applied Organic Coatings Using an Ultrasonic Coating Thickness Gage; American Society for Testing and Materials: West Conshohocken, PA, USA, 2017.
- ASTM D4541-17 Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers; American Society for Testing and Materials: West Conshohocken, PA, USA, 2017.
- ASTM D4060-19 Standard Test Method for Abrasion Resistance of Organic Coatings by the Taber Abraser; American Society for Testing and Materials: West Conshohocken, PA, USA, 2019.
- ISO 1519 Paints and Varnishes—Bend Test (Cylindrical Mandrel); International Organization for Standardization (ISO): Geneva, Switzerland, 2011.
- ISO 1520 Paints and Varnishes—Cupping Test; International Organization for Standardization (ISO): Geneva, Switzerland, 2006.
- Chupin, L.; Motillon, C.; Bouhtoury, F.C.-E.; Pizzi, A.; Charrier, B. Characterisation of maritime pine (Pinus pinaster) bark tannins extracted under different conditions by spectroscopic methods, FTIR and HPLC. Ind. Crops Prod. 2013, 49, 897–903. [Google Scholar] [CrossRef]
- El-Zaher, N.A.; Osiris, W.G. Thermal and structural properties of poly(vinyl alcohol) doped with hydroxypropyl cellulose. J. Appl. Polym. Sci. 2005, 96, 1914–1923. [Google Scholar] [CrossRef]
- Giraud, S.; Bourbigot, S.; Rochery, M.; Vroman, I.; Tighzert, L.; Delobel, R. Microencapsulation of phosphate. Polym. Degrad. Stab. 2002, 77, 285–297. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kwon, D.-J.; Shin, P.-S.; Baek, Y.-M.; Park, H.-S.; Devries, K.L.; Park, J.-M. The evaluation of the interfacial and flame retardant properties of glass fiber/unsaturated polyester composites with ammonium dihydrogen phosphate. Compos. Part B Eng. 2019, 167, 221–230. [Google Scholar] [CrossRef]
- Lim, W.P.; Mariatti, M.; Chow, W.; Mar, K. Effect of intumescent ammonium polyphosphate (APP) and melamine cyanurate (MC) on the properties of epoxy/glass fiber composites. Compos. Part B Eng. 2012, 43, 124–128. [Google Scholar] [CrossRef]
- ISO 5660-1 Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement)); International Organization for Standardization (ISO): Geneva, Switzerland, 2015.
- ISO 9705-1 Reaction to Fire Tests—Room Corner Test for Wall and Ceiling Lining Products—Part 1: Test Method for a Small Room Configuration; International Organization for Standardization (ISO): Geneva, Switzerland, 2016.




| Formulations | F-Blank | F-3% McMAP | F-9% McMAP |
|---|---|---|---|
| Components | |||
| Water | 27.80 | 27.74 | 27.80 |
| Caolín (Opacit) | 5.12 | 4.75 | 4.75 |
| Calcium carbonate (Hifill) | 13.33 | 12.35 | 3.01 |
| L-MWT | 15.50 | 15.00 | 15.00 |
| McMAP | 0.00 | 3.01 | 9.03 |
| Foamaster MO 2134 | 0.16 | 0.14 | 0.14 |
| Acronal S716 1.09 | 31.00 | 26.00 | 26.00 |
| Vibration Signal | Melamine | PVA | MAP | McMAP |
|---|---|---|---|---|
| Wavenumber (cm−1) | ||||
| N−H Stretching Primary Amine | 3500 3440 | – | – | – |
| N=C Stretching | 1630 | – | – | – |
| N−H Bending Primary Amine | 1560 | – | – | 1560 |
| N−C Stretching | 1020 | – | – | 1138 |
| O−H Stretching Alcohol | – | 3249 | – | 3200 |
| C−H Stretching sp2 sp3 | – | 2936 2906 | – | – |
| C=C Stretching alkene | – | 1664 | – | 1664 |
| C−H Bending alkane | – | 1430 | – | – |
| O−H Bending | – | 1330 | – | – |
| C=C Bending alkene | – | 840 | – | – |
| NH4+ | – | – | 3200 | – |
| P=O Stretching | – | – | 1256 | 1256 |
| P−O Symmetric Stretching | – | – | 1075 | – |
| P−O Asymmetric Stretching | – | – | 880 | 880 |
| Sample | T5% | Tmax1 | Tmax2 | Tmax3 | Residual Mass (%) | ||
|---|---|---|---|---|---|---|---|
| 400 °C | 500 °C | 600 °C | |||||
| McMAP | 90.00 °C | 103.40 °C | 337.20 °C | 468.20 °C | 50% | 40% | 27% |
| Formulations | PVC | CPVC | λ |
|---|---|---|---|
| F-Blank | 49.90 | 36.53 | 1.37 |
| F-3% McMAP | 58.70 | 39.52 | 1.49 |
| F-9% McMAP | 65.42 | 43.46 | 1.51 |
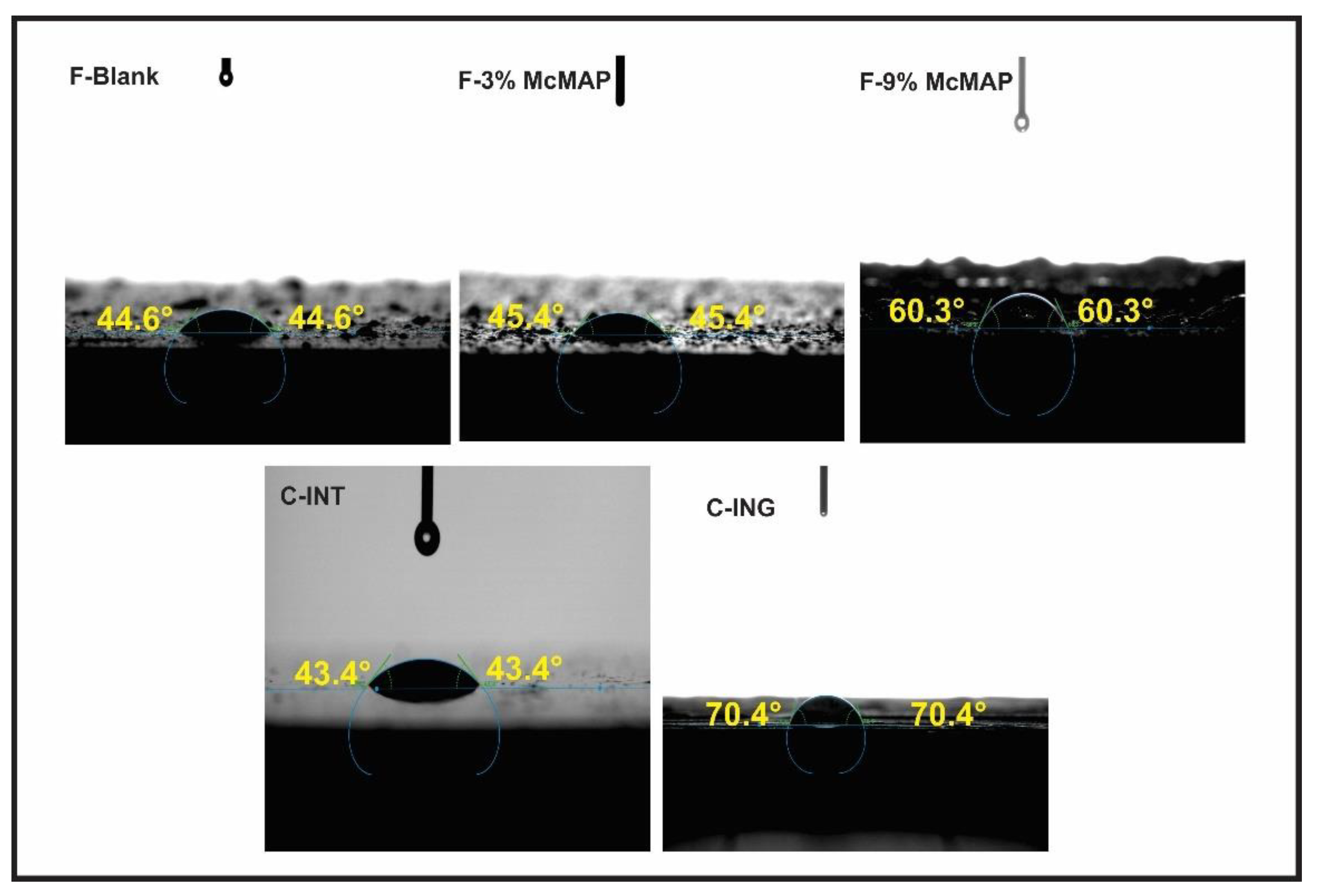
| Formulations | Viscosity (cp) | Steel Substrate | Wood Substrate | Contact Angles |
|---|---|---|---|---|
| Dry Film Thickness (µm) | Dry Film Thickness (µm) | ° | ||
| F-Blank | 381.00 | 153.50 (± 15.40) | 125.10 (± 0.07) | 44.56 (± 1.10) |
| F-3% McMAP | 1743.00 | 190.80 (± 27.40) | 99.10 (± 0.49) | 45.38 (± 4.78) |
| F-9% McMAP | 2402.00 | 154.90 (± 13.40) | 65.55 (± 2.68) | 60.27 (± 6.02) |
| C-INT | – | 256.86 (± 43.35) | 65.73 (± 1.73) | 43.42 (± 9.06) |
| C-IGN | – | 49.46 (± 5.48) | 61.65 (± 0.49) | 70.34 (± 2.77) |
| Formulations | Abrasion | Flexibility | Cupping | Adhesion | |
|---|---|---|---|---|---|
| Wear Index (mg) | Diameter Mandrel without Fail (mm) | Impact Resistance (mm) | MPa | Type Failure | |
| F-Blank | 193.40 | 12 | 1.00 (± 0.68) | 1.77 (± 0.03) | 100% cohesive |
| F-3% McMAP | 179.10 | 12 | 0.59 (± 0.04) | 0.74 (± 0.04) | 90% cohesive |
| F-9% McMAP | – | – | – | – | – |
| C-INT | 342.70 | 16 | 1.70 (± 0.12) | 1.06 (± 0.04) | 90% cohesive |
| C-IGN | 274.22 | 2 | 8.38 (± 0.04) | 3.50 (± 0.69) | 98% adhesive |
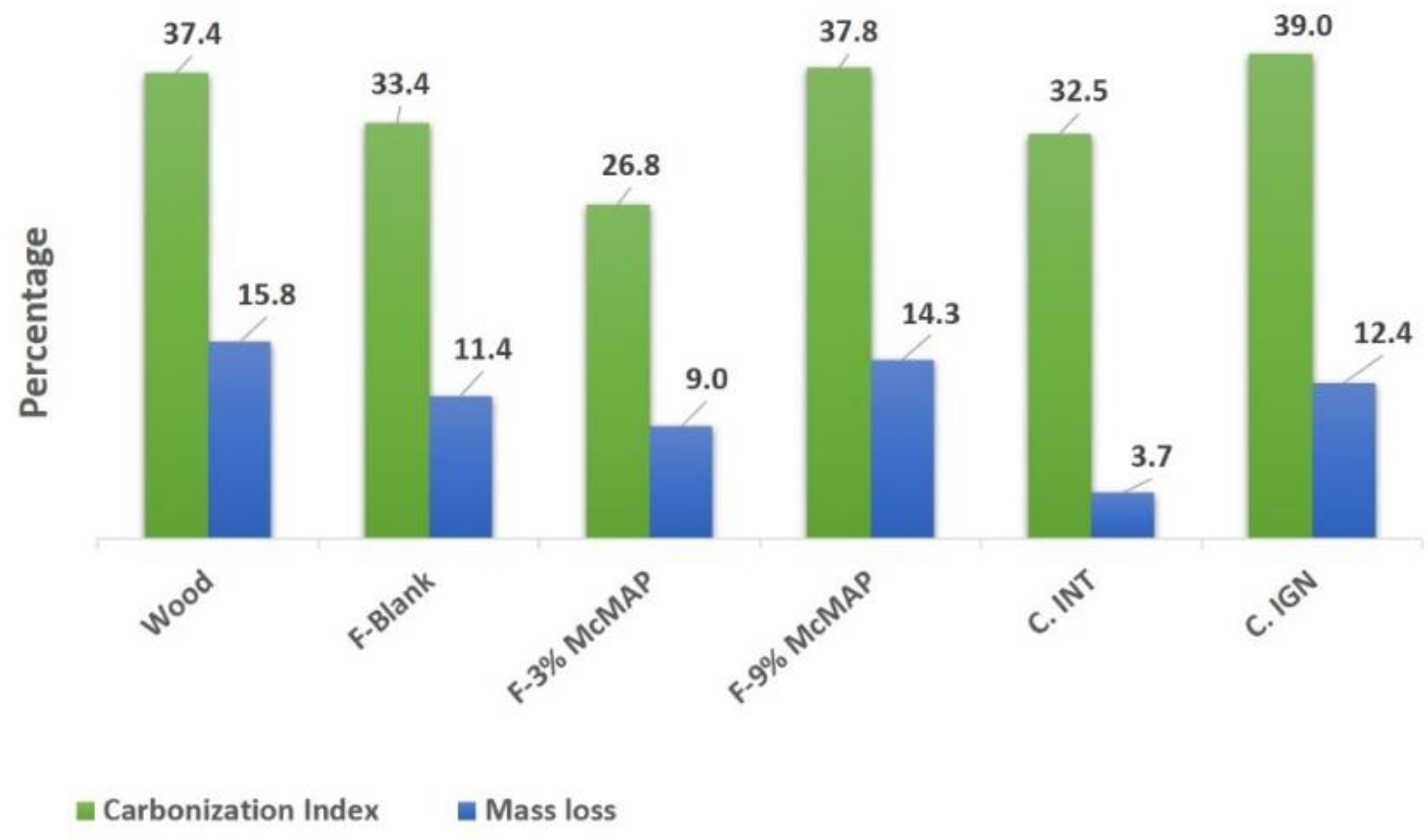
| Formulations | % Carbonization Index | % Mass Loss |
|---|---|---|
| Uncoated wood | 37.40 | 15.80 |
| F-Blank | 33.40 | 11.40 |
| F-3% McMAP | 26.80 | 9.00 |
| F-9% McMAP | 37.80 | 14.30 |
| C-INT | 32.50 | 3.70 |
| C-IGN | 39.00 | 12.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo, A.F.; Díaz-Gómez, A.; Ramirez, J.; Berrio, M.E.; Cornejo, V.; Rojas, D.; Montoya, L.F.; Mera, A.; Melendrez, M.F. Eco-Friendly Fire-Resistant Coatings Containing Dihydrogen Ammonium Phosphate Microcapsules and Tannins. Coatings 2021, 11, 280. https://doi.org/10.3390/coatings11030280
Jaramillo AF, Díaz-Gómez A, Ramirez J, Berrio ME, Cornejo V, Rojas D, Montoya LF, Mera A, Melendrez MF. Eco-Friendly Fire-Resistant Coatings Containing Dihydrogen Ammonium Phosphate Microcapsules and Tannins. Coatings. 2021; 11(3):280. https://doi.org/10.3390/coatings11030280
Chicago/Turabian StyleJaramillo, Andrés Felipe, Andrés Díaz-Gómez, Jesús Ramirez, María Elizabeth Berrio, Vanessa Cornejo, David Rojas, Luis Felipe Montoya, Adriana Mera, and Manuel Francisco Melendrez. 2021. "Eco-Friendly Fire-Resistant Coatings Containing Dihydrogen Ammonium Phosphate Microcapsules and Tannins" Coatings 11, no. 3: 280. https://doi.org/10.3390/coatings11030280
APA StyleJaramillo, A. F., Díaz-Gómez, A., Ramirez, J., Berrio, M. E., Cornejo, V., Rojas, D., Montoya, L. F., Mera, A., & Melendrez, M. F. (2021). Eco-Friendly Fire-Resistant Coatings Containing Dihydrogen Ammonium Phosphate Microcapsules and Tannins. Coatings, 11(3), 280. https://doi.org/10.3390/coatings11030280






