Metal Deposition Induced by the Step Region of Si (111)-(7 × 7) Surface
Abstract
:1. Introduction
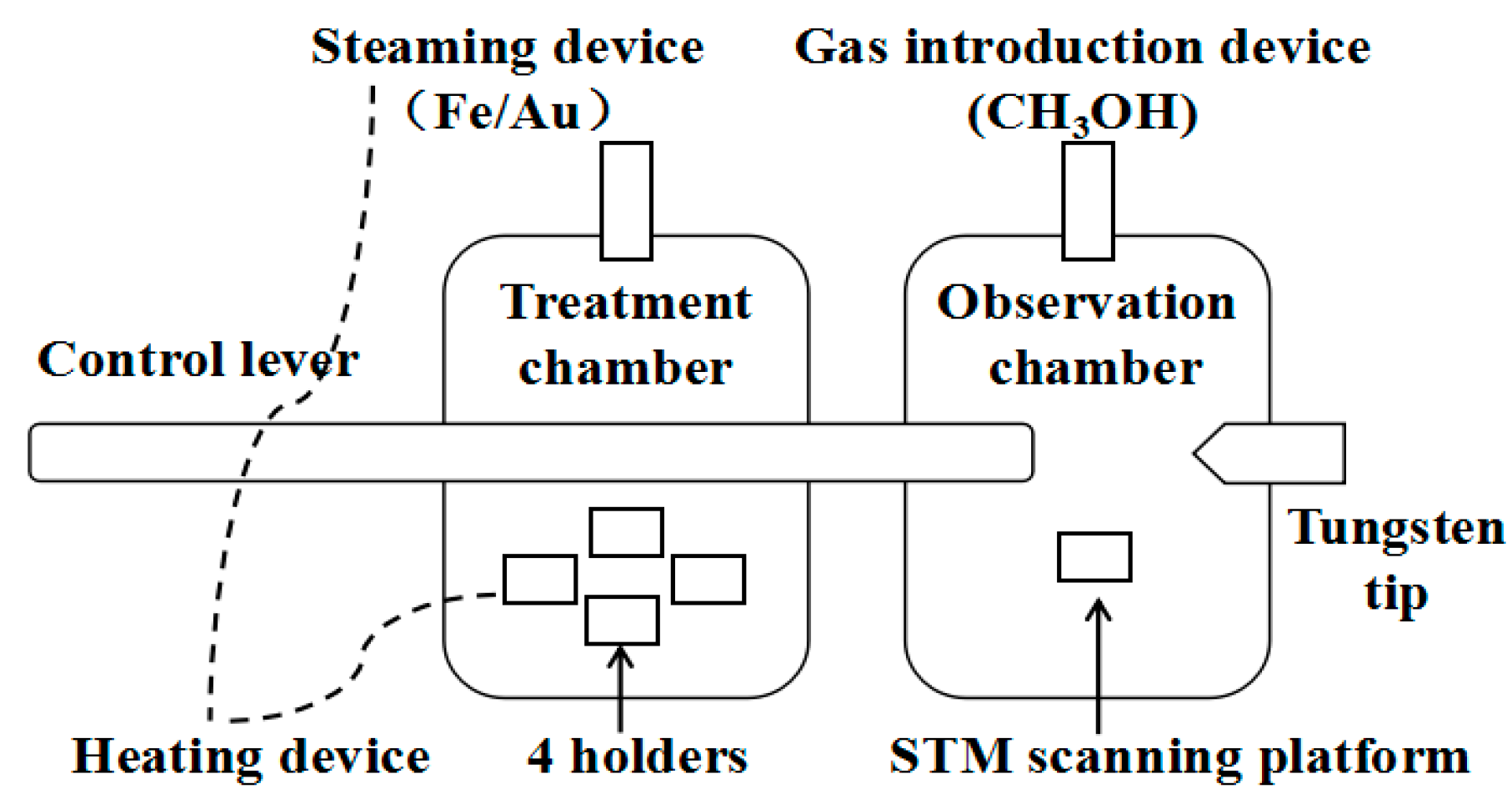
2. Materials and Methods
3. Results
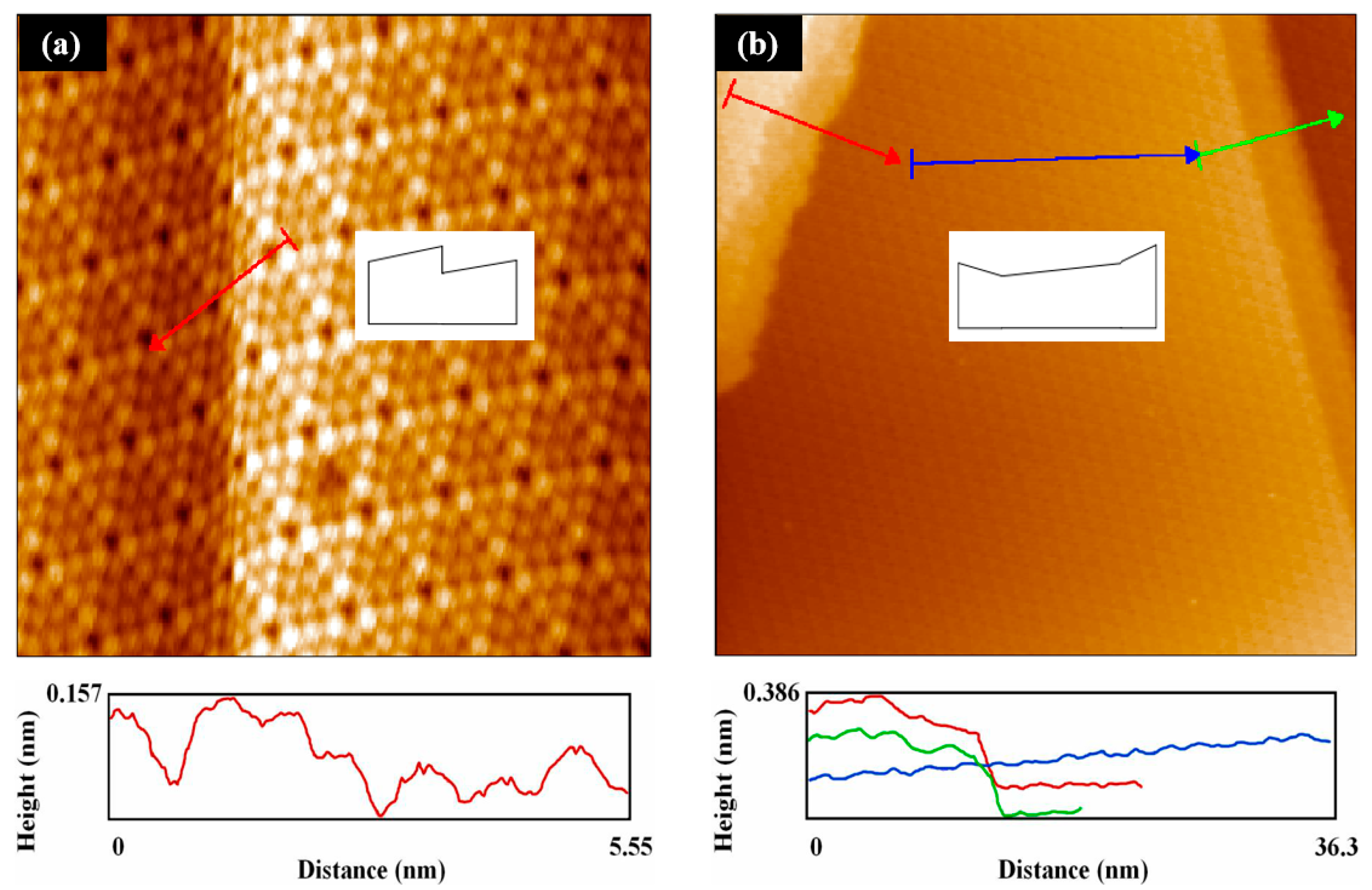
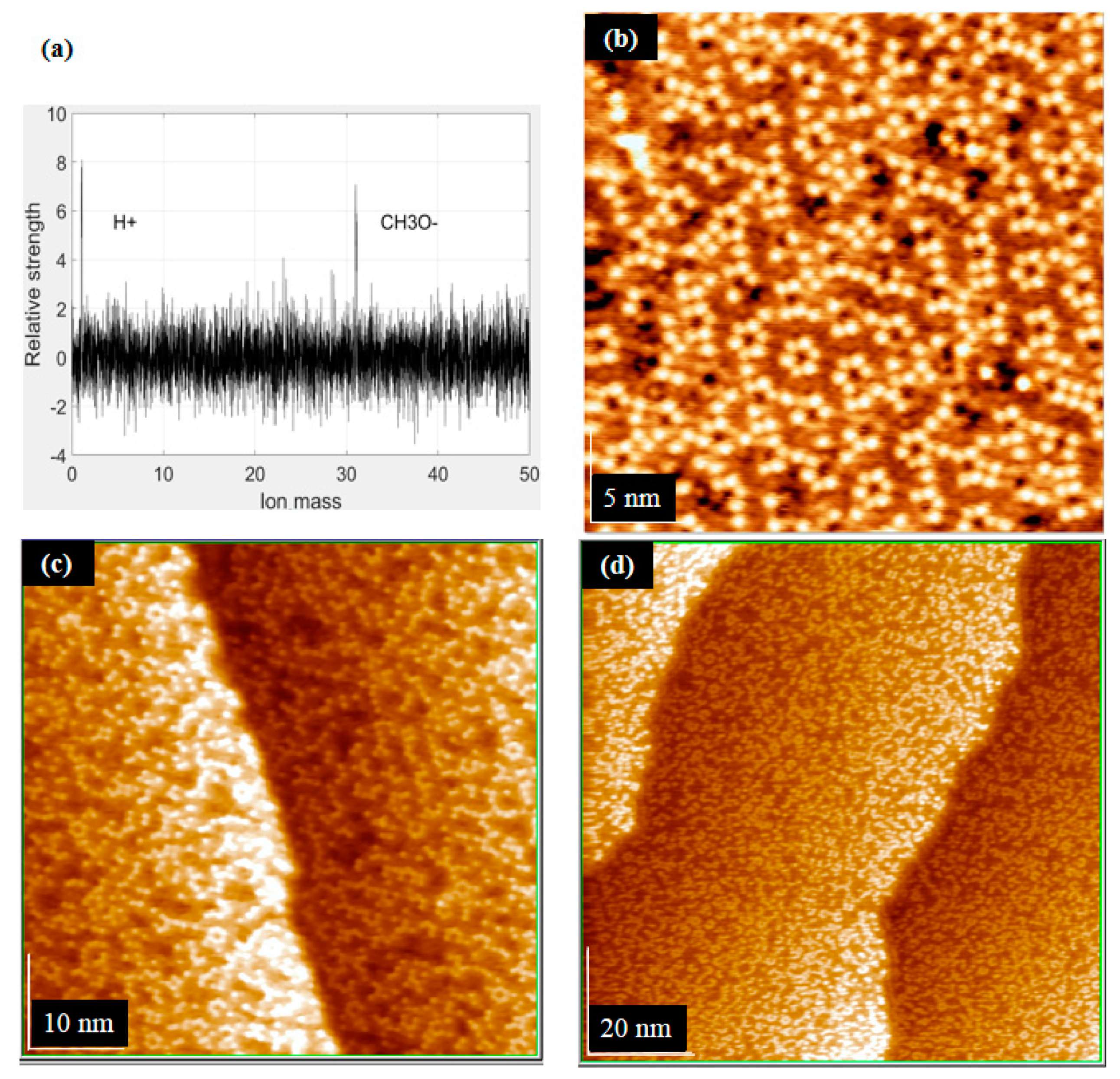
3.1. Metal Deposition on the Step Region
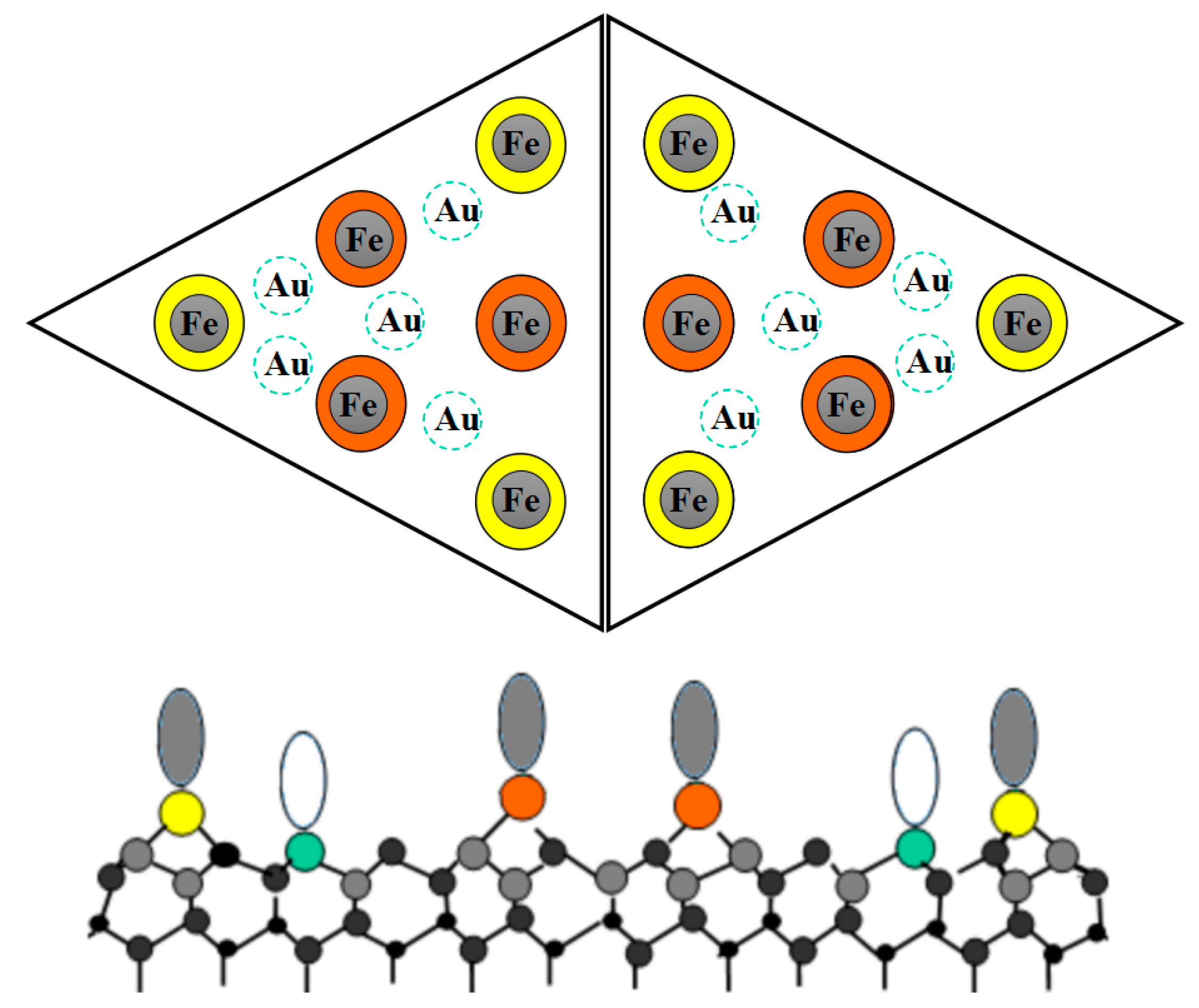
3.2. Classification Model and CH3OH Adsorption
3.2.1. Deduction of the Theoretical Prediction
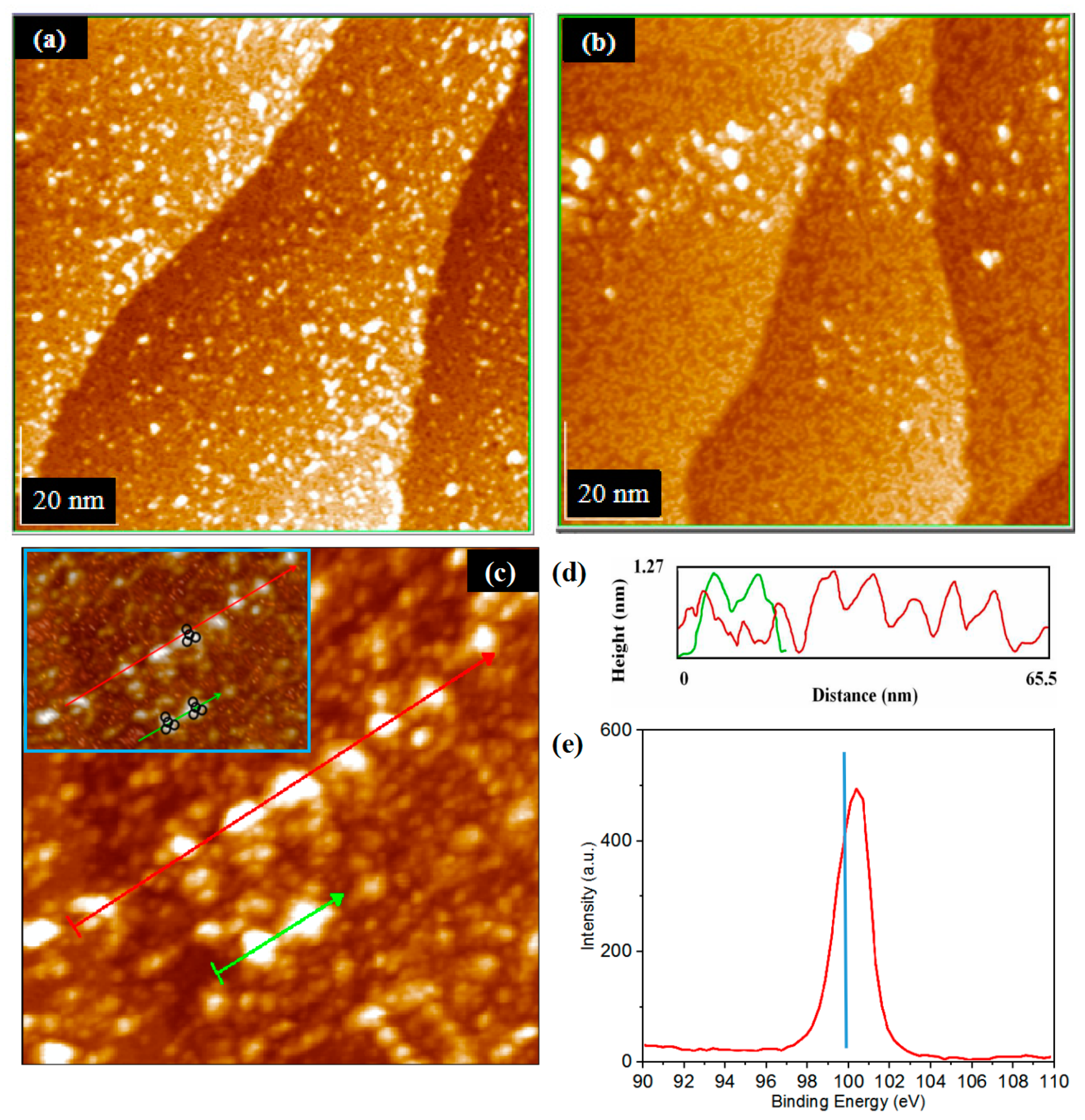
3.2.2. CH3OH Adsorption on the Step Region
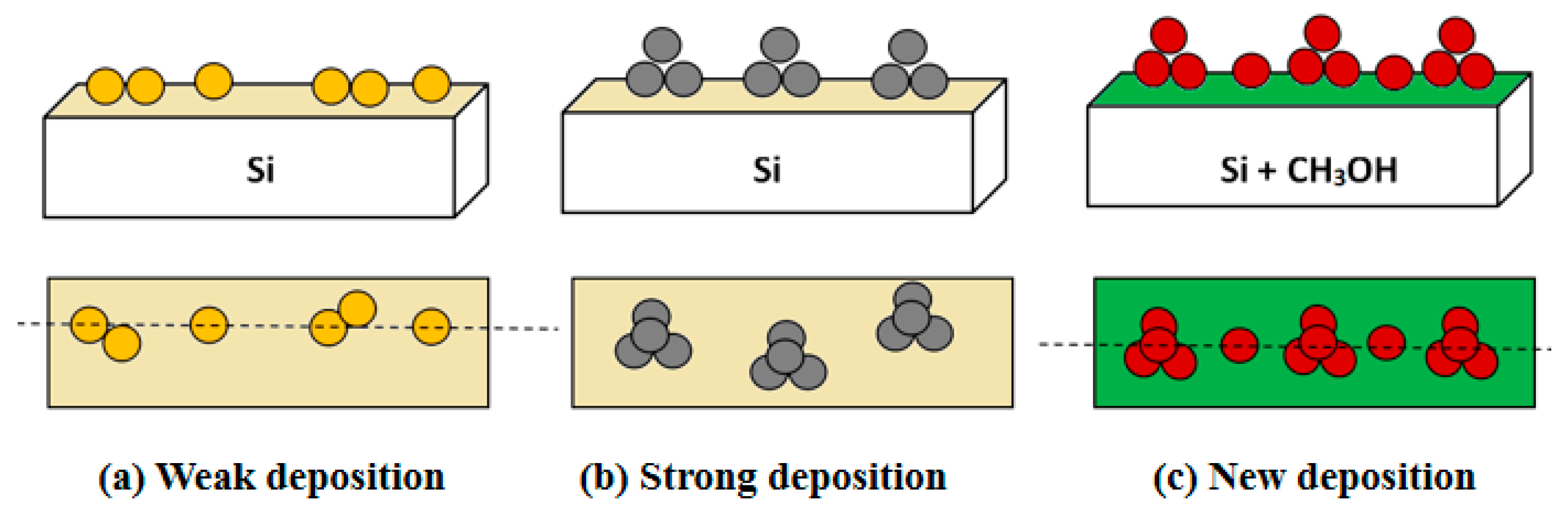
3.3. The Realization of Quasi Deposition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaya, D.; Cobley, R.J.; Palmer, R.E. Combining scanning tunneling microscope (STM) imaging and local manipulation to probe the high dose oxidation structure of the Si(111)-7 × 7 surface. Nano Res. 2020, 13, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Gomes, S.; Assy, A.; Chapuis, P.O. Scanning thermal microscopy: A review. Phys. Status Solidi 2015, 212, 477–494. [Google Scholar] [CrossRef]
- Karbivska, L.I.; Karbivskii, V.L.; Romansky, A.A.; Kuznetsova, O.Y.; Artemyuk, V.A. Nanostructures of Cu, Au and In on the silicon single crystal surfaces at their thermal deposition. In Proceedings of the IEEE 39th International Conference on Electronics and Nanotechnology, Kyiv, Ukraine, 16–18 April 2019. [Google Scholar]
- Tripathi, J.K.; Markovich, G.; Goldfarb, I. Self-ordered magnetic α-FeSi2 nano-stripes on Si (111). Appl. Phys. Lett. 2013, 102, 251604. [Google Scholar] [CrossRef] [Green Version]
- Aulbach, J.; Erwin, S.C.; Claessen, R. Spin chains and electron transfer at stepped silicon surfaces. Nano Lett. 2016, 16, 2698–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biedermann, K.; Regensburger, S.; Fauster, T. Spin-split silicon states at step edges of Si (553)-Au. Phys. Rev. B 2012, 85, 245413. [Google Scholar] [CrossRef] [Green Version]
- Utecht, M.; Gaebel, T.; Klamroth, T. Desorption induced by low energy charge carriers on Si (111)-7 × 7: First principles molecular dynamics for benzene derivates. J. Comput. Chem. 2018, 39, 2517–2525. [Google Scholar] [CrossRef]
- Mao, H.; Guan, D.; Chen, M.; Dou, W.; Song, F.; Zhang, H.; Li, H.; He, P.; Bao, S. Adsorption geometry of tetracene on SIO2/Si (111) substrate with the balance of molecule–substrate and intermolecular interaction. Phys. Rev. B 2010, 405, 990–995. [Google Scholar] [CrossRef]
- Batsaikhan, E.; Lee, C.; Hsu, H.; Wu, C.; Peng, J.; Ma, M.; Deleg, S.; Li, W. Largely enhanced ferromagnetism in bare CuO nanoparticles by a small size effect. ACS Omega 2020, 5, 3849–3856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiaoqing, Z.; Xiaohu, Y.; Yugang, S. Scale effect on flexural wave propagation in embedded single-walled carbon nanotubes: Nonlocal small-size effect and surface effect. J. Comput. Theor. Nanos 2015, 12, 2356–2365. [Google Scholar] [CrossRef]
- Ding, W.; Ju, D.; Guo, Y. Formation of linearly linked Fe clusters on Si (111)-7 × 7-C2H5OH surface. Nanoscale Res. Lett. 2014, 9, 377. [Google Scholar] [CrossRef] [Green Version]
- Mackus, A.; Merkx, M.; Kessels, W. From the bottom-up: Toward area-selective atomic layer deposition with high selectivity. J. Mater. Chem. 2019, 31, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.I.; Xie, Z.X.; Egawa, T. Dynamics of Sn and Zn atoms on a Si (111)-7 × 7 surface. J. Mol. Catal. A Chem. 2003, 199, 19–26. [Google Scholar] [CrossRef]
- Xie, Z.X.; Tanaka, K. Tin atoms adsorbed on a Si (111)-(7 × 7) surface. Surf. Sci. 2001, 479, 26–32. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, G.; Wang, K. Experimental and theoretical investigation of single Cu, Ag, and Au atoms adsorbed on Si (111)−(7 × 7). Phys. Rev. Lett. 2005, 94, 176104. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Ding, W.; Ju, D.; Tanaka, K.I.; Komori, F. Study on formation process and models of linear Fe cluster structure on a Si (111)-7 × 7-CH3OH surface. Materials 2018, 11, 1593. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Nomoto, Y.; Xie, Z.X. Dissociation mechanism of 2-propanol on a Si (111)-(7 × 7) surface studied by scanning tunneling microscopy. J. Chem. Phys. 2004, 120, 4486–4491. [Google Scholar] [CrossRef]
- Tanaka, K.; Xie, Z.X. Adsorption kinetics and patterning of a Si (111)-7 × 7 surface by dissociation of methanol. J. Chem. Phys. 2005, 122, 054706. [Google Scholar] [CrossRef] [PubMed]
- Schlenhoff, A.; Lindner, P.; Friedlein, J. Magnetic nano-skyrmion lattice observed in a Si-wafer-based multilayer system. ACS Nano 2015, 9, 5908–5912. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Guo, L. Iron triad (Fe, Co, Ni) nanomaterials: Structural design, functionalization and their applications. Chem. Soc. Rev. 2015, 44, 6697–6707. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K. Dynamic Chemical Processes on Solid Surfaces; Springer: Singapore, 2017. [Google Scholar]
- Tanaka, K.; Jiang, X.; Shimojo, M. Growth of nano-crystalline metal dots on the Si (111)-7 × 7 surface saturated with C2H5OH. Surf. Sci. 2007, 601, 5093–5097. [Google Scholar] [CrossRef]
- Li, W.; Ding, W.; Ju, D.; Tanaka, K.I.; Komori, F. Formation process and mechanism of iron-nitride compounds on Si (111)-7 × 7-CH3OH surface. Chem. Phys. Lett. 2018, 703, 17–22. [Google Scholar] [CrossRef]
- Šljivančanin, Ž.; Pasquarello, A. Supported Fe nanoclusters: Evolution of magnetic properties with cluster size. Phys. Rev. Lett. 2003, 90, 247202. [Google Scholar] [CrossRef]
- Sadowski, J.T.; Nagao, T.; Yaginuma, S.; Fujikawa, Y.; Ohno, T. Stability of the quasicubic phase in the initial stage of the growth of bismuth films on Si(111)-7 × 7. J. Appl. Phys 2006, 99, 2782. [Google Scholar] [CrossRef]
- Takahashi, Y.; Miyamachi, T.; Nakashima, S.; Kawamura, N.; Takagi, Y.; Uozumi, M.; Antonov, V.N.; Yokoyama, T.; Ernst, A.; Komori, F. Thickness-dependent electronic and magnetic properties of γ′−Fe4N atomic layers on Cu (001). Phys. Rev. B 2017, 95, 224417. [Google Scholar] [CrossRef] [Green Version]
- Alam, K.; Disseler, S.M.; Ratcliff, W.D.; Borchers, J.A.; Ponce-Pérez, R.; Cocoletzi, G.H. Structural and magnetic phase transitions in chromium nitride thin films grown by RF nitrogen plasma molecular beam epitaxy. Phys. Rev. B 2017, 96, 104433. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Ding, W.; Gong, Y.; Ju, D. Metal Deposition Induced by the Step Region of Si (111)-(7 × 7) Surface. Coatings 2021, 11, 281. https://doi.org/10.3390/coatings11030281
Li W, Ding W, Gong Y, Ju D. Metal Deposition Induced by the Step Region of Si (111)-(7 × 7) Surface. Coatings. 2021; 11(3):281. https://doi.org/10.3390/coatings11030281
Chicago/Turabian StyleLi, Wenxin, Wanyu Ding, Youping Gong, and Dongying Ju. 2021. "Metal Deposition Induced by the Step Region of Si (111)-(7 × 7) Surface" Coatings 11, no. 3: 281. https://doi.org/10.3390/coatings11030281
APA StyleLi, W., Ding, W., Gong, Y., & Ju, D. (2021). Metal Deposition Induced by the Step Region of Si (111)-(7 × 7) Surface. Coatings, 11(3), 281. https://doi.org/10.3390/coatings11030281





