Comparative Study of Corrosion Properties of Different Graphene Nanoplate/Epoxy Composite Coatings for Enhanced Surface Barrier Protection
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Gnps/Epoxy Coatings on Steel Samples
2.3. Characterization of Gnps/Epoxy Composite Coatings
2.4. Pull-Off Adhesion Testing
2.5. Water Contact Angle and Absorption Testing
2.6. Salt Spray Testing
2.7. Electrochemical Experiments
3. Results
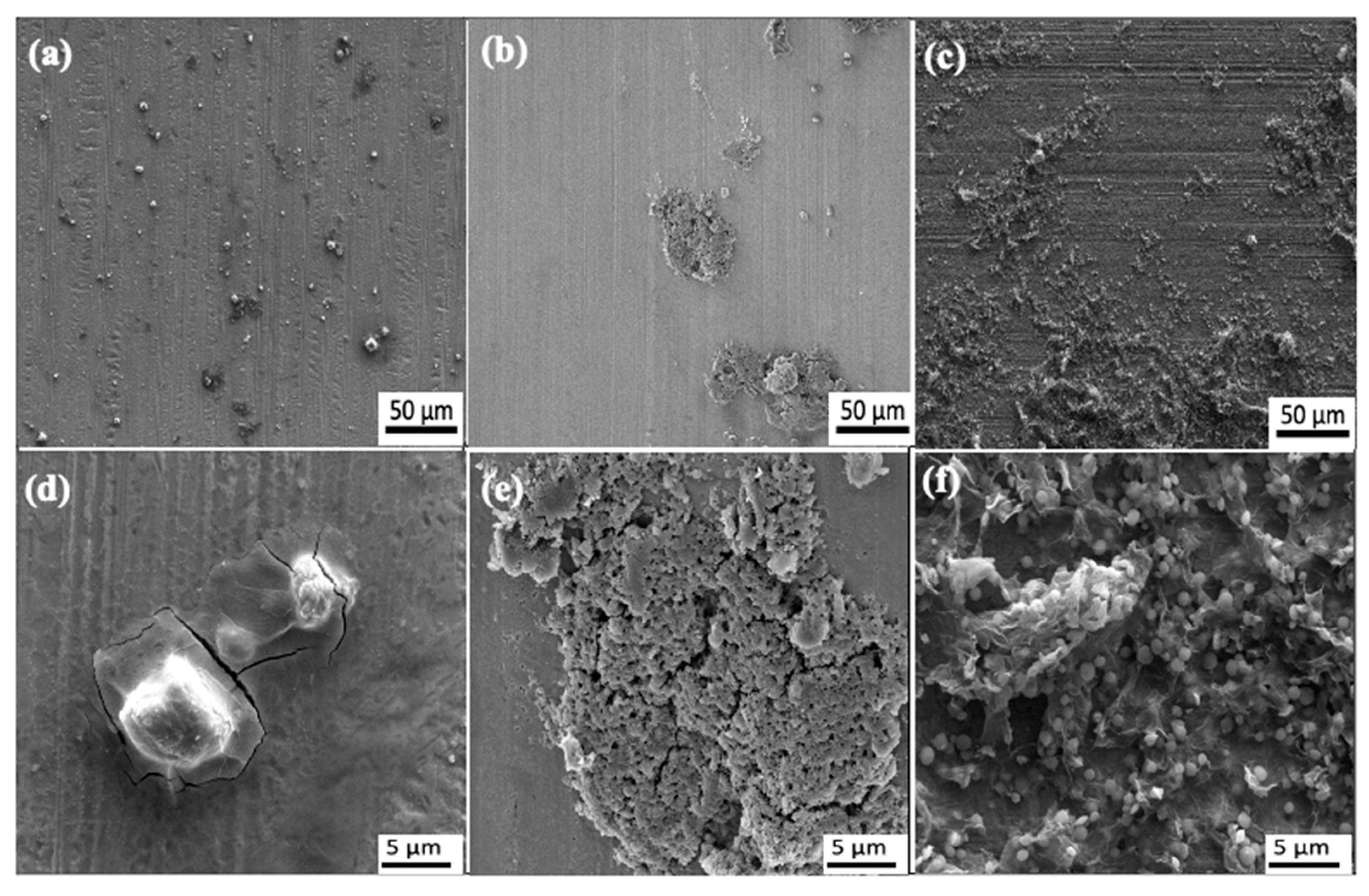
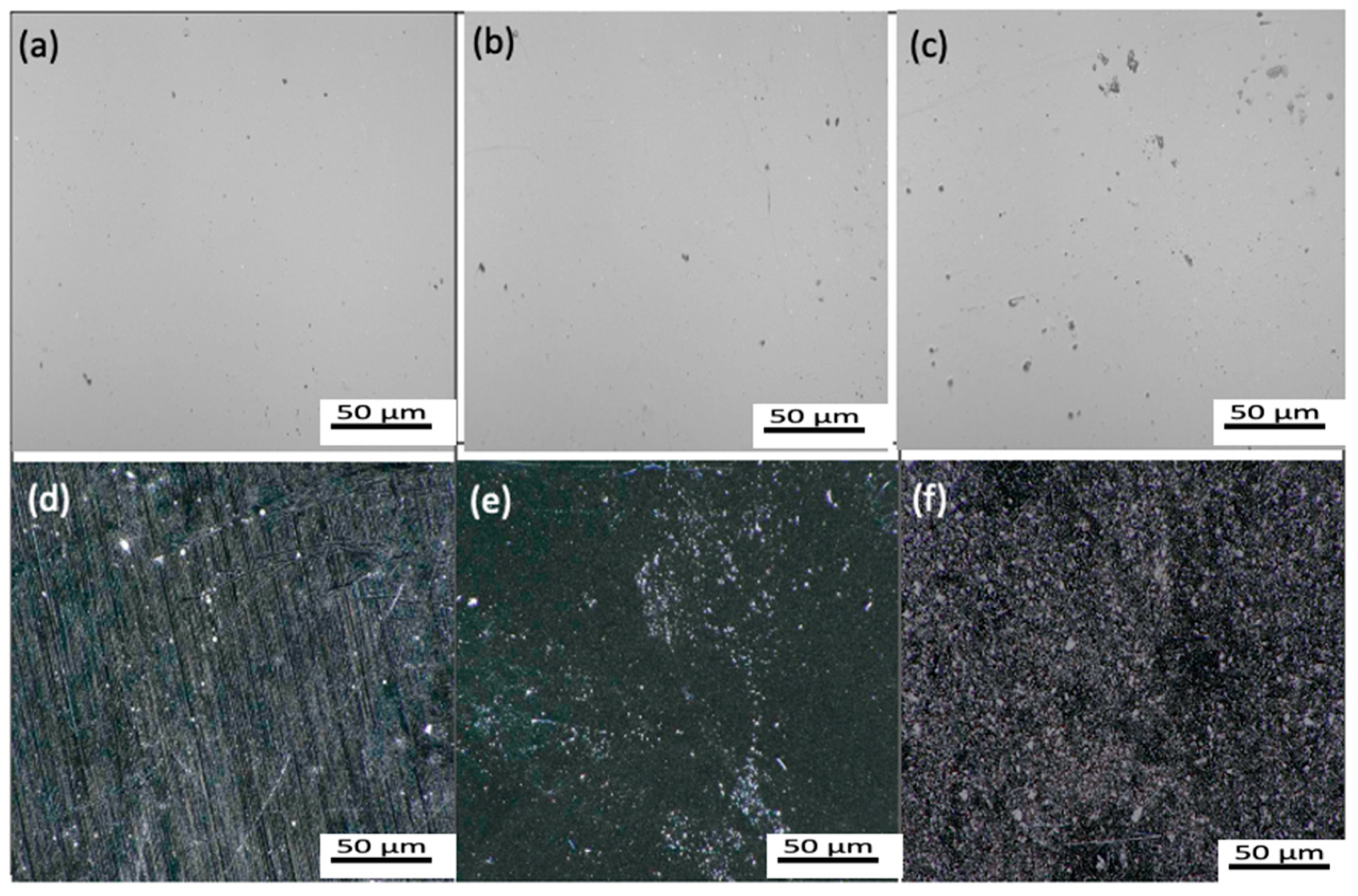
3.1. Characterization of Gnps/Epoxy Composite Coatings
3.2. Physical Properties of Gnps/Epoxy Composite Coatings
3.2.1. Adhesion Testing
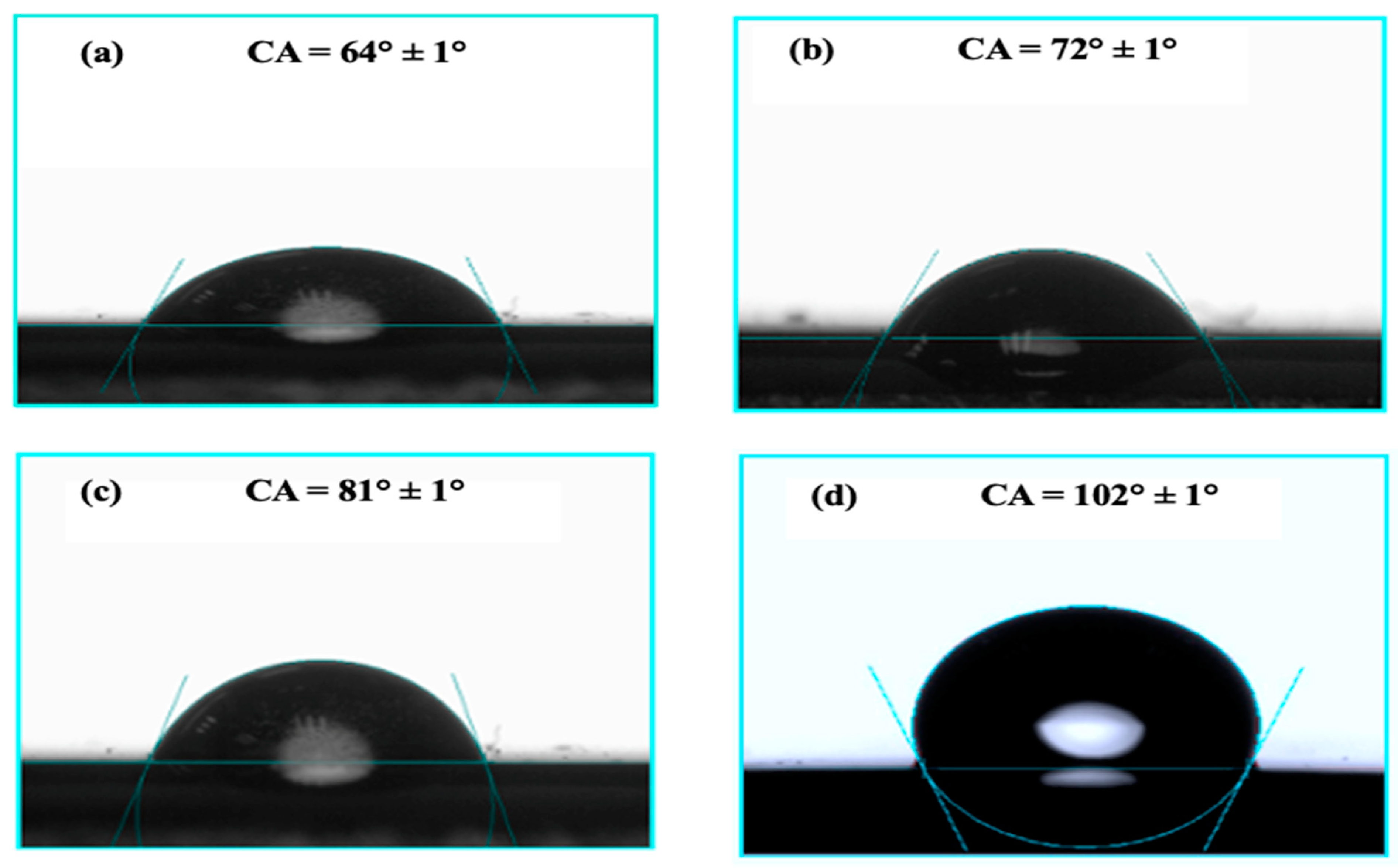
3.2.2. Water Contact Angle Testing
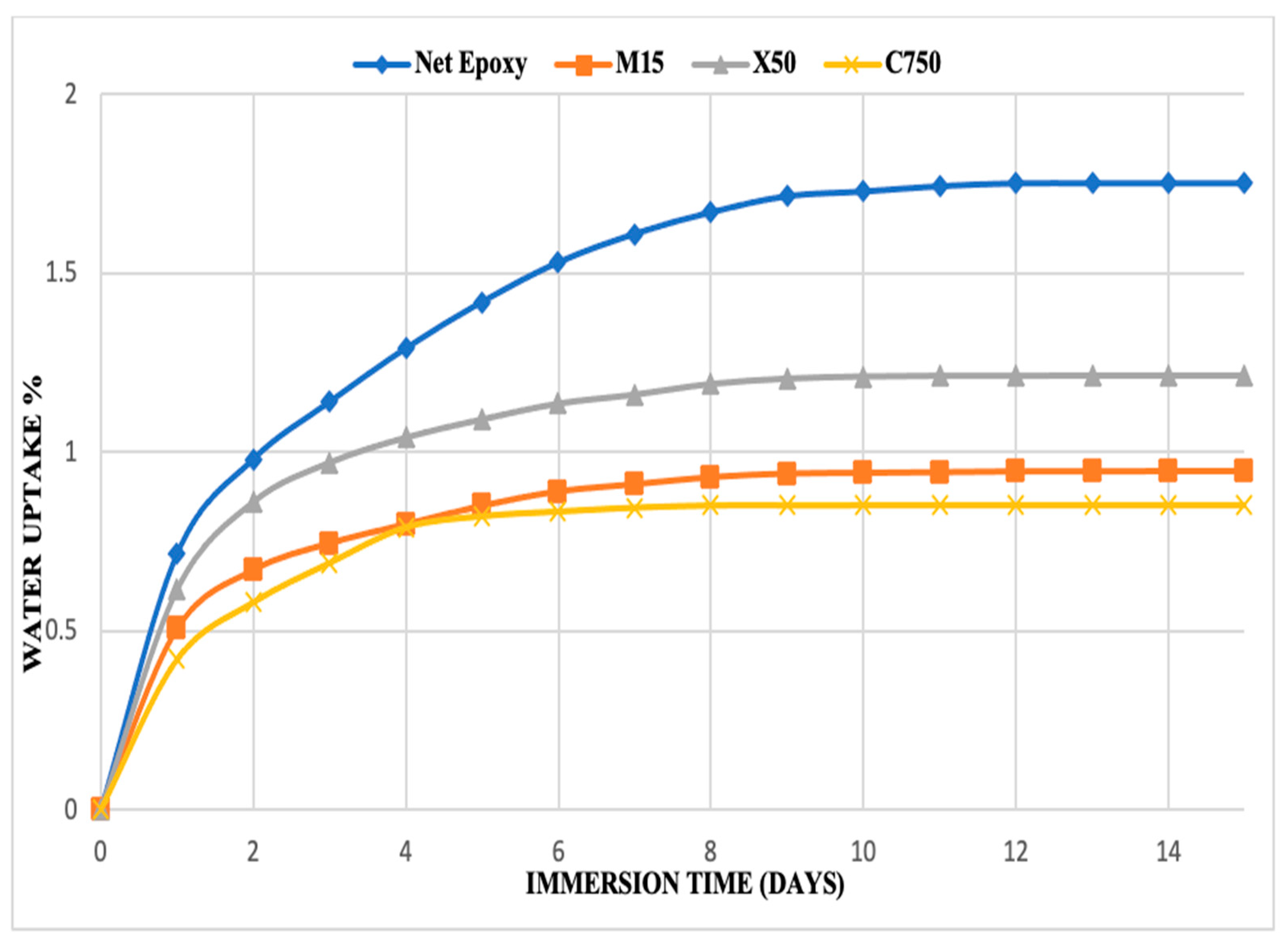
3.2.3. Water Absorption Testing
3.3. Corrosion Properties of Gnps/Epoxy Composite Coatings
3.3.1. Salt Spray Testing
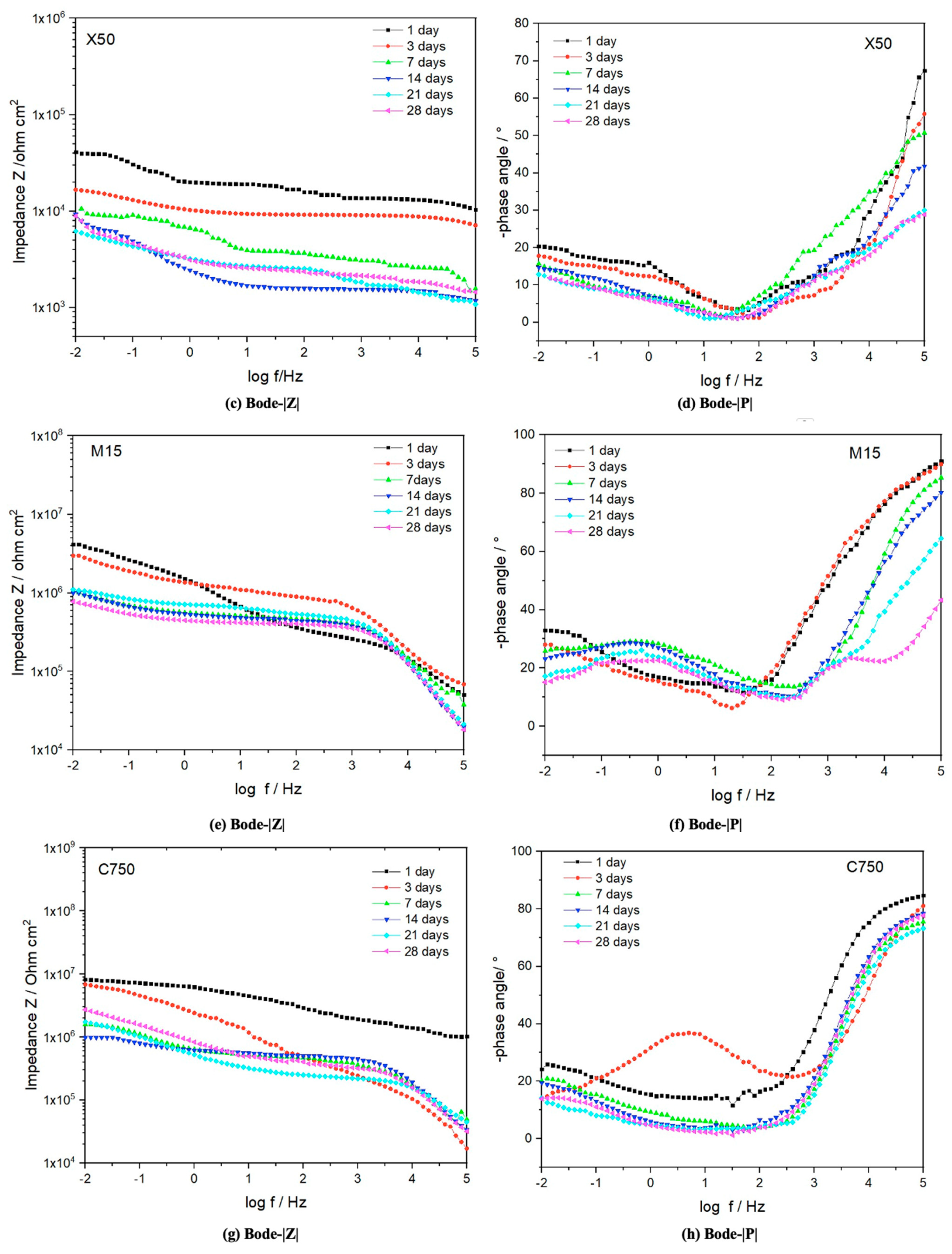
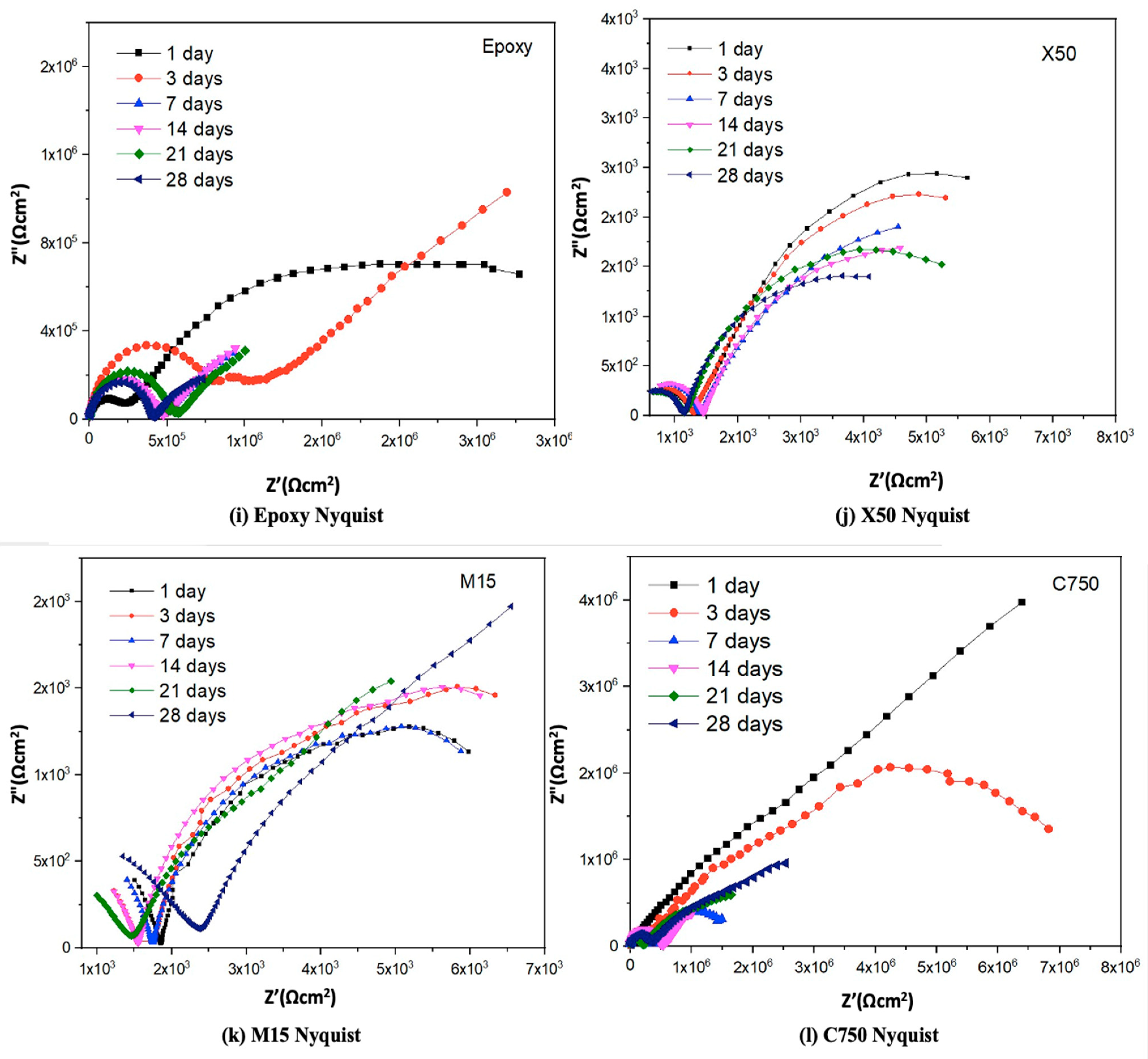
3.3.2. EIS Characterization
4. Discussion
4.1. Morphological Influence on the Corrosion Properties of Gnps/Epoxy Composite Coatings
4.2. Mechanism for Enhanced Anti-Corrosion Resistance of Gnps/Epoxy Composite Coatings
5. Conclusions
- C750 Gnp of a smaller particle size and higher average surface area were highly favorable to initiate an efficient pathway that strongly suppressed the deeper penetration of corrosive agents. On the contrary, when agglomeration occurred due to difficult dispersion caused by larger size Gnps (X50 and M15), the nanoparticles were unable to fill the micro pores and voids in the epoxy composites thus caused the coating’s poor corrosion properties.
- The Gnps provided an excellent anti-corrosion mechanism by means of forming a passive protecting layer on the coating’s interface that hindered with the diffusion rate of corrosive media like O2, H2O, H+, and Cl−. However, the increased corrosion resistance of the Gnps/epoxy composite is attributed to the improved surface barrier’s influence on the coating’s anti-corrosion resistance and water uptake performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, M.; Kannan, K.; Parangusan, H.; Eldeib, S.; Shehata, O.; Ismail, M.; Zarandah, R.; Sadasivuni, K.K. Enhanced corrosion protection of epoxy/ZnO-NiO nanocomposite coatings on steel. Coatings 2020, 10, 783. [Google Scholar] [CrossRef]
- Xavier, J.R. Investigation on the anticorrosion, adhesion and mechanical performance of epoxy nanocomposite coatings containing epoxy-silane treated nano-MoO3 on mild steel. J. Adhes. Sci. Technol. 2020, 34, 115–134. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, H.; Zhu, R.; Chen, R.; Sheng, X.; Xie, D.; Mei, Y. Green modification of graphene oxide with phytic acid and its application in anticorrosive water-borne epoxy coatings. Prog. Org. Coat. 2020, 143. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Ma, I.; Farah, Z.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. Studies on SiO2-hybrid polymeric nanocomposite coatings with superior corrosion protection and hydrophobicity. Surf. Coat. Technol. 2017, 324, 536–545. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Haeri, Z.; Ramezanzadeh, M. A facile route of making silica nanoparticles-covered graphene oxide nanohybrids (SiO2-GO); fabrication of SiO2-GO/epoxy composite coating with superior barrier and corrosion protection performance. Chem. Eng. J. 2016, 303, 511–528. [Google Scholar] [CrossRef]
- Golru, S.S.; Attar, M.M.; Ramezanzadeh, B. Studying the influence of nano-Al2O3 particles on the corrosion performance and hydrolytic degradation resistance of an epoxy/polyamide coating on AA-1050. Prog. Org. 2014, 77, 1391–1399. [Google Scholar] [CrossRef]
- Işın, D.; Kayaman-Apohan, N.; Güngör, A. Preparation and characterization of UV-curable epoxy/silica nanocomposite coatings. Prog. Org. 2009, 65, 477–483. [Google Scholar] [CrossRef]
- Ammar, S.; Cheng, C.H.; Ma, I.A.W.; Baig, S.B.; Kasi, R.; Subramaniam, R.; Balakrishnan, V. Effects of TiO2 nanoparticles on the overall performance and corrosion protection ability of neat epoxy and PDMS modified epoxy coating systems. Front. Mater. 2020, 6, 336. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M. Studying the corrosion resistance and hydrolytic degradation of an epoxy coating containing ZnO nanoparticles. Mater. Chem. Phys. 2011, 13, 1208–1219. [Google Scholar] [CrossRef]
- Dhoke, S.K.; Khanna, A.S. Effect of nano-Fe2O3 particles on the corrosion behavior of alkyd based waterborne coatings. Corros. Sci. 2009, 51, 6–20. [Google Scholar] [CrossRef]
- Shi, H.; Liu, F.; Yang, L.; Han, E. Characterization of protective performance of epoxy reinforced with nanometer-sized TiO2 and SiO2. Prog. Org. Coat. 2008, 62, 359–368. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, W.; Liu, S.; Cen, Q.; Xue, Q. Comparative tribological and corrosion resistance properties of epoxy composite coatings reinforced with functionalized fullerene C60 and graphene. Surf. Coat. Technol. 2016, 286, 354–364. [Google Scholar] [CrossRef]
- Kumar, C.M.P.; Venkatesha, T.V.; Shabadi, R. Preparation and corrosion behavior of Ni and Ni–graphene composite coatings. Mater. Res. Bull. 2013, 48, 1477–1483. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.A.; Bagherzadeh, M.R. Exploring corrosion protection properties of solvent based epoxy-graphene oxide nanocomposite coatings on mild steel. Corros. Sci. 2016, 115, 78–92. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef]
- Glover, C.F.; Richards, C.; Baker, J.; Williams, G.; McMurray, H.N. In-coating graphene nano-platelets for environmentally-friendly corrosion protection of iron. Corros. Sci. 2017, 114, 169–172. [Google Scholar] [CrossRef] [Green Version]
- King, J.A.; Klimek, D.R.; Miskioglu, I.; Odegard, G.M. Mechanical properties of graphene nanoplatelet/epoxy composites. J. Compos. Mater. 2014, 49, 659–668. [Google Scholar] [CrossRef]
- Yadav, S.K.; Cho, J.W. Functionalized graphene nanoplatelets for enhanced mechanical and thermal properties of polyurethane nanocomposites. Appl. Surf. Sci. 2013, 266, 360–367. [Google Scholar] [CrossRef]
- Kumar, A.M.; Khan, A.; Suleiman, R.; Qamar, M.; Saravanan, S.; Dafalla, H. Bifunctional CuO/TiO2 nanocomposite as nanofiller for improved corrosion resistance and antibacterial protection. Prog. Org. Coat. 2018, 114, 9–18. [Google Scholar] [CrossRef]
- Frigione, M.; Lettieri, M. Recent advances and trends of nanofilled/nanostructured epoxies. Materials 2020, 13, 3415. [Google Scholar] [CrossRef]
- Monetta, T.; Acquesta, A.; Bellucci, F. Graphene/epoxy coating as multifunctional material for aircraft structures. Aerospace 2015, 2, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Prolongo, S.G.; Jiménez-Suárez, A.; Moriche, R.; Ureña, A. Influence of thickness and lateral size of graphene nanoplatelets on water uptake in epoxy/graphene nanocomposites. Appl. Sci. 2018, 8, 1550. [Google Scholar] [CrossRef] [Green Version]
- Kuo, W.-S.; Tai, N.-H.; Chang, T.-W. Deformation and fracture in graphene nanosheets. Compos. Part A Appl. Sci. 2013, 51, 56–61. [Google Scholar] [CrossRef]
- Liu, S.; Gu, L.; Zhao, H.; Chen, J.; Yu, H. Corrosion resistance of graphene-reinforced waterborne epoxy coatings. J. Mater. Sci Technol. 2016, 32, 425–431. [Google Scholar] [CrossRef]
- Starkova, O.; Buschhorn, S.T.; Mannov, E.; Schulte, K.; Aniskevich, A. Water transport in epoxy/MWCNT composites. Eur. Polym. J. 2013, 49, 2138–2148. [Google Scholar] [CrossRef]
- Um, J.G.; Jun, Y.-S.; Alhumade, H.; Krithivasan, H.; Lui, G.; Yu, A. Investigation of the size effect of graphene nano-platelets (GnPs) on the anti-corrosion performance of polyurethane/GnP composites. RSC Adv. 2018, 8, 17091–17100. [Google Scholar] [CrossRef] [Green Version]
- Ziat, Y.; Hammi, M.; Zarhri, Z.; Laghlimi, C. Epoxy coating modified with graphene: A promising composite against corrosion behavior of copper surface in marine media. J. Alloys Compd. 2020, 820. [Google Scholar] [CrossRef]
- Q235B Chinese GB Standard Equivalent, Properties, Specification and Composition. Available online: https://www.theworldmaterials.com/chinese-gb-standard/ (accessed on 28 February 2021).
- ASTM D4541-17, Standard Test Method for Pull-off Strength of Coatings Using Portable Adhesion Testers; ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D7490-13, Standard Test Method for Measurement of the Surface Tension of Solid Coatings, Substrates and Pigments using Contact Angle Measurements; ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM B117-19, Standard Practice for Operating Salt Spray (Fog) Apparatus; ASTM International: West Conshohocken, PA, USA, 2019.
- ISO 4628-1:2016, Paints and Varnishes-Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance—Part 1: General Introduction and Designation System; International Organization for Standardization: Geneva, Switzerland, 2016.
- Cao, X.; Huang, F.; Huang, C.; Liu, J.; Cheng, Y.F. Preparation of graphene nanoplate added zinc-rich epoxy coatings for enhanced sacrificial anode-based corrosion protection. Corros. Sci. 2019, 159. [Google Scholar] [CrossRef]
- Johnson, D.W.; Dobson, B.P.; Coleman, K.S. A manufacturing perspective on graphene dispersions. Curr. Opin. Colloid Interface Sci 2015, 20, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Naik, R.B.; Jagtap, S.B.; Ratna, D. Effect of carbon nanofillers on anticorrosive and physico-mechanical properties of hyperbranched urethane alkyd coatings. Prog. Org. Coat. 2015, 87, 28–35. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Li, J.-L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Qiu, S.; Cui, M.; Qin, S.; Yan, G.; Zhao, H.; Wang, L.; Xue, Q. Achieving high performance corrosion and wear resistant epoxy coatings via incorporation of noncovalent functionalized graphene. Carbon 2017, 114, 356–366. [Google Scholar] [CrossRef]
- Salom, C.; Prolongo, M.G.; Toribio, A.; Martínez-Martínez, A.J.; Aguirre de Cárce, I.; Prolongo, S.G. Mechanical properties and adhesive behavior of epoxy-graphene nanocomposites. Int. J. Adhes Adhes 2018, 84, 119–125. [Google Scholar] [CrossRef]
- Volovitch, P.; Vu, T.N.; Allély, C.; Abdel Aal, A.; Ogle, K. Understanding corrosion via corrosion product characterization: II. Role of alloying elements in improving the corrosion resistance of Zn–Al–Mg coatings on steel. Corros. Sci. 2011, 53, 2437–2445. [Google Scholar] [CrossRef]
- Jüttner, K. Electrochemical impedance spectroscopy (EIS) of corrosion process on inhomogeneous surfaces. Electrochim. Acta 1990, 35, 1501–1508. [Google Scholar] [CrossRef]
- Ghanbari, A.; Attar, M.M. A study on the anticorrosion performance of epoxy nanocomposite coatings containing epoxy-silane treated nano-silica on mild steel substrate. J. Ind. Eng. Chem. 2015, 23, 145–153. [Google Scholar] [CrossRef]
- Wang, X.; Tang, F.; Qi, X.; Lin, Z. Mechanical, electrochemical, and durability behavior of graphene nano-platelet loaded epoxy-resin composite coatings. Compos. B Eng. 2019, 176. [Google Scholar] [CrossRef]
- Parhizkar, N.; Ramezanzadeh, B.; Shahrabi, T. Corrosion protection and adhesion properties of the epoxy coating applied on the steel substrate pre-treated by a sol-gel based silane coating filled with amino and isocyanate silane functionalized graphene oxide nanosheets. Appl. Surf. Sci. 2018, 439, 45–59. [Google Scholar] [CrossRef]
- Prolongo, S.G.; Gude, M.R.; Ureña, A. Water uptake of epoxy composites reinforced with carbon nanofillers. Compos. Part A Appl. Sci. Manuf. 2012, 43, 2169–2175. [Google Scholar] [CrossRef]
- Qi, K.; Sun, Y.; Duan, H.; Guo, X. A corrosion-protective coating based on a solution-processable polymer-grafted graphene oxide nanocomposite. Corros. Sci. 2015, 98, 500–506. [Google Scholar] [CrossRef]
- Dorri Moghadam, A.; Omran, E.; Menezes, P.L.; Rohatgi, P.K. Mechanical and tribological properties of self-lubricating metal matrix nanocomposites reinforced by carbon nanotubes (CNTs) and graphene—A review. Compos. B Eng. 2015, 77, 402–420. [Google Scholar] [CrossRef]
- Montazeri, A.; Chitsazzadeh, M. Effect of sonication parameters on the mechanical properties of multi-walled carbon nanotube/epoxy composites. Mater. Des. 2014, 56, 500–508. [Google Scholar] [CrossRef]








| Grade | Diameter (μm) | |
|---|---|---|
| C750 Gnp | <2 | 750 |
| M15 Gnp | <15 | 150 |
| X50 Gnp | 150 | 50–80 |
| Coating Sample | Neat epoxy | C750 Gnp | M15 Gnp | X50 Gnp |
|---|---|---|---|---|
| Dry pull-off strength (MPa) | 5.99 | 9.42 | 6.78 | 6.90 |
| Wet Pull-off strength (MPa) | 3.77 | 8.51 | 5.79 | 5.54 |
| Pull-off strength % loss | 37% | 9.6% | 14.6% | 19% |
| Coating Samples | Neat epoxy | C750 Gnp | M15 Gnp | X50 Gnp |
|---|---|---|---|---|
| Maximum absorbed water content % | 1.751 | 0.851 | 0.946 | 1.213 |
| Coating Sample | Degree of Blistering | Degree of Blistering Size | Degree of Delamination |
|---|---|---|---|
| Neat Epoxy | 5 | 5 | Severe |
| C750 Gnp/epoxy | 1 | 1 | Very Slight |
| M15 Gnp/epoxy | 2 | 2 | Slight |
| X50 Gnp/epoxy | 3 | 3 | Considerable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abakah, R.R.; Huang, F.; Hu, Q.; Wang, Y.; Liu, J. Comparative Study of Corrosion Properties of Different Graphene Nanoplate/Epoxy Composite Coatings for Enhanced Surface Barrier Protection. Coatings 2021, 11, 285. https://doi.org/10.3390/coatings11030285
Abakah RR, Huang F, Hu Q, Wang Y, Liu J. Comparative Study of Corrosion Properties of Different Graphene Nanoplate/Epoxy Composite Coatings for Enhanced Surface Barrier Protection. Coatings. 2021; 11(3):285. https://doi.org/10.3390/coatings11030285
Chicago/Turabian StyleAbakah, Randy Raymond, Feng Huang, Qian Hu, Yicong Wang, and Jing Liu. 2021. "Comparative Study of Corrosion Properties of Different Graphene Nanoplate/Epoxy Composite Coatings for Enhanced Surface Barrier Protection" Coatings 11, no. 3: 285. https://doi.org/10.3390/coatings11030285






