Prediction of Thermal Spray Coatings Performance in Marine Environments by Combination of Laboratory and Field Tests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thermal Spray Samples
2.2. Simulated Environment Tests
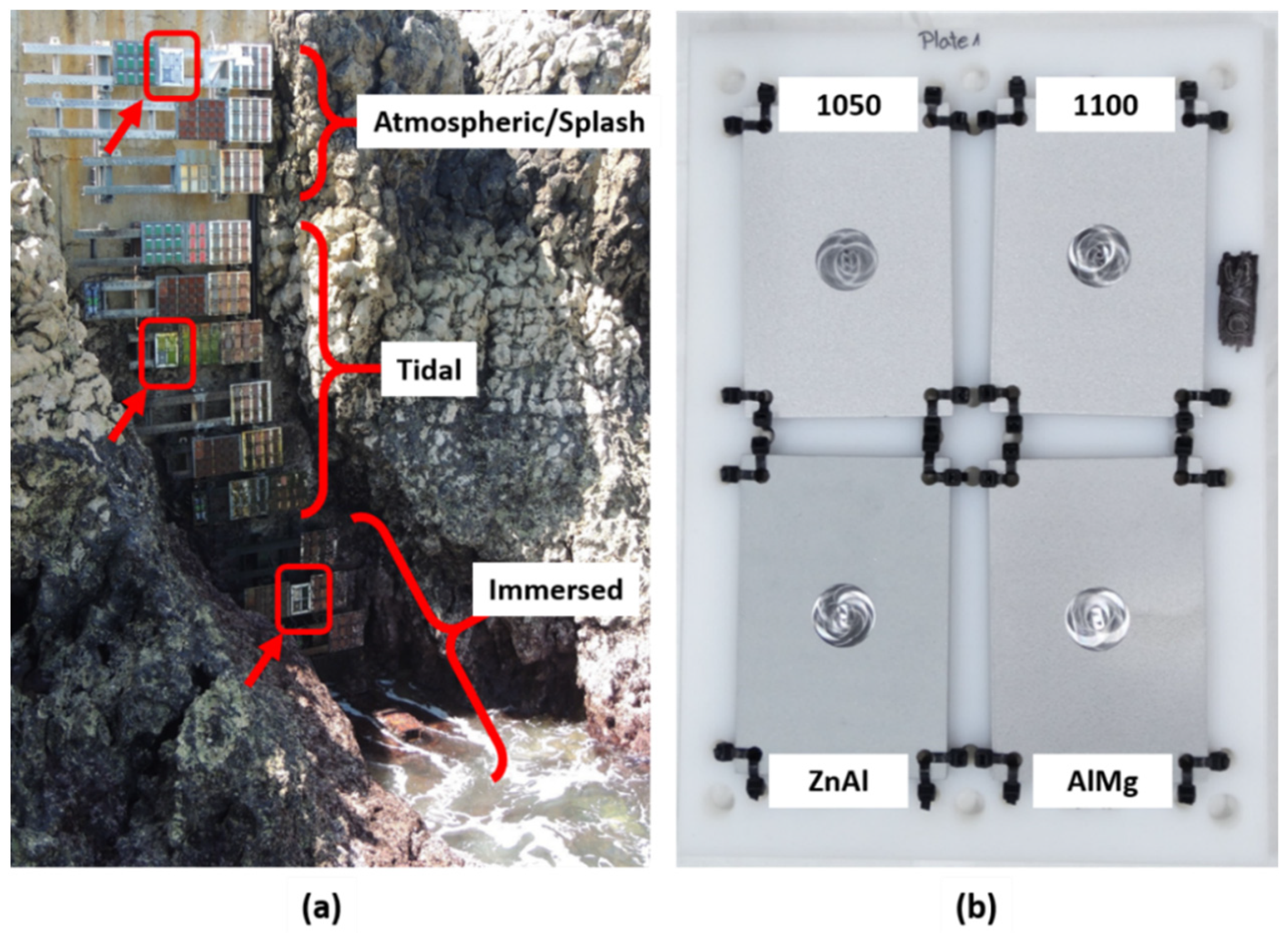
2.3. Field Trials
2.4. Surface Characterization
3. Results
3.1. Simulated Environments Tests
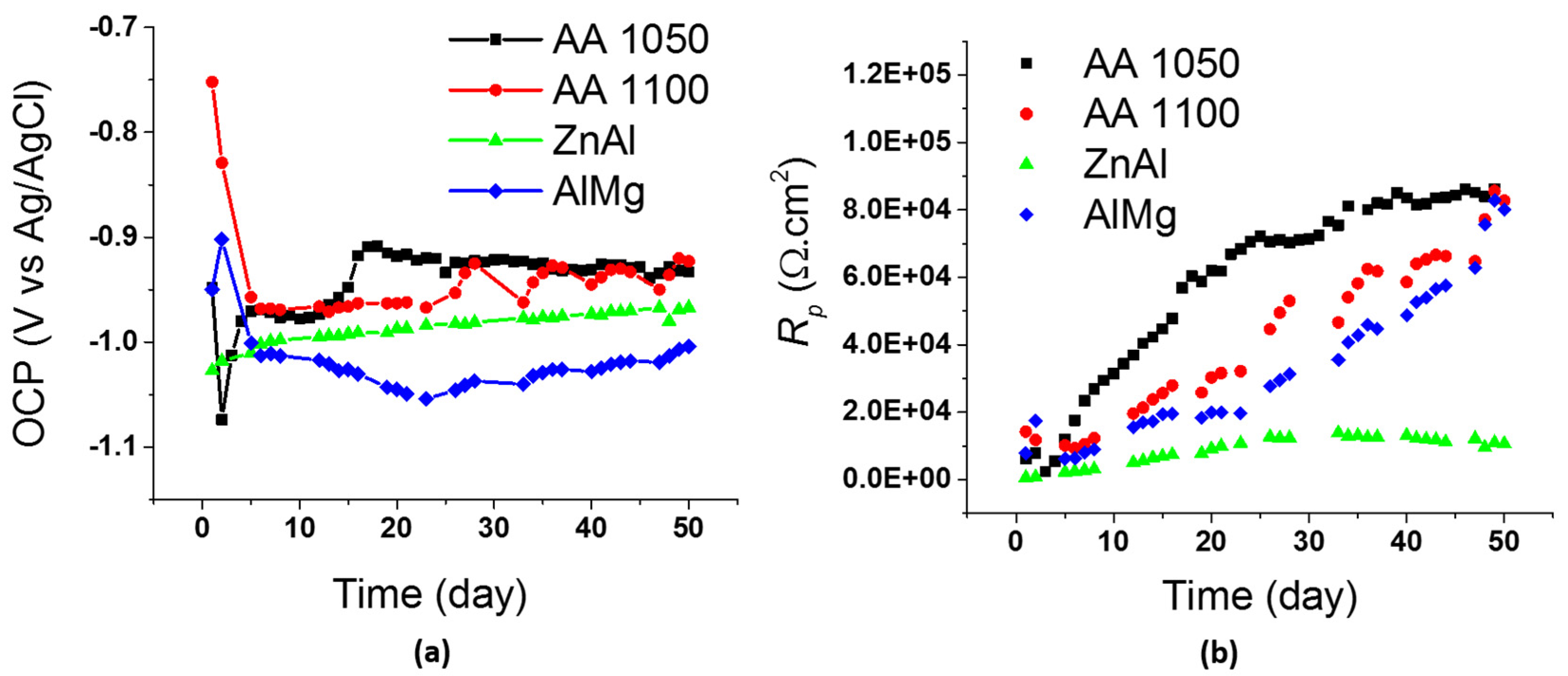
3.1.1. Electrochemical Characterization of Coatings
3.1.2. Long-Term Tests
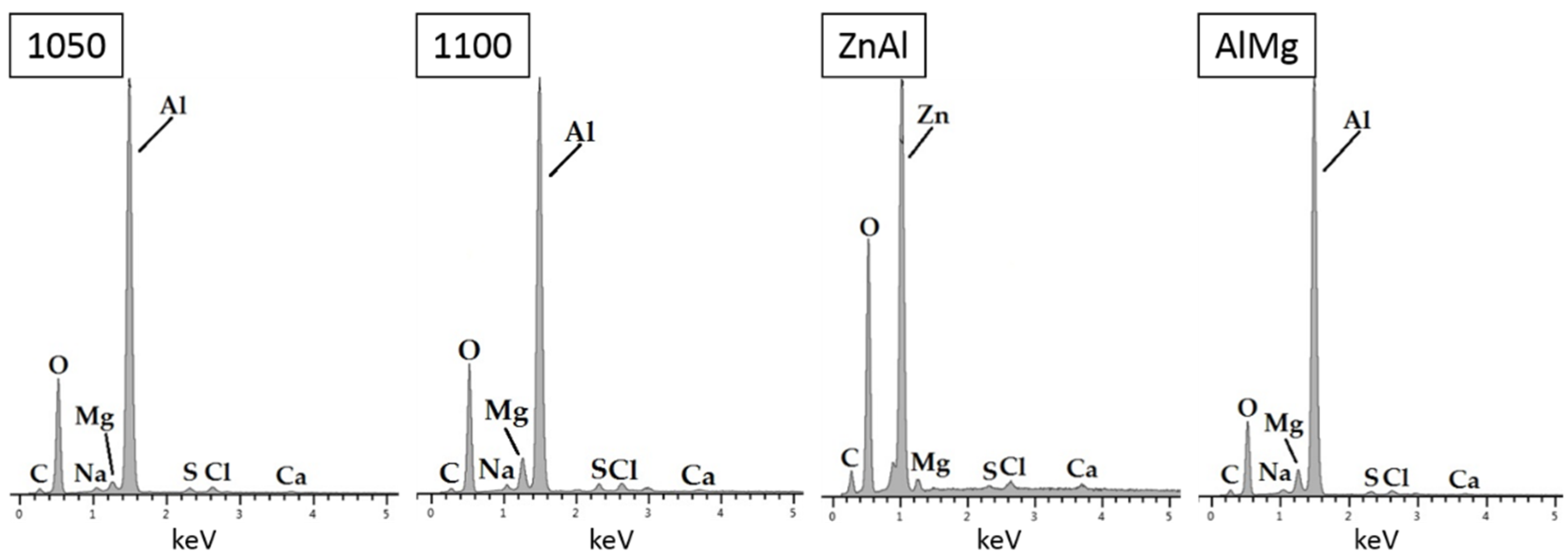
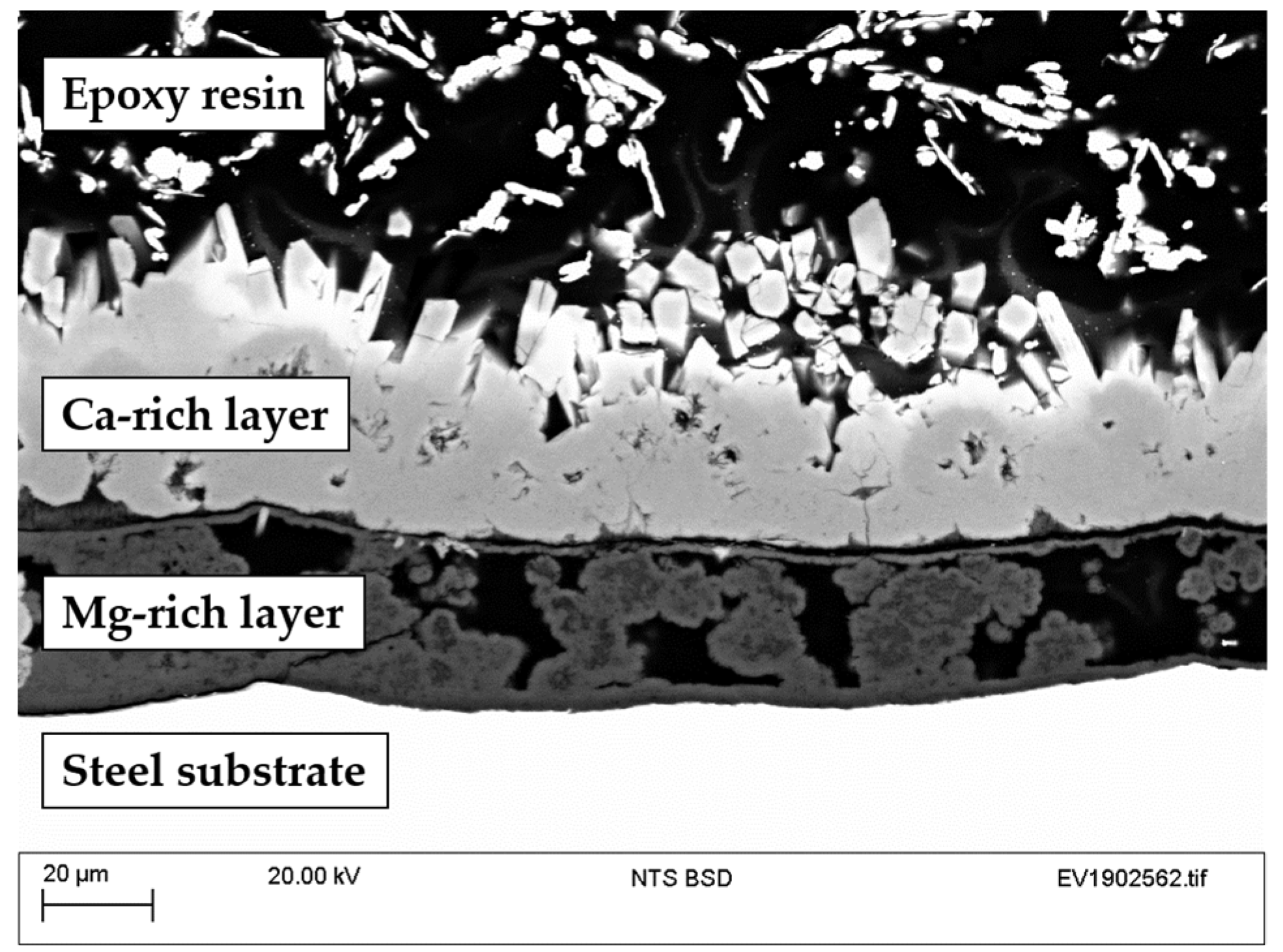
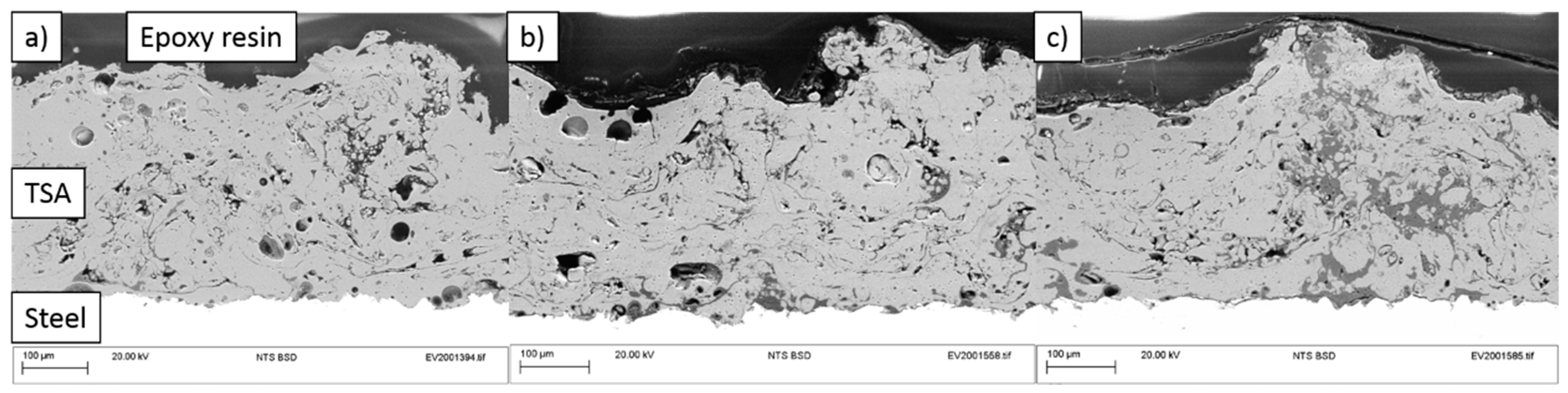
3.1.3. Surface Characterization
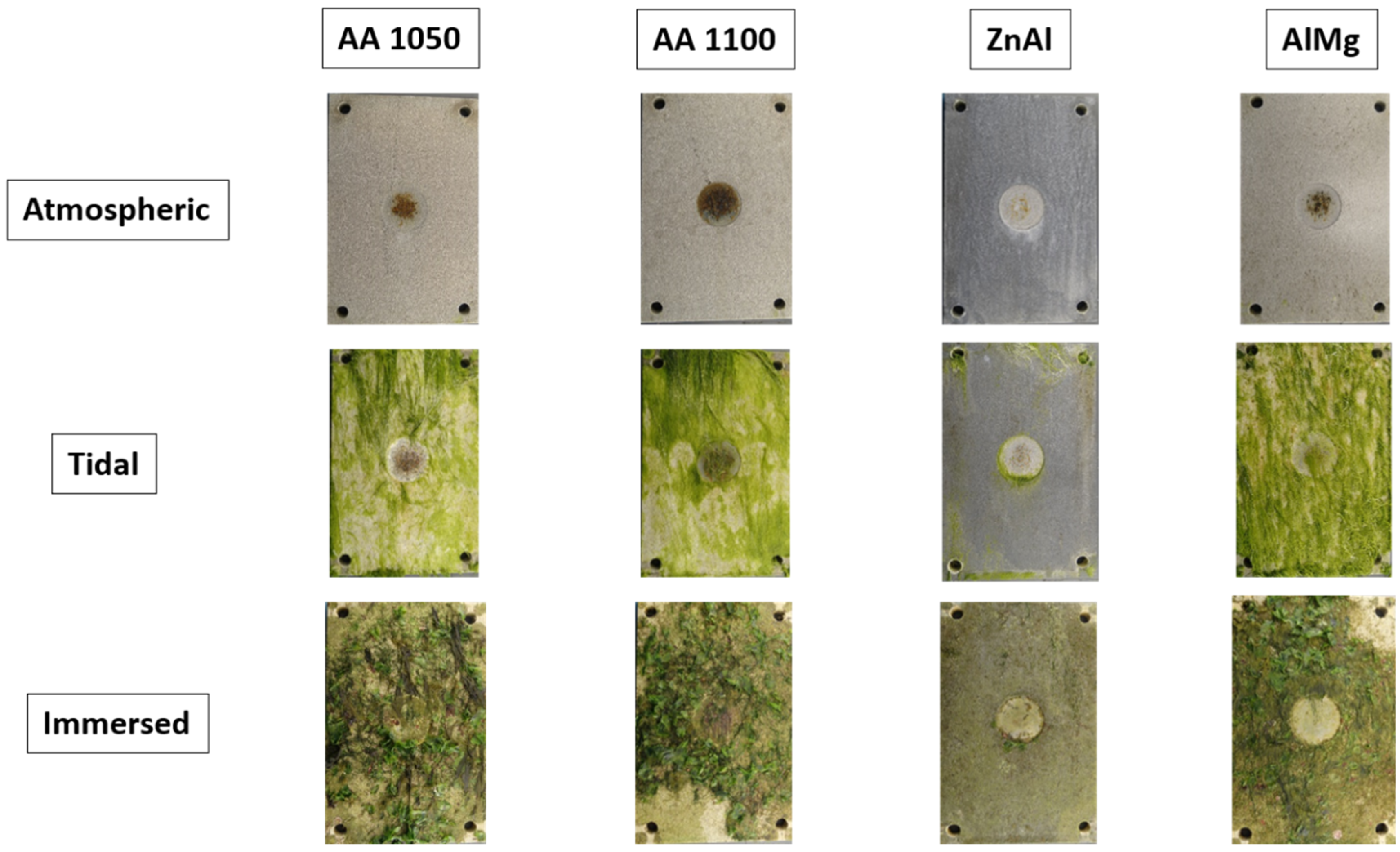
3.2. Field Trials
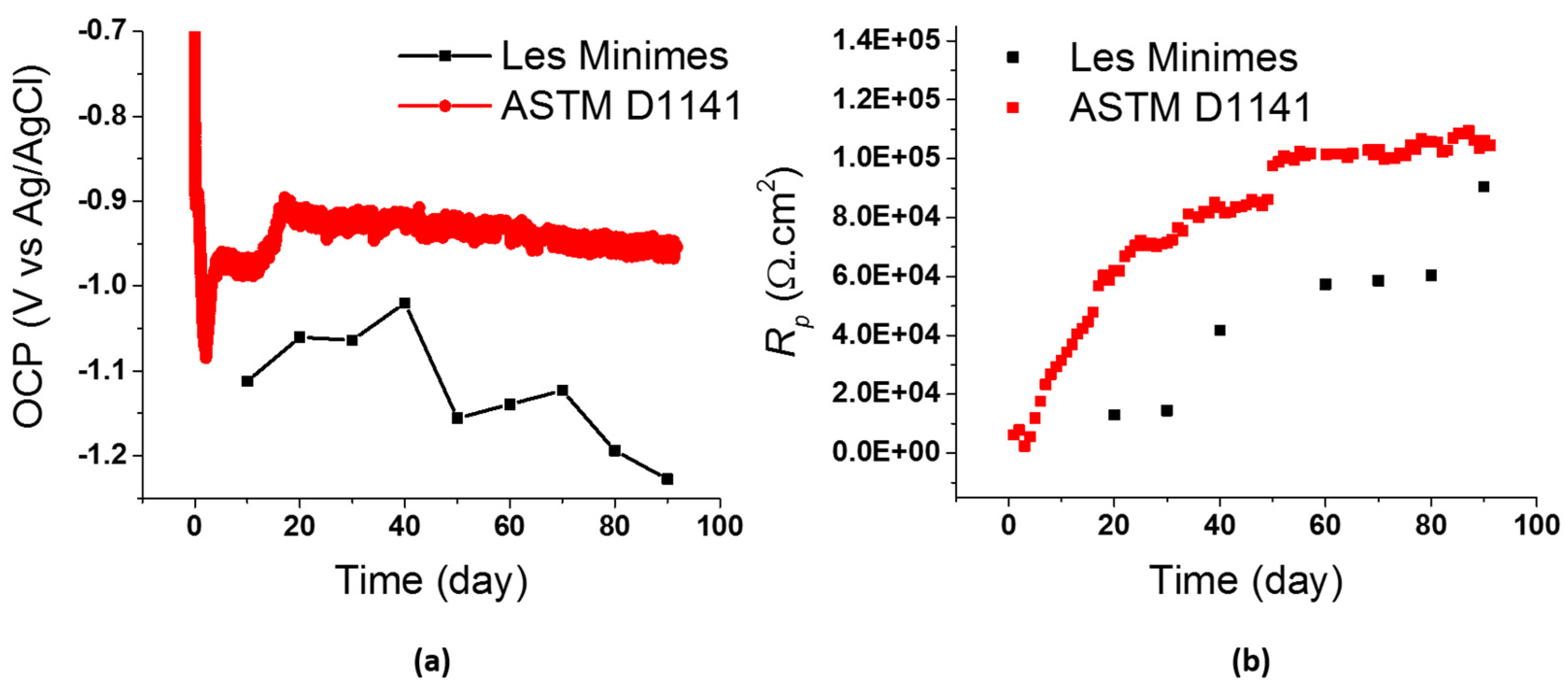
3.2.1. Electrochemical Tests In Situ
3.2.2. Long-Term Exposure of TS Coatings
4. Conclusions
- The four coating types tested showed that they provided cathodic protection for steel in marine environments based on laboratory and field tests.
- The laboratory tests performed with different Al alloys (AA1050, AA1100, Al-5Mg) and Zn-15Al coatings confirmed that although TS Zn-15Al is able to polarize steel to more negative potentials, it provides a shorter lifetime under immersed conditions as evidenced from the Rp values. Therefore, for the design of offshore structures, the exposure zone (atmospheric, tidal or immersed) should be considered in order to choose the coating that provides the best protection.
- Surface characterization of samples revealed the formation of aluminum and zinc oxides/hydroxides on top of the coatings and calcareous deposits on the steel surface in the defect areas.
- The comparison of electrochemical data for TS AA1050 in controlled laboratory tests and field tests substantiates a good correlation in terms of OCP and Rp.
- TSA and TSZ (Zn-15Al) samples with defects showed no significant signs of corrosion after a 6-month exposure to the different zones of marine environments (atmospheric, tidal and immersed).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Presuel-Moreno, F.; Jakab, M.; Tailleart, N.; Goldman, M.; Scully, J. Corrosion-resistant metallic coatings. Mater. Today 2008, 11, 14–23. [Google Scholar] [CrossRef]
- Momber, A.; Plagemann, P.; Stenzel, V. Performance and integrity of protective coating systems for offshore wind power structures after three years under offshore site conditions. Renew. Energy 2015, 74, 606–617. [Google Scholar] [CrossRef]
- Lee, H.S.; Ismail, M.A.; Choe, H.B. Arc thermal metal spray for the protection of steel structures: An overview. Corros. Rev. 2015, 33, 31–61. [Google Scholar] [CrossRef]
- Herman, H.; Sulit, R.A. Thermal spray coatings. Weld. Brazing Solder. 1993, 6, 1004–1009. [Google Scholar] [CrossRef]
- Shibli, S.; Meena, B.; Remya, R. A review on recent approaches in the field of hot dip zinc galvanizing process. Surf. Coat. Technol. 2015, 262, 210–215. [Google Scholar] [CrossRef]
- Paredes, R.; Amico, S.; D’Oliveira, A. The effect of roughness and pre-heating of the substrate on the morphology of aluminium coatings deposited by thermal spraying. Surf. Coat. Technol. 2006, 200, 3049–3055. [Google Scholar] [CrossRef]
- Shrestha, S.; Sturgeon, A. Characteristics and electrochemical corrosion behaviour of thermal sprayed aluminium (TSA) coat-ings prepared by various wire thermal spray processes electrochemical polarization. EUROCORR 2005, 2005, 1–8. [Google Scholar]
- López-Ortega, A.; Arana, J.; Rodríguez, E.; Bayón, R. Corrosion, wear and tribocorrosion performance of a thermally sprayed aluminum coating modified by plasma electrolytic oxidation technique for offshore submerged components protection. Corros. Sci. 2018, 143, 258–280. [Google Scholar] [CrossRef]
- López-Ortega, A.; Bayón, R.; Arana, J. Evaluation of protective coatings for offshore applications. Corrosion and tribocorrosion behavior in synthetic seawater. Surf. Coat. Technol. 2018, 349, 1083–1097. [Google Scholar] [CrossRef]
- Malek, M.H.A.; Saad, N.H.; Abas, S.K.; Roselina, N.N.; Shah, N.M. Performance and microstructure analysis of 99.5% aluminium coating by thermal arc spray technique. Procedia Eng. 2013, 68, 558–565. [Google Scholar] [CrossRef] [Green Version]
- Moon, K.-M.; Kim, Y.-H.; Lee, M.-H.; Baek, T.-S. Polarization characteristics of four types of coating films by thermal spray in seawater solution. Mod. Phys. Lett. B 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Azevedo, M.S.; Allély, C.; Ogle, K.; Volovitch, P. Corrosion mechanisms of Zn (Mg, Al) coated steel: 2. The effect of Mg and Al alloying on the formation and properties of corrosion products in different electrolytes. Corros. Sci. 2015, 90, 482–490. [Google Scholar] [CrossRef]
- Katayama, H.; Kuroda, S. Long-term atmospheric corrosion properties of thermally sprayed Zn, Al and Zn–Al coatings exposed in a coastal area. Corros. Sci. 2013, 76, 35–41. [Google Scholar] [CrossRef]
- Quale, G.; Årtun, L.; Iannuzzi, M.; Johnsen, R. Cathodic protection by distributed sacrificial anodes—A new cost-effective solution to prevent corrosion of subsea structures. Corros. NACE 2017, 2017, 1–15. [Google Scholar]
- Fischer, K.P.; Thomason, W.H.; Rosbrook, T.; Murali, J. Performance history of thermal-sprayed aluminum coatings in off-shore service. Mater. Perform. 1995, 34, 27–35. [Google Scholar]
- Frost, R.L.; Weier, M.L.; Kloprogge, J.T. Raman spectroscopy of some natural hydrotalcites with sulphate and carbonate in the interlayer. J. Raman Spectrosc. 2003, 34, 760–768. [Google Scholar] [CrossRef] [Green Version]
- Hartt, W.H.; Culberson, C.H.; Smith, S.W. Calcareous deposits on metal surfaces in seawater—A critical review. Corrosion 1984, 40, 609–618. [Google Scholar] [CrossRef]
- Echaniz, R.G.; Paul, S.; Thornton, R. Effect of seawater constituents on the performance of thermal spray aluminum in marine environments. Mater. Corros. 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ce, N.; Paul, S. The effect of temperature and local pH on calcareous deposit formation in damaged thermal spray aluminum (TSA) coatings and its implication on corrosion mitigation of offshore steel structures. Coatings 2017, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- BS EN 10025-1:2004. Hot Rolled Products of Structural Steels—Part 1: General Technical Delivery Conditions; BSI: London, UK, 2004. [Google Scholar]
- ASTM D1141-98. Standard Practice for the Preparation of Substitute Ocean Water; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Gates-rector, S.; Blanton, T. The powder diffraction file: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-S.; Singh, J.K.; Park, J.H. Pore blocking characteristics of corrosion products formed on aluminum coating produced by arc thermal metal spray process in 3.5 wt.% NaCl solution. Constr. Build. Mater. 2016, 113, 905–916. [Google Scholar] [CrossRef]
- Dehnavi, V.; Shoesmith, D.W.; Luan, B.L.; Yari, M.; Liu, X.Y.; Rohani, S. Corrosion properties of plasma electrolytic oxidation coatings on an aluminium alloy–The effect of the PEO process stage. Mater. Chem. Phys. 2015, 161, 49–58. [Google Scholar] [CrossRef]
- Silva, F.; Bedoya, J.; Dosta, S.; Cinca, N.; Cano, I.; Guilemany, J.; Benedetti, A. Corrosion characteristics of cold gas spray coatings of reinforced aluminum deposited onto carbon steel. Corros. Sci. 2017, 114, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Esfahani, E.A.; Salimijazi, H.; Golozar, M.A.; Mostaghimi, J.; Pershin, L. Study of corrosion behavior of arc sprayed aluminum coating on mild steel. J. Therm. Spray Technol. 2012, 21, 1195–1202. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, X.; Zhao, H.; Sun, H.; Chen, J. Corrosion performance of zinc coated steel in seawater environment. Chin. J. Oceanol. Limnol. 2016, 35, 423–430. [Google Scholar] [CrossRef]
- De La Pierre, M.; Carteret, C.; Maschio, L.; André, E.; Orlando, R.; Dovesi, R. The raman spectrum of CaCO3 polymorphs calcite and aragonite: A combined experimental and computational study. J. Chem. Phys. 2014, 140, 164509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Refait, P.; Jeannin, M.; Sabot, R.; Antony, H.; Pineau, S. Electrochemical formation and transformation of corrosion products on carbon steel under cathodic protection in seawater. Corros. Sci. 2013, 71, 32–36. [Google Scholar] [CrossRef]
- Barchiche, C.; Deslouis, C.; Festy, D.; Gil, O.; Refait, P.; Touzain, S.; Tribollet, B. Characterization of calcareous deposits in artificial seawater by impedance techniques: 3-Deposit of CaCO3 in the presence of Mg (II). Electrochim. Acta 2003, 48, 1645–1654. [Google Scholar] [CrossRef]





| Elements | C | Mn | Si | P | S | Cr | Ni | Cu | Al | Mo |
|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 0.150 | 1.350 | 0.030 | 0.016 | 0.005 | 0.080 | 0.060 | 0.170 | 0.035 | 0.014 |
| Alloy | Al (wt.%) | Zn (wt.%) | Mg (wt.%) | Cu (wt.%) |
|---|---|---|---|---|
| AA1050 | 99.5 min | 0.05 | 0.05 | 0.02 |
| AA1100 | 99.0 min | 0.1 | - | 0.2 max |
| ZnAl | 15.0 | 85.0 | - | - |
| AlMg | 95.0 | - | 5.0 | - |
| Alloy | Wire Diameter (mm) | Wire Feed Rate (m min−1) | Spray Distance (mm) | Increment Step (mm) | Traverse Speed (m s−1) | Voltage (V) | Current (A) |
|---|---|---|---|---|---|---|---|
| AA1050 AA1100 AlMg | 2.3 | 5.0 | 95.0 | 10.0 | 0.45 | 33.0 | 200 |
| ZnAl | 2.3 | 5.0 | 95.0 | 8.0 | 0.45 | 32.0 | 160 |
| Coating Alloy | Ecorr (V) | jcorr (A cm−2) | ba (mV/dec) | bc (mV/dec) |
|---|---|---|---|---|
| AA1050 | −0.98 | 5.50 × 10−6 | 570 | 180 |
| AA1100 | −0.97 | 6.20 × 10−6 | 510 | 140 |
| ZnAl | −1.10 | 1.50 × 10−4 | 90 | 130 |
| AlMg | −0.98 | 5.30 × 10−6 | 380 | 150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grinon-Echaniz, R.; Paul, S.; Thornton, R.; Refait, P.; Jeannin, M.; Rodriguez, A. Prediction of Thermal Spray Coatings Performance in Marine Environments by Combination of Laboratory and Field Tests. Coatings 2021, 11, 320. https://doi.org/10.3390/coatings11030320
Grinon-Echaniz R, Paul S, Thornton R, Refait P, Jeannin M, Rodriguez A. Prediction of Thermal Spray Coatings Performance in Marine Environments by Combination of Laboratory and Field Tests. Coatings. 2021; 11(3):320. https://doi.org/10.3390/coatings11030320
Chicago/Turabian StyleGrinon-Echaniz, Rosa, Shiladitya Paul, Rob Thornton, Philippe Refait, Marc Jeannin, and Alvaro Rodriguez. 2021. "Prediction of Thermal Spray Coatings Performance in Marine Environments by Combination of Laboratory and Field Tests" Coatings 11, no. 3: 320. https://doi.org/10.3390/coatings11030320









