RETRACTED: Therapeutic and Ameliorative Effects of Active Compounds of Combretum molle in the Treatment and Relief from Wounds in a Diabetes Mellitus Experimental Model
Abstract
:1. Introduction
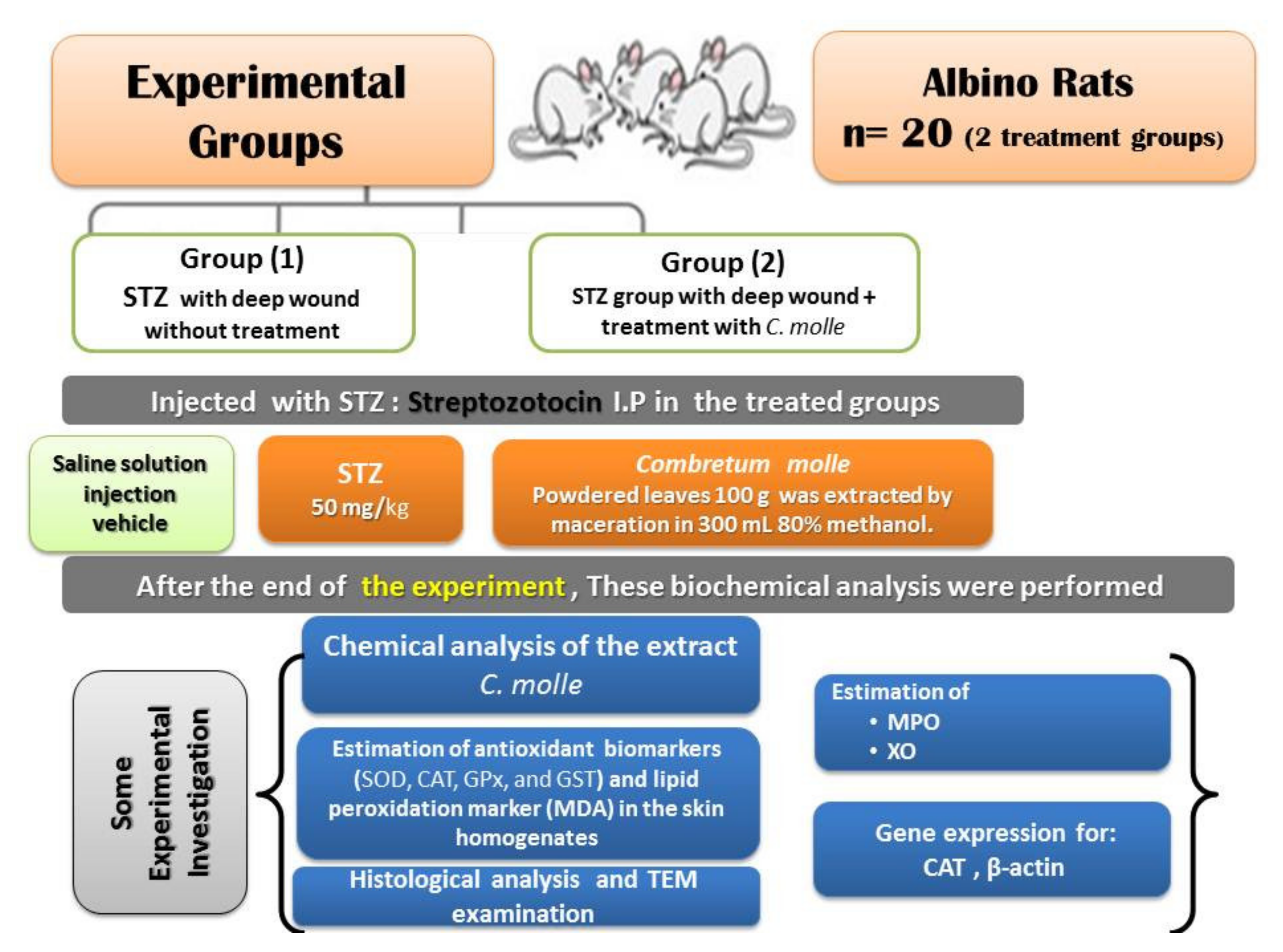
2. Materials and Methods
2.1. Combretum molle Material Preparation
2.2. Chemical Composition of the Combretum molle Plant Extract
- Alkaloids:
- Saponins:
- Steroids:
- Flavonoids:
- Cardiac glycosides:
- Anthraquinones:
- Tannins:
2.3. HPLC-UV of the Plant Extract Combretum molle
2.4. Experimental Animals
2.4.1. Experimental Animal Design
- Diabetic deep wound—positive control group.
- Diabetic deep wound—Combretum molle extract group.
2.4.2. Diabetes Mellitus Experimental Model
2.5. Blood Sample Collection
2.6. Tissue Homogenate Preparation for the Measurement of Oxidative Stress Markers
2.7. Determination of Markers of Oxidative Stress
2.8. Estimation of Inflammation Biomarkers
2.9. Mitochondrial Function
2.9.1. Evaluation of Succinate Dehydrogenase (SDH, Complex II) Activity
2.9.2. Mitochondrial ROS Assay
2.9.3. Mitochondria Membrane Potential (MMP, ΔΨm) Assay
2.9.4. Mitochondrial Membrane Potential via Fluorescent Detection
2.9.5. Evaluation of Swelling of the Mitochondria
2.9.6. Cytochrome C Oxidase Release Assay
2.9.7. ATP Content Assay
2.10. Transmission Electron Microscopic Study (TEM)
2.11. Histological Analysis of Skin Tissues
2.12. RNA Isolation and Quantitative Reverse Transcription Polymerase Chain Reaction
2.13. Statistical Analysis
2.14. Sample Size
3. Results
3.1. Chemical Composition of the Novel Ointment
3.2. Biological Results
3.2.1. Morphological Aspects of the Wound Healing in the Different Treated Groups
3.2.2. Oxidative Stress Biomarkers
3.2.3. CRP Levels in the Serum
3.2.4. SDH, ROS, Cytochrome-C, ATP, and MMP
3.2.5. Mitochondrial Membrane Potential
3.2.6. Gene Expression Levels in the Dermal Tissues of the Two Groups
3.2.7. Histological Examination
3.2.8. TEM Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merkin, A.; Mesele, B.; Tsige, K. Effect of Combretum molle (Combretaceae) seed extract on hematological and biochemical parameters. J. Med. Plants Res. 2018, 12, 55–63. [Google Scholar] [CrossRef]
- Eloff, J.N.; Katerere, D.R.; Mcgaw, L.J. The biological activity and chemistry of the southern African Combretaceae. J. Ethnopharmacol. 2008, 119, 686–699. [Google Scholar] [CrossRef]
- Fyhrquist, P.; Mwasumbi, L.; Haeggstro, C.A.; Vuorela, H.; Hiltunen, R.; Vuorela, P. Ethnobotanical and antimicrobial investigation on some species of Terminalia and Combretum (Combretaceae) growing in Tanzania. J. Ethnopharmacol. 2012, 79, 169–177. [Google Scholar] [CrossRef]
- Sahlu, T. Antibacterial Activities and Preliminary Phytochemical Investigation of Four Selected Medicinal Plants. Master’s Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2013. [Google Scholar]
- Amare, T.; Tadesse, E. In vitro Antibacterial Effect of Combretum molle and Fr1 against Staphylococcus aureus and E. coli Isolated from Bovine Mastitis. World J. Biol. Med. Sci. 2016, 3, 115–131. [Google Scholar]
- Ademola, O.; Eloff, N. In vitro anthelmintic activity of Combretum molle ex (Combretaceae) against Haemonchus contortusova and larvae. Vet. Parasitol. 2010, 169, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Sanon, S.; Gansane, A.; Ouattara, L.P.; Traore, A.; Ouedraogo, I.N.; Tiono, A.; Taramelli, D.; Basilico, N.; Sirima, S.B. In vitro antiplasmodial and cytotoxic properties of some medicinal plants from western Burkina Faso. Afr. J. Lab Med. 2013, 2, 81. [Google Scholar] [CrossRef]
- John, K.M.; Mustapha, A.; Yakubu, J. Aqueous Co-extract Mixture of Combretum molle (stembark) and Xylopia aethiopica (fruit) show Phytochemical Synergy in its Anti-fungal and Antioxidant Bioactivities. Adv. Complement Alt. Med. 2020, 6, 560–575. [Google Scholar]
- Trikkalinou, A.; Papazafiropoulou, A.K.; Melidonis, A. Type 2 diabetes and quality of life. World J. Diabetes 2017, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Qianjin, X.; Xiaqiang, C.; Zenghui, L.; Ying, W.; Xu, D.; Yan, X. Preparation and characterization of epigallocatechin gallate, ascorbic acid, gelatin, chitosan nanoparticles and their beneficial effect on wound healing of diabetic mice. Int. J. Biol. Macromol. 2020, 148, 777–784. [Google Scholar] [CrossRef]
- Paul, E.J.; Padmapriya, B. A pragmatic review on the property, role and significance of polymers in treating diabetic foot ulcer. Mater. Today Proc. 2020, 23, 91–99. [Google Scholar] [CrossRef]
- Fard, A.S.; Esmaelzadeh, M.; Larijani, B. Assessment and treatment of diabetic foot ulcer. Int. J. Clin. Pract. 2007, 61, 1931–1938. [Google Scholar] [CrossRef]
- ADM. American Diabetes Association. Standards of medical care in diabetes-2014. Diabetes Care 2014, 37, 14–80. [Google Scholar] [CrossRef]
- Soliman, A.M.; Teoh, S.L.; Norzana, A.; Ghafar, S.D. Virgin coconut oil and diabetic wound healing: Histopathological and biochemical analysis. Eur. J. Anat. 2018, 22, 135–144. [Google Scholar]
- Shahbazian, H.; Yazdanpanah, L.; Latifi, S.M. Risk assessment of patients with diabetes for foot ul-cers according to risk classification consensus of Inter-national Working Group on Diabetic Foot (IWGDF). Pak. J. Med. Sci. 2013, 29, 730–734. [Google Scholar] [CrossRef]
- Gansane, A.; Sanon, S.; Ouattara, L.P.; Traore, A.; Hutter, S.; Ollivier, E.; Azas, N.; Traore, A.S.; Guissou, I.P.; Sirima, S.B. Antiplasmodial activity and toxicity of crude extracts from alternatives parts of plants widely used for the treatment of malaria in Burkina Faso: Contribution for their preservation. Parasitol. Res. 2010, 106, 335–340. [Google Scholar] [CrossRef]
- Miaffo, D.; Wansi, S.L.; Mbiantcha, M.; Poualeu, S.L.K.; Guessom, O.K. Toxicological Evaluation of Aqueous and Acetone Extracts of Combretum molle Twigs in Wistar Rats. Electron. J. Biol. 2015, 11, 33–45. [Google Scholar]
- Snyder, R.J.; Hanft, J.R. Diabetic foot ulcers—Effects on QOL, costs, and mortality and the role of standard wound care and advanced-care therapies. Ostomy Wound Manag. 2009, 55, 28–38. [Google Scholar]
- Nasab, M.E.; Nasrin, T.; Partow, M.S.; Alireza, P. In vitro antioxidant activity and in vivo wound-healing effect of lecithin liposomes: A comparative study. J. Comp. Eff. Res. 2019, 8, 633–643. [Google Scholar] [CrossRef]
- Pazyar, N.; Yaghoobi, R.; Rafiee, E.; Mehrabian, A.; Feily, A. Skin wound healing and phytomedicine: A review. Skin Pharmacol. Physiol. 2014, 27, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.N.; Da Silva, A.L. A standard burn model using rats. Acta Cir Bras 1999, 14. [Google Scholar] [CrossRef]
- Yang, D.J.; Moh, S.H.; Son, D.H.; You, S.; Kinyua, A.W.; Ko, C.M.; Song, M.; Yeo, J.; Choi, Y.H.; Kim, K.W. Gallic Acid Promotes Wound Healing in Normal and Hyperglucidic Conditions. Molecules 2016, 21, 899. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Hamza, R.Z.; Gobouri, A.A.; Refat, M.S. Synthesis of new antidiabetic agent by complexity between vanadyl (II) sulfate and vitamin B1: Structural, characterization, anti-DNA damage, structural alterations and antioxidative damage studies. Appl. Organomet. Chem. 2019, 33, e4892. [Google Scholar] [CrossRef]
- Litwack, G.; Bothwell, J.W.; Williams, J.N.; Elvehjem, C.A. A colorimetric assay for xanthine oxidase in rat liver homogenates. J. Biol. Chem. 1953, 200, 303. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. J. Anal. Biochem. 1968, 25, 192. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351. [Google Scholar] [CrossRef]
- Beers, J.R.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133. [Google Scholar] [CrossRef]
- Couri, D.; Abdel-Rahman, M.S. Effect of chlorine dioxide and metabolites on glutathione dependent system in rat, mouse and chicken blood. J. Environ. Pathol. Toxicol. 1979, 3, 451–460. [Google Scholar] [PubMed]
- Goldberg, D.M.; Spooner, R.J. Methods of Enzymatic Analysis, 3rd ed.; Bergmeyen, H.V., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 1983; Volume 3, pp. 258–265. [Google Scholar]
- Wener, M.H.; Daum, P.R.; McQuillin, G.M. The influence of age, sex, and race on the upper reference limit of serum C-reactive protein concentration. J. Rheumatol. 2000, 27, 2351–2359. [Google Scholar]
- Suzuki, K.; Ota, H.; Sasagawa, S.; Sakatani, T.; Fujikura, T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983, 132, 345–352. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, L.; Liu, H.; Xia, Q.; Zhang, Y.; Yang, X.; Wang, K. Vanadium compounds induced mitochondria permeability transition pore (PTP) opening related to oxidative stress. J. Inorg. Biochem. 2010, 104, 371–378. [Google Scholar] [CrossRef]
- Ayoubi, M.; Naserzadeh, P.; Hashemi, M.T.; Rostami, M.R.; Tamjid, E.; Tavakoli, M.M.; Simchi, A. Biochemical mechanisms of dose-dependent cytotoxicity and ROS-mediated apoptosis induced by lead sulfide/graphene oxide quantum dots for potential bioimaging applications. Sci. Rep. 2017, 7, 12896. [Google Scholar] [CrossRef]
- Naserzadeh, P.; Ansari, E.F.; Kaviani, M.; Ashtari, K.; Kheirbakhsh, R.; Salimi, A.; Pourahmad, J. Single-walled carbon nanotube, multiwalled carbon nanotube, and Fe2O3 nanoparticles induced mitochondria-mediated apoptosis in melanoma cells. Cutan. Ocul. Toxicol. 2018, 37, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Tafreshi, N.; Hosseinkhani, S.; Sadeghizadeh, M.; Sadeghi, M.; Ranjbar, B.; Naderi-Manesh, H. The influence of insertion of a critical residue (Arg356) in structure and bioluminescence spectra of firefly luciferase. J. Biol. Chem. 2007, 282, 8641–8647. [Google Scholar] [CrossRef]
- Hayat, M.A. Basic Techniques for Transmission Electron Microscopy, 1st ed.; Hayat, M.A., Ed.; Macmillan Press: New York, NY, USA, 1986; ISBN 9780123339263. [Google Scholar]
- Petrie, A.; Sabin, C. Medical Statistics at a Glance, 3rd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; Volume 23. [Google Scholar]
- Hailing, L.; Hoyong, C. Self-Healing Properties of Lignin-Containing Nanocomposite: Synthesis of Lignin-graft-poly (5-acetylaminopentyl acrylate) via RAFT and Click Chemistry. Macromolecules 2016, 49, 7246–7256. [Google Scholar]
- Fukui, M.; Choi, H.J.; Zhu, B.T. Mechanism for the protective effect of resveratrol against oxidative stress-induced neuronal death. Free. Radic. Biol. Med. 2010, 49, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Maresca, V.; Flori, E.; Briganti, S.; Mastrofrancesco, A.; Fabbri, C.; Mileo, A.M.; Paggi, M.G.; Picardo, M. Correlation between melanogenic and catalase activity in in vitro human melanocytes: A synergic strategy against oxidative stress. Pigment. Cell Melanoma Res. 2008, 21, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Fyhrquist, P.; Laakso, I.; Garcia Marco, S.; Julkunen-Tiitto, R.; Hiltunen, R. Antimycobacterial activity of ellagitannin and ellagic acid derivative rich crude extracts and fractions of five selected species of Terminalia used for treatment of infectious diseases in African traditional medicine. S. Afr. J. Bot. 2014, 90, 1–16. [Google Scholar] [CrossRef]
- Refat, M.S.; Hamza, R.Z.; Adam, A.A.; Saad, H.A.; Gobouri, A.A.; Al-Harbi, F.S.; Al-Salmi, F.A.; Altalhi, T.; El-Megharbel, S.M. Quercetin/Zinc complex and stem cells: A new drug therapy to ameliorate glycometabolic control and pulmonary dysfunction in diabetes mellitus: Structural characterization and genetic studies. PLoS ONE. 2021, 16, e0246265. [Google Scholar] [CrossRef] [PubMed]













| No. | Chemical Group | Reagent (Test) Used to Detect | Results of Screening (+ or −) of the Leaf Powder |
|---|---|---|---|
| 1 | Cardiac glycosides | Keller Kelliani’s test | − |
| 2 | Alkaloids | Mayer’s test | + |
| Wagner’s test | + | ||
| Dragendroff’s test | + | ||
| 3 | Carbohydrates | Molisch’s test | + |
| 4 | Saponin glycosides | Froth test | + |
| Foam test | + | ||
| 5 | Anthraquinone glycosides | Modified Borntrager’s test | + |
| 6 | Flavonoids | Lead acetate test | + |
| Alkaline reagent test | + | ||
| 7 | Unsaturated sterols and/or triterpenes | Libermann Burchard’s test | + |
| Salkowski’s test | + | ||
| 8 | Phenols (tannins) | Ferric chloride test | + |
| 9 | Proteins and amino acids | Ninhydrin test | + |
| 10 | Lignin | Phloroglucinol | + |
| No. | Compounds | Molecular Composition | Rt-UHPLC (min) |
|---|---|---|---|
| 1 | Gallic acid | C7H6O5 | 1.370 |
| 2 | Gallic acid derivative | - | 2.343 |
| 3 | Protocatechuic acid | C7H6O4 | 2.722 |
| 4 | O-hydroxycinnamic-acid-like substance | - | 2.990 |
| 5 | Ellagitannin | C44H32O27 | 3.023 |
| 6 | Unknown ellagitannin | - | 3.630 |
| 7 | Corilagin derivative | C27H22O18 | 3.703 |
| 8 | Punicacortein D | C48H28O30 | 3.863 |
| 9 | Unknown ellagitannin | - | 4.083 |
| 10 | Unknown ellagitannin | - | 4.233 |
| 11 | Sanguiin H-4 | C27H22O18 | 4.462 |
| 13 | Gallotannin | C76H52O46 | 5.030 |
| 14 | Β-punicalagin | C48H28O30 | 5.299 |
| 15 | An epigallocatechin-like substance | - | 5.367 |
| 16 | Ellagic acid derivative | - | 5.517 |
| 17 | Lignan | C25H30O8 | 5.743 |
| 18 | Ellagic acid derivative | - | 5.897 |
| 20 | A procyanidin-B-3-like substance | C30H26O12 | 6.890 |
| Oxidative/Antioxidant Parameters | Control Group (n = 10) | Treated Group (n = 10) | t | p-Value |
|---|---|---|---|---|
| CAT (U/g) | 0.74 ± 0.2 | 3.2 ± 0.8 | 9.4 | <0.001 * |
| SOD (U/g) | 8.1 ± 0.4 | 20.9 ± 4.2 | 9.6 | <0.001 * |
| MDA (U/g) | 69.8 ± 4.1 | 9.3 ± 0.2 | 46.6 | <0.001 * |
| GPX (U/g) | 4.1 ± 0.1 | 14.2 ± 2.4 | 13.3 | <0.001 * |
| GST (U/g) | 4.2 ± 0.4 | 12.2 ± 2.8 | 8.9 | <0.001 * |
| Oxidative/Antioxidant Parameters | Control Group (n = 10) | Treated Group (n = 10) | t | p-Value |
|---|---|---|---|---|
| MPO (nmol/min/mL) | 28.1 ± 2.2 | 14.4 ± 2.3 | 13.6 | <0.001 * |
| XO (U/g) | 38.1 ± 6.2 | 12.2 ± 2.1 | 12.5 | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, R.Z.; Al-Motaani, S.E.; Al-Talhi, T. RETRACTED: Therapeutic and Ameliorative Effects of Active Compounds of Combretum molle in the Treatment and Relief from Wounds in a Diabetes Mellitus Experimental Model. Coatings 2021, 11, 324. https://doi.org/10.3390/coatings11030324
Hamza RZ, Al-Motaani SE, Al-Talhi T. RETRACTED: Therapeutic and Ameliorative Effects of Active Compounds of Combretum molle in the Treatment and Relief from Wounds in a Diabetes Mellitus Experimental Model. Coatings. 2021; 11(3):324. https://doi.org/10.3390/coatings11030324
Chicago/Turabian StyleHamza, Reham Z., Shaden E. Al-Motaani, and Tarek Al-Talhi. 2021. "RETRACTED: Therapeutic and Ameliorative Effects of Active Compounds of Combretum molle in the Treatment and Relief from Wounds in a Diabetes Mellitus Experimental Model" Coatings 11, no. 3: 324. https://doi.org/10.3390/coatings11030324





