Investigation into Microstructure, Wear Resistance in Air and NaCl Solution of AlCrCoNiFeCTax High-Entropy Alloy Coatings Fabricated by Laser Cladding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Coatings
2.2. Microstructure Characterization
2.3. Mechanical Properties
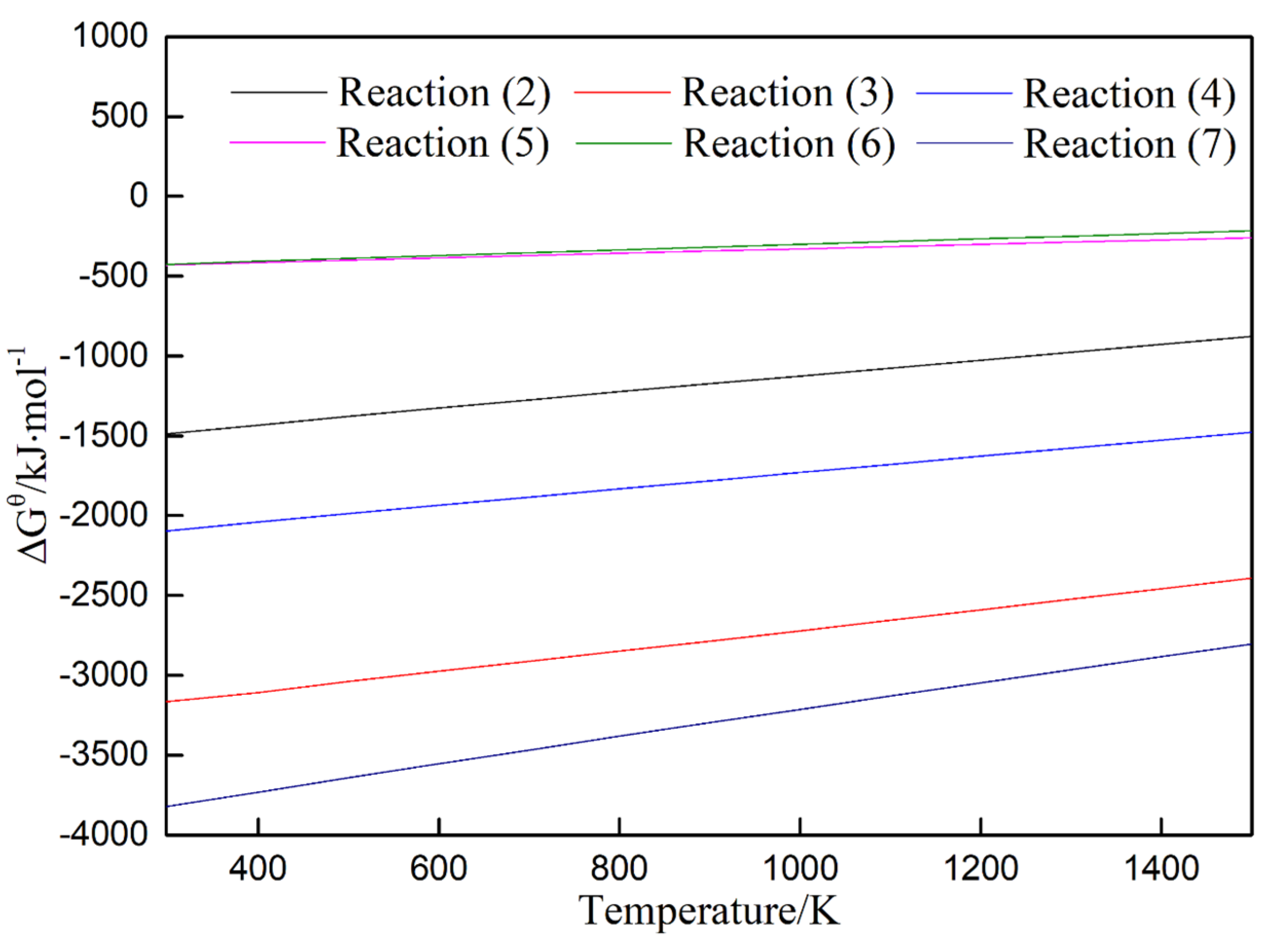
3. Results and Discussion
3.1. XRD Analyses
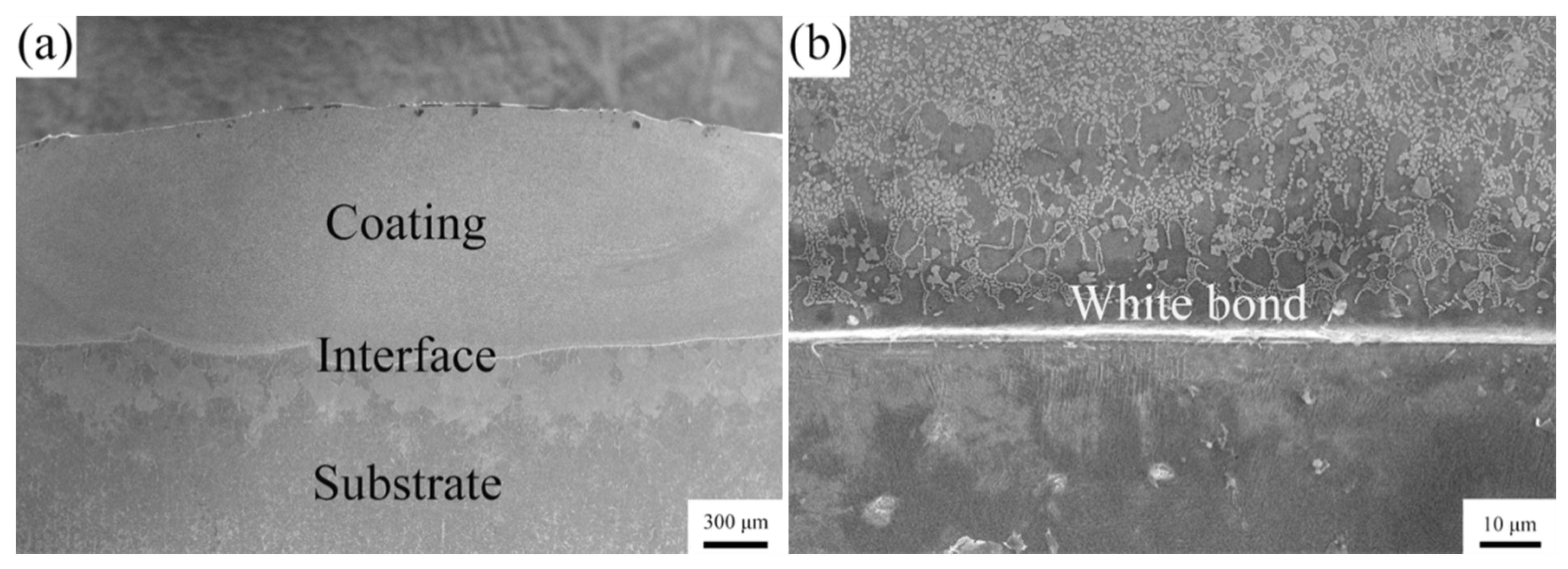
3.2. Microstructural Characterization
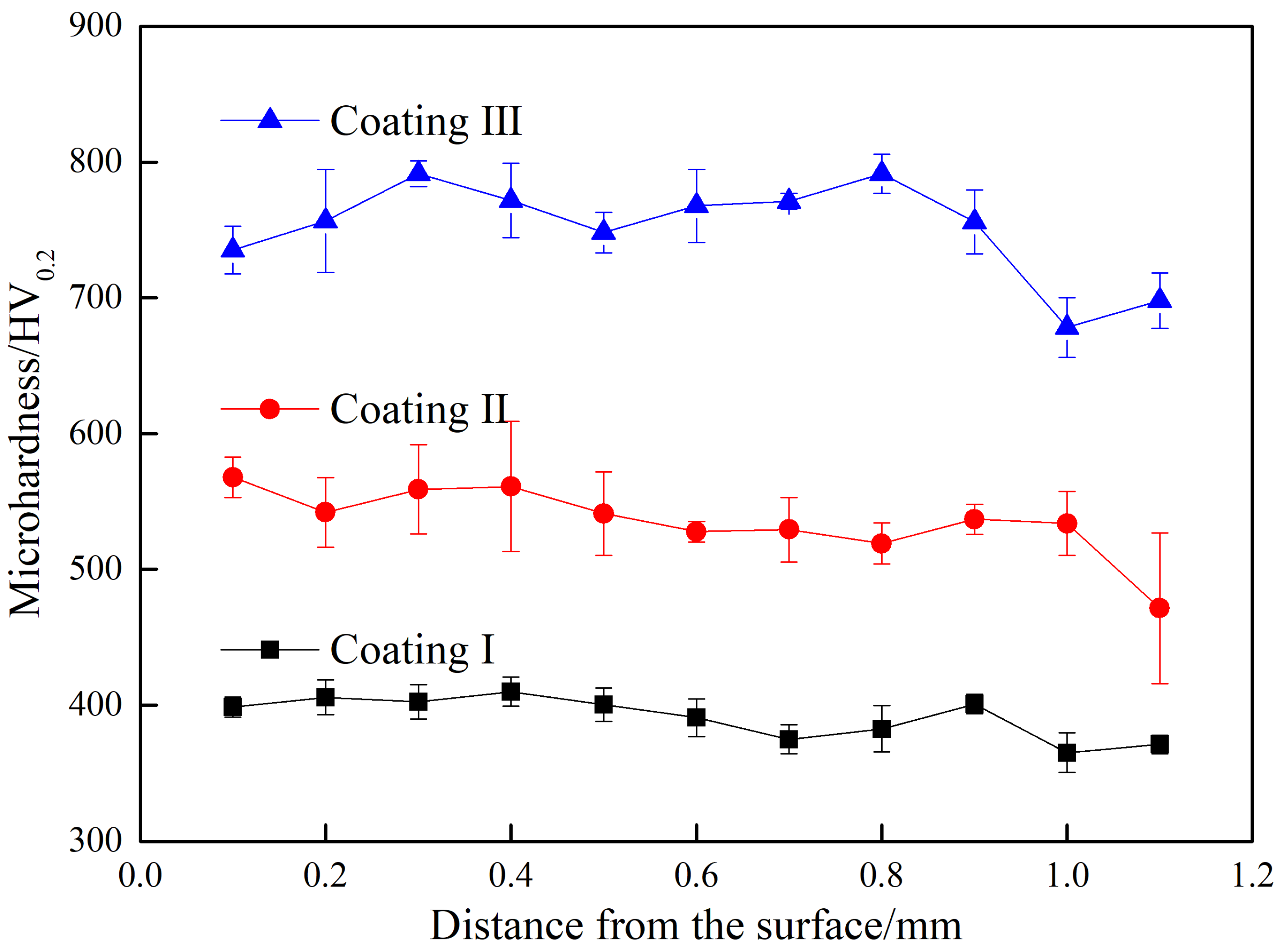
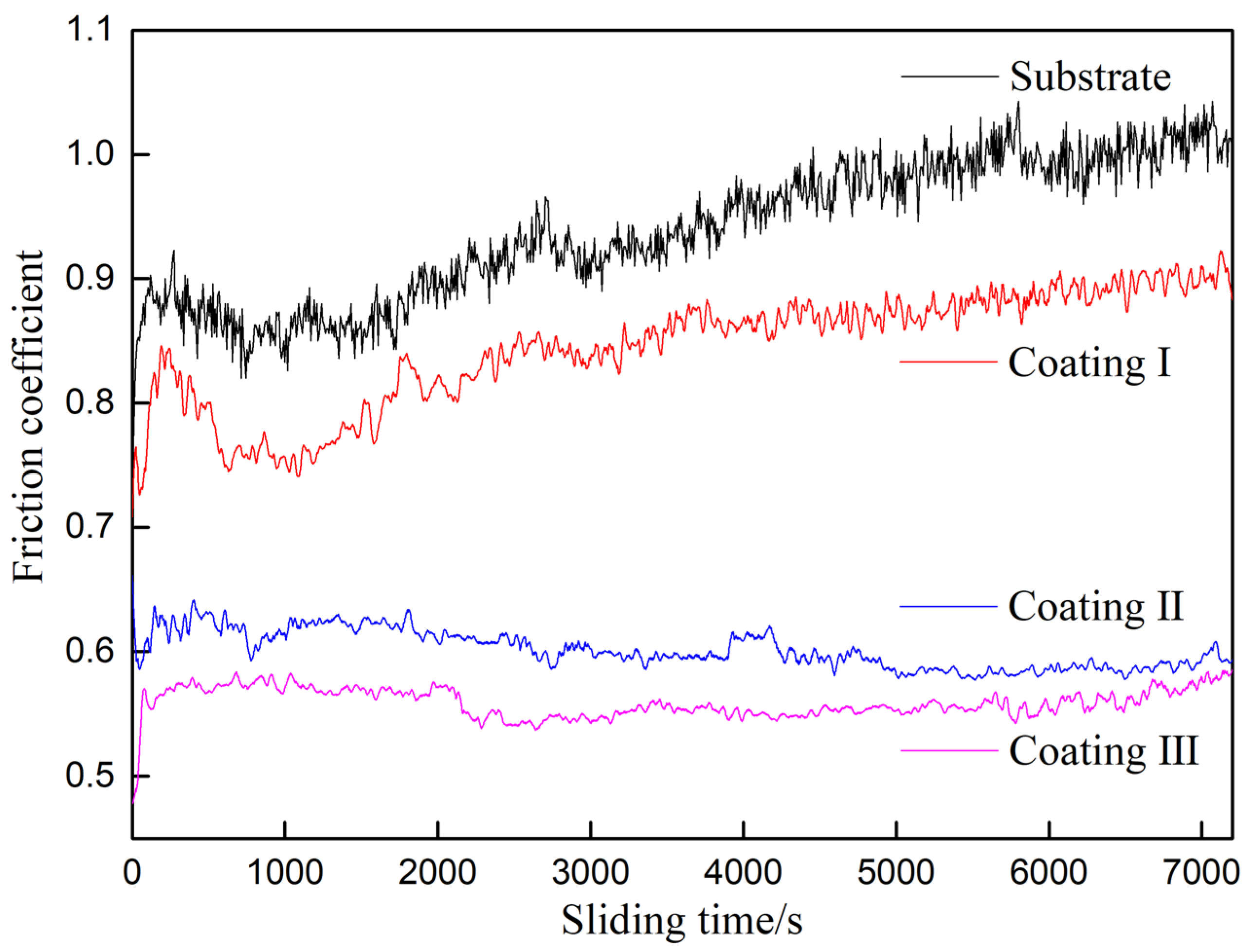
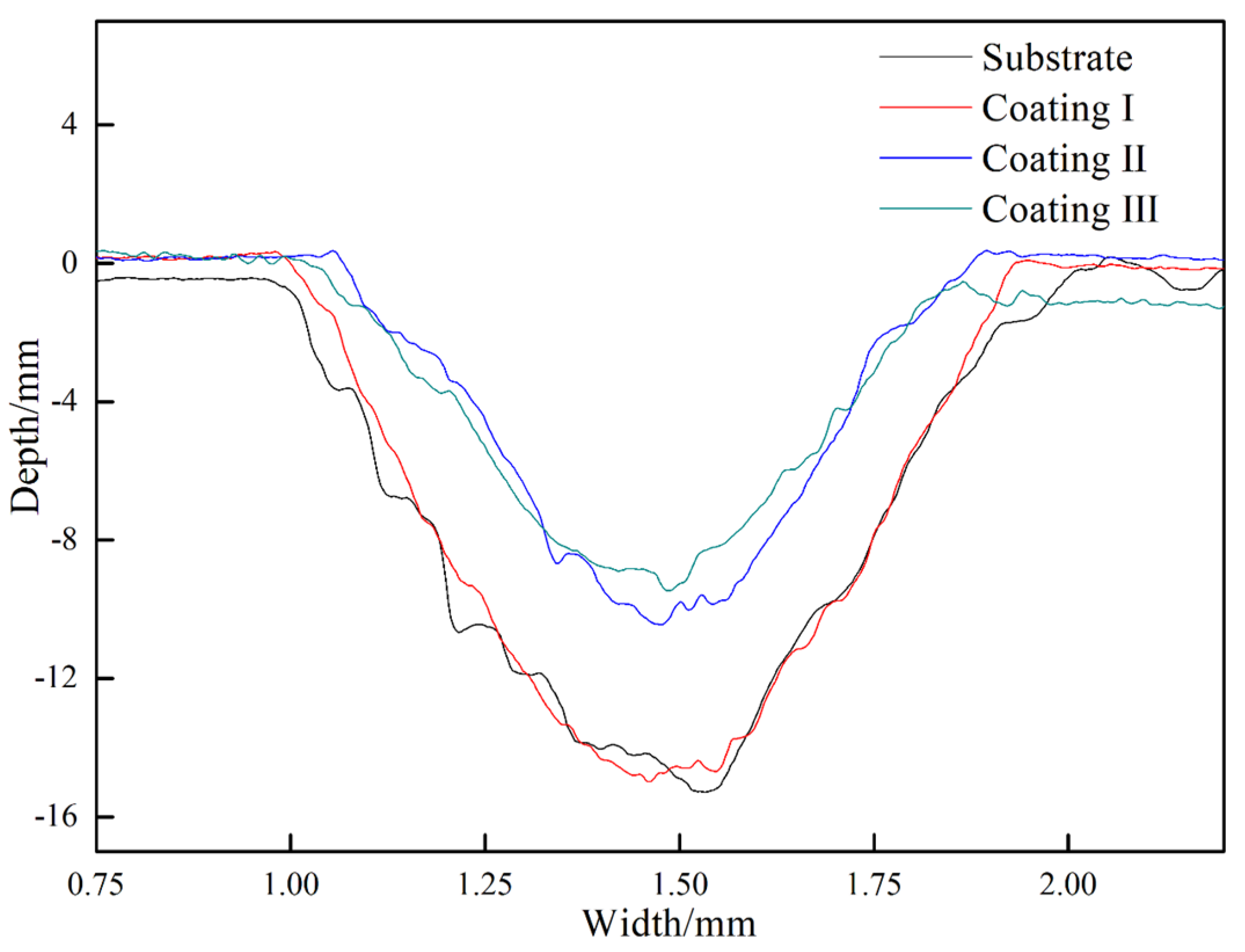
3.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.X.; Lu, Z.C.; Wang, S.L.; Wu, Y.; Lu, Z.P. Fe-based bulk metallic glasses: Glass formation, fabrication, properties and applications. Prog. Mater. Sci. 2019, 103, 235–318. [Google Scholar] [CrossRef]
- Liu, H.X.; Xu, Q.; Wang, C.Q.; Zhang, X.W. Corrosion and wear behavior of Ni60CuMoW coatings fabricated by combination of laser cladding and mechanical vibration processing. J. Alloy. Compd. 2015, 621, 357–363. [Google Scholar] [CrossRef]
- Shang, F.M.; Chen, S.Y.; Zhang, C.Y.; Liang, J.; Liu, C.S.; Wang, M. The effect of Si and B on formability and wear resistance of preset-powder laser cladding W10V5Co4 alloy steel coating. Opt. Laser Technol. 2021, 134, 106590. [Google Scholar] [CrossRef]
- Panda, J.N.; Wong, B.C.; Medvedovski, E.; Egberts, P. Enhancement of tribo-corrosion performance of carbon steel through boronizing and BN-based coatings. Tribo. Int. 2021, 153, 106666. [Google Scholar] [CrossRef]
- Kossman, S.; Coelho, L.B.; Mejias, A.; Montagne, A.; Gorp, A.V.; Coorevits, T.; Touzin, M.; Poorteman, M.; Olivier, M.G.; Iost, A.; et al. Impact of industrially applied surface finishing processes on tribocorrosion performance of 316L stainless steel. Wear 2020, 456–457, 203341. [Google Scholar] [CrossRef]
- Zheng, K.; Wang, Y.R.; Wang, R.Y.; Wang, Y.L.; Cheng, F.; Ma, Y.; Hei, H.J.; Gao, J.; Zhou, B.; Wang, Y.S.; et al. Microstructure, oxidation behavior and adhesion of a CoNiCrAlTaY coating deposited on a high Nb–TiAl alloy by plasma surface metallizing technique. Vacuum 2020, 179, 109494. [Google Scholar] [CrossRef]
- Attard, B.; Leyland, A.; Matthews, A.; Gutmanas, E.Y.; Gotman, I.; Cassar, G. Improving the surface characteristics of Ti-6Al-4V and Timetal 834 using PIRAC nitriding treatments. Surf. Coat. Technol. 2018, 339, 208–223. [Google Scholar] [CrossRef]
- Li, G.M.; Liang, Y.L.; Yin, C.H.; Sun, H.; Zhu, Z.L. Study of M50NiL steel under carburizing and nitriding duplex treatment. Surf. Coat. Technol. 2019, 375, 132–142. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, Q.; Li, S.X.; Qin, Z. Microstructure, corrosion and tribological properties of Ti(CN) multilayer coatings on 35CrMo steel by CVD. Rare Met. 2020, 39, 1314–1320. [Google Scholar] [CrossRef]
- Dong, C.M.; Cui, Q.F.; Gao, X.M.; Jiang, D.; Fu, Y.L.; Wang, D.S.; Weng, L.J.; Hu, M.; Sun, J.Y. Tribological property of MoS2-Cr3O4 nanocomposite films prepared by PVD and liquid phase synthesis. Surf. Coat. Technol. 2020, 403, 126382. [Google Scholar] [CrossRef]
- Wang, Y.W.; Wang, X.Y.; Wang, X.L.; Yang, Y.; Cui, Y.H.; Ma, Y.D.; Sun, W.W. Microstructure and properties of in-situ composite coatings prepared by plasma spraying MoO3–Al composite powders. Ceram. Int. 2021, 47, 1109–1120. [Google Scholar] [CrossRef]
- Sassi, W.; Boubaker, H.; Bahar, S.; Othman, M.; Ghorbal, A.; Zrelli, R.; Hihn, J.-Y. A challenge to succeed the electroplating of nanocomposite Ni–Cr alloy onto porous substrate under ultrasonic waves and from a continuous flow titanium nanofluids. J. Alloy. Compd. 2020, 828, 154437. [Google Scholar] [CrossRef]
- Ding, Z.L.; Zhou, Q.; Wang, Y.; Ding, Z.Y.; Tang, Y.H.; He, Q.G. Microstructure and properties of monolayer, bilayer and multilayer Ta2O5-based coatings on biomedical Ti-6Al-4V alloy by magnetron sputtering. Ceram. Int. 2021, 47, 1133–1144. [Google Scholar] [CrossRef]
- Katinas, E.; Jankauskas, V.; Kazak, N.; Michailov, V. Improving Abrasive Wear Resistance for Steel Hardox 400 by Electro-Spark Deposition. J. Frict. Wear 2019, 40, 100–106. [Google Scholar] [CrossRef]
- Guan, Q.F.; Zhang, S.H.; Zheng, H.H.; Li, C.; Zhang, C.L.; Lv, P. Cr surface alloying of 45# steel by high-current pulsed electron beam treatment. J. Jilin Univ. 2018, 48, 1161–1168. [Google Scholar]
- Gromov, V.E.; Gorbunov, S.V.; Ivanov, Y.F.; Vorobiev, S.V.; Konovalov, S.V. Formation of surface gradient structural-phase states under electron-beam treatment of stainless steel. J. Surf. Invest. 2011, 5, 974–978. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, J.; Grosdidier, T.; Dong, C. Formation and evolution of craters in carbon steels during low-energy high-current pulsed electron-beam treatment. J. Vac. Sci. Technol. A Vac. Surf. Film. 2009, 27, 1217–1226. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Dubey, A.K. Recent trends in laser cladding and surface alloying. Opt. Laser Technol. 2021, 134, 106619. [Google Scholar] [CrossRef]
- Li, N.; Huang, S.; Zhang, G.D.; Qin, R.Y.; Liu, W.; Xiong, H.P.; Shi, G.Q.; Blackburn, J. Progress in additive manufacturing on new materials: A review. J. Mater. Sci. Technol. 2019, 35, 242–269. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Ding, Q.Q.; Zhang, Y.; Chen, X.; Fu, X.Q.; Chen, D.K.; Chen, S.J.; Gu, L.; Wei, F.; Bei, H.B.; Gao, Y.F.; et al. Tuning element distribution, structure and properties by composition in high-entropy alloys. Nature 2019, 574, 223–227. [Google Scholar] [CrossRef]
- Juan, Y.F.; Li, J.; Jiang, Y.Q.; Jia, W.L.; Lu, Z.J. Modified criterions for phase prediction in the multi-component laser-clad coatings and investigations into microstructural evolution/wear resistance of FeCrCoNiAlMox laser-clad coatings. Appl. Surf. Sci. 2019, 465, 700–714. [Google Scholar] [CrossRef]
- Qiu, X.W. Microstructure and mechanical properties of CoCrFeNiMo high-entropy alloy coatings. J. Mater. Res. Technol. 2020, 9, 5127–5133. [Google Scholar] [CrossRef]
- Liang, H.; Miao, J.W.; Gao, B.Y.; Deng, D.W.; Wang, T.M.; Lu, Y.P.; Cao, Z.Q.; Jiang, H.; Li, T.J.; Kang, H.J. Microstructure and tribological properties of AlCrFe2Ni2W0.2Mo0.75 high-entropy alloy coating prepared by laser cladding in seawater, NaCl solution and deionized water. Surf. Coat. Technol. 2020, 400, 126214. [Google Scholar] [CrossRef]
- Wen, X.; Cui, X.F.; Jin, G.; Zhang, X.R.; Zhang, Y.; Zhang, D.; Fang, Y.C. Design and characterization of FeCrCoAlMn0.5Mo0.1 high-entropy alloy coating by ultrasonic assisted laser cladding. J. Alloy. Compd. 2020, 835, 155449. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, T.F.; Xiao, M.; Shen, Y.F. Effect of process parameters on the microstructure and properties of laser-clad FeNiCoCrTi0.5 high-entropy alloy coating. Int. J. Min. Met. Mater. 2020, 27, 630–639. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, T.F.; Xiao, M.; Shen, Y.F. Effect of Nb content on microstructure and properties of laser cladding FeNiCoCrTi0.5Nbx high-entropy alloy coating. Optik 2019, 198, 163316. [Google Scholar] [CrossRef]
- Chen, W.; Dejun, K. Effects of Al mass fraction on corrosive wear and electrochemical corrosion of laser cladded AlFeCoCr amorphous coating in 3.5 wt% NaCl solution. Mater. Res. Express 2019, 6, 076552. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.J.; Liu, J.; Gao, W.P.; Du, X.T.; Hao, J.B. Microstructural evolution and properties of dual-layer CoCrFeMnTi0.2 high-entropy alloy coating fabricated by laser cladding. Opt. Laser Technol. 2021, 134, 106646. [Google Scholar] [CrossRef]
- Hu, L.F.; Li, J.; Tao, Y.F.; Lv, Y.H. Corrosion behaviors of TiNi/Ti2Ni matrix coatings in the environment rich in Cl ions. Surf. Coatings Technol. 2017, 311, 295–306. [Google Scholar] [CrossRef]
- Yan, L. Production Practice of Converter Continuous Casting for 45~# Steel. Anhui Metal. 2001, 1, 19–23. [Google Scholar]
- Wüstholz, T.; Reinhardt, H.W. Deformation behaviour of self-compacting concrete under tensile loading. Mater. Struct. 2007, 40, 965–977. [Google Scholar]
- Gao, K.; Guo, J.Z.; Qin, X.P. Kinetics modeling for austenite transformation inAISI 1045 steel during rapid heating under high frequency electromagnetic field. J. Cent. South Univ. 2020, 27, 1543–1556. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and Rubbing of Flat Surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Lv, Y.H.; Li, J.; Tao, Y.F.; Hu, L.F. High-temperature wear and oxidation behaviors of TiNi/Ti2Ni matrix composite coatings with TaC addition prepared on Ti6Al4V by laser cladding. Appl. Surf. Sci. 2017, 402, 478–494. [Google Scholar] [CrossRef]
- Ren, P.; Wen, M.; Zhang, K.; Du, S.X.; Zhang, Y.D.; Chen, J.H.; Zheng, W.T. Self-assembly of TaC@Ta core-shell-like nanocomposite film via solid-state dewetting: Toward superior wear and corrosion resistance. Acta Mater. 2018, 160, 72–84. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.Y.; Li, W.G.; Zhang, G.J. Microstructure and properties of in situ synthesized TiB2+WC reinforced composite coatings. Rare Met. 2008, 27, 451–456. [Google Scholar] [CrossRef]
- Yang, S.; Zhong, M.L.; Liu, W.J. TiC particulate composite coating produced in situ by laser cladding. Mater. Sci. Eng. A 2003, 343, 57–62. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, L.; Huang, Y.; Xu, S. A novel method to fabricate Ni/WC composite coatings by laser wire deposition: Processing characteristics, microstructural evolution and mechanical properties under different wire transfer modes. Addit. Manuf. 2021, 38, 101738. [Google Scholar] [CrossRef]
- Li, Z.Y.; Yan, H.; Zhang, P.L.; Guo, J.L.; Yu, Z.S.; Ringsberg, J.W. Improving surface resistance to wear and corrosion of nickel-aluminum bronze by laser-clad TaC/Co-based alloy composite coatings. Surf. Coat. Technol. 2021, 405, 126592. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Li, M.; Chi, J.; Wang, S.F.; Fang, M.; Zhou, C.; Liang, B.; Xue, J.X. In-situ (Ti,Nb)C reinforced Ni-based coatings: New insights into microstructure evolution, enhanced phase configuration behavior and tribological properties. Ceram. Int. 2020, 46, 14161–14172. [Google Scholar] [CrossRef]
- Kuang, S.H.; Zhou, F.; Zheng, S.S.; Liu, Q.B. Annealing-induced microstructure and properties evolution of refractory MoFeCrTiWAlNb3 eutectic high-entropy alloy coating by laser cladding. Intermetallics 2021, 129, 107039. [Google Scholar] [CrossRef]
- Wen, X.; Cui, X.F.; Jin, G.; Liu, Y.F.; Zhang, Y.; Fang, Y.C. In-situ synthesis of nano-lamellar Ni1.5CrCoFe0.5Mo0.1Nbx eutectic high-entropy alloy coatings by laser cladding: Alloy design and microstructure evolution. Surf. Coat. Technol. 2021, 405, 126728. [Google Scholar] [CrossRef]
- Muvvala, G.; Mullick, S.; Nath, A.K. Development of process maps based on molten pool thermal history during laser cladding of Inconel 718/TiC metal matrix composite coatings. Surf. Coat. Technol. 2020, 399, 126100. [Google Scholar] [CrossRef]
- Miao, J.W.; Liang, H.; Zhang, A.J.; He, J.Y.; Meng, J.H.; Lu, Y.P. Tribological behavior of an AlCoCrFeNi2.1 eutectic high entropy alloy sliding against different counterfaces. Tribol. Int. 2021, 153, 106599. [Google Scholar] [CrossRef]
- Liu, H.; Sun, S.F.; Zhang, T.; Zhang, G.Z.; Yang, H.F.; Hao, J.B. Effect of Si addition on microstructure and wear behavior of AlCoCrFeNi high-entropy alloy coatings prepared by laser cladding. Surf. Coat. Technol. 2021, 405, 126522. [Google Scholar] [CrossRef]
- Moravcikova-Gouvea, L.; Moravcik, I.; Omasta, M.; Veselý, J.; Cizek, J.; Minárik, P.; Cupera, J.; Záděra, A.; Jan, V.; Dlouhy, I. High-strength Al0.2Co1.5CrFeNi1.5Ti high-entropy alloy produced by powder metallurgy and casting: A comparison of microstructures, mechanical and tribological properties. Mater. Charact. 2020, 159, 110046. [Google Scholar] [CrossRef]
- Li, Y.Z.; Shi, Y. Microhardness, wear resistance, and corrosion resistance of AlxCrFeCoNiCu high-entropy alloy coatings on aluminum by laser cladding. Opt. Laser Technol. 2021, 134, 106632. [Google Scholar] [CrossRef]
- Xiao, J.K.; Wu, Y.Q.; Chen, J.; Zhang, C. Microstructure and tribological properties of plasma sprayed FeCoNiCrSiAlx high entropy alloy coatings. Wear 2020, 448–449, 203209. [Google Scholar] [CrossRef]
- Xiao, J.K.; Tan, H.; Chen, J.; Martini, A.; Zhang, C. Effect of carbon content on microstructure, hardness and wear resistance of CoCrFeMnNiCx high-entropy alloys. J. Alloy. Compd. 2020, 847, 156533. [Google Scholar] [CrossRef]
- Ayyagari, A.; Barthelemy, C.; Gwalani, B.; Banerjee, R.; Scharf, T.W.; Mukherjee, S. Reciprocating sliding wear behavior of high entropy alloys in dry and marine environments. Mater. Chem. Phys. 2018, 210, 162–169. [Google Scholar] [CrossRef]
- Zhao, D.C.; Yamaguchi, T.; Shu, J.F.; Tokunaga, T.; Danjo, T. Rapid fabrication of the continuous AlFeCrCoNi high entropy alloy coating on aluminum alloy by resistance seam welding. Appl. Surf. Sci. 2020, 517, 145980. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, N.N.; Zhu, S.Y.; Qiao, Z.H.; Zhang, J.B.; Yang, J.; Liu, W.M. A novel Cu-doped high entropy alloy with excellent comprehensive performances for marine application. J. Mater. Sci. Technol. 2021, 69, 48–59. [Google Scholar] [CrossRef]






| Crystal Face | Theoretical d Value in JCPDS Card: NO.03-065-4899/nm | Experimental d Value/nm | ||
|---|---|---|---|---|
| Coating I | Coating II | Coating III | ||
| (110) | 2.02728 | 2.01894 | 2.03948 | 2.04060 |
| (200) | 1.43350 | 1.39924 | 1.44172 | 1.44290 |
| (211) | 1.17045 | 1.16809 | 1.17514 | 1.17840 |
| (220) | 1.01364 | 1.0114 | 1.01753 | 1.01888 |
| Coating | Zones | Elements (at.%) | ||||||
|---|---|---|---|---|---|---|---|---|
| C | Al | Cr | Co | Ni | Fe | Ta | ||
| Coating I | 1 | 1.00 | 6.00 | 6.93 | 9.57 | 6.85 | 69.63 | 0 |
| 2 | 1.58 | 7.90 | 11.30 | 11.31 | 6.74 | 61.16 | 0 | |
| 3 | 39.02 | 3.91 | 5.43 | 6.00 | 3.88 | 41.75 | 0 | |
| Coating II | 4 | 2.32 | 5.68 | 9.38 | 12.64 | 7.28 | 60.26 | 2.44 |
| 5 | 50.67 | 2.54 | 3.77 | 5.62 | 2.50 | 17.82 | 17.08 | |
| 6 | 51.94 | 6.16 | 1.43 | 2.20 | 0.75 | 5.55 | 31.97 | |
| Coating III | 7 | 1.56 | 9.70 | 9.25 | 11.42 | 8.62 | 56.93 | 2.51 |
| 8 | 38.66 | 3.94 | 5.63 | 7.41 | 5.08 | 31.40 | 7.89 | |
| 9 | 67.5 | 1.41 | 1.36 | 0.55 | 0 | 4.98 | 24.19 | |
| Friction Circumstance | Sample | Wear Volume/mm3 | Wear Rate/mm3·N−1·m−1 |
|---|---|---|---|
| Air | Substrate | 0.0247 | 7.62 × 10−6 |
| Coating I | 0.0242 | 7.48 × 10−6 | |
| Coating II | 0.0144 | 4.44 × 10−6 | |
| Coating III | 0.0121 | 3.73 × 10−6 | |
| NaCl solution | Substrate | 0.0718 | 2.22 × 10−5 |
| Coating I | 0.0541 | 1.67 × 10−5 | |
| Coating II | 0.0119 | 3.67 × 10−6 | |
| Coating III | 0.0107 | 3.30 × 10−6 |
| Coatings | Environment | Wear Rate/mm3·N−1·m−1 | Refs. |
|---|---|---|---|
| AlCoCrFeNi2.1 | Air | 3.27 × 10−4 | [45] |
| AlCoCrFeNiSi0.5 | Air | 1.48 × 10−4 | [46] |
| Al0.2Co1.5CrFeNi1.5Ti | Air | 2.50 × 10−5 | [47] |
| AlCrFeCoNiCu | Air | 9.74 × 10−6 | [48] |
| FeCoNiCrSiAl | Air | 6.70 × 10−6 | [49] |
| CoCrFeMnNiC0.6 | Air | 4.70 × 10−6 | [50] |
| AlCrCoNiFeCTa | Air | 3.73×10−6 | Our work |
| Friction Circumstance | Samples | Zones | Elements (at.%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Fe | Al | Cr | Co | Ni | Ta | |||
| Air | Substrate | 1 | 13.90 | 17.64 | 68.46 | - | - | - | - | - |
| 2 | 9.42 | 62.51 | 28.07 | - | - | - | - | - | ||
| Coating III | 3 | 17.17 | 7.40 | 57.23 | 3.32 | 4.43 | 2.82 | 3.11 | 4.52 | |
| 4 | 12.45 | 53.40 | 27.04 | 1.75 | 1.76 | 1.52 | 2.07 | 0 | ||
| NaCl solution | Substrate | 5 | 25.38 | 13.73 | 60.09 | - | - | - | - | - |
| Coating III | 6 | 37.66 | 11.06 | 30.46 | 2.99 | 4.33 | 3.84 | 4.40 | 5.26 | |
| Coatings | Environment | Wear Rate/mm3·N−1·m−1 | Refs. |
|---|---|---|---|
| Al0.1CoCrFeNi | Seawater | 4.40 × 10−4 | [51] |
| AlFeCrCoNi | NaCl | 4.40 × 10−5 | [52] |
| AlCrFe2Ni2W0.2Mo0.75 | NaCl | 1.74 × 10−5 | [24] |
| AlCoCrFeNiCu0.5 | Seawater | 8.8 × 10−6 | [53] |
| AlCrCoNiFeCTa | NaCl | 3.73 × 10−6 | Our work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Li, J.; Lei, R.; Yuan, B.; Xia, M.; Li, X.; Zhang, Y. Investigation into Microstructure, Wear Resistance in Air and NaCl Solution of AlCrCoNiFeCTax High-Entropy Alloy Coatings Fabricated by Laser Cladding. Coatings 2021, 11, 358. https://doi.org/10.3390/coatings11030358
Zhao P, Li J, Lei R, Yuan B, Xia M, Li X, Zhang Y. Investigation into Microstructure, Wear Resistance in Air and NaCl Solution of AlCrCoNiFeCTax High-Entropy Alloy Coatings Fabricated by Laser Cladding. Coatings. 2021; 11(3):358. https://doi.org/10.3390/coatings11030358
Chicago/Turabian StyleZhao, Peng, Jun Li, Ruyan Lei, Baige Yuan, Manman Xia, Xiao Li, and Ying Zhang. 2021. "Investigation into Microstructure, Wear Resistance in Air and NaCl Solution of AlCrCoNiFeCTax High-Entropy Alloy Coatings Fabricated by Laser Cladding" Coatings 11, no. 3: 358. https://doi.org/10.3390/coatings11030358
APA StyleZhao, P., Li, J., Lei, R., Yuan, B., Xia, M., Li, X., & Zhang, Y. (2021). Investigation into Microstructure, Wear Resistance in Air and NaCl Solution of AlCrCoNiFeCTax High-Entropy Alloy Coatings Fabricated by Laser Cladding. Coatings, 11(3), 358. https://doi.org/10.3390/coatings11030358






