Corrosion Behavior of Alloyed Cast Iron in Ethylene Glycol-Based Engine Coolants at Elevated Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Corrosion Testing
3. Results
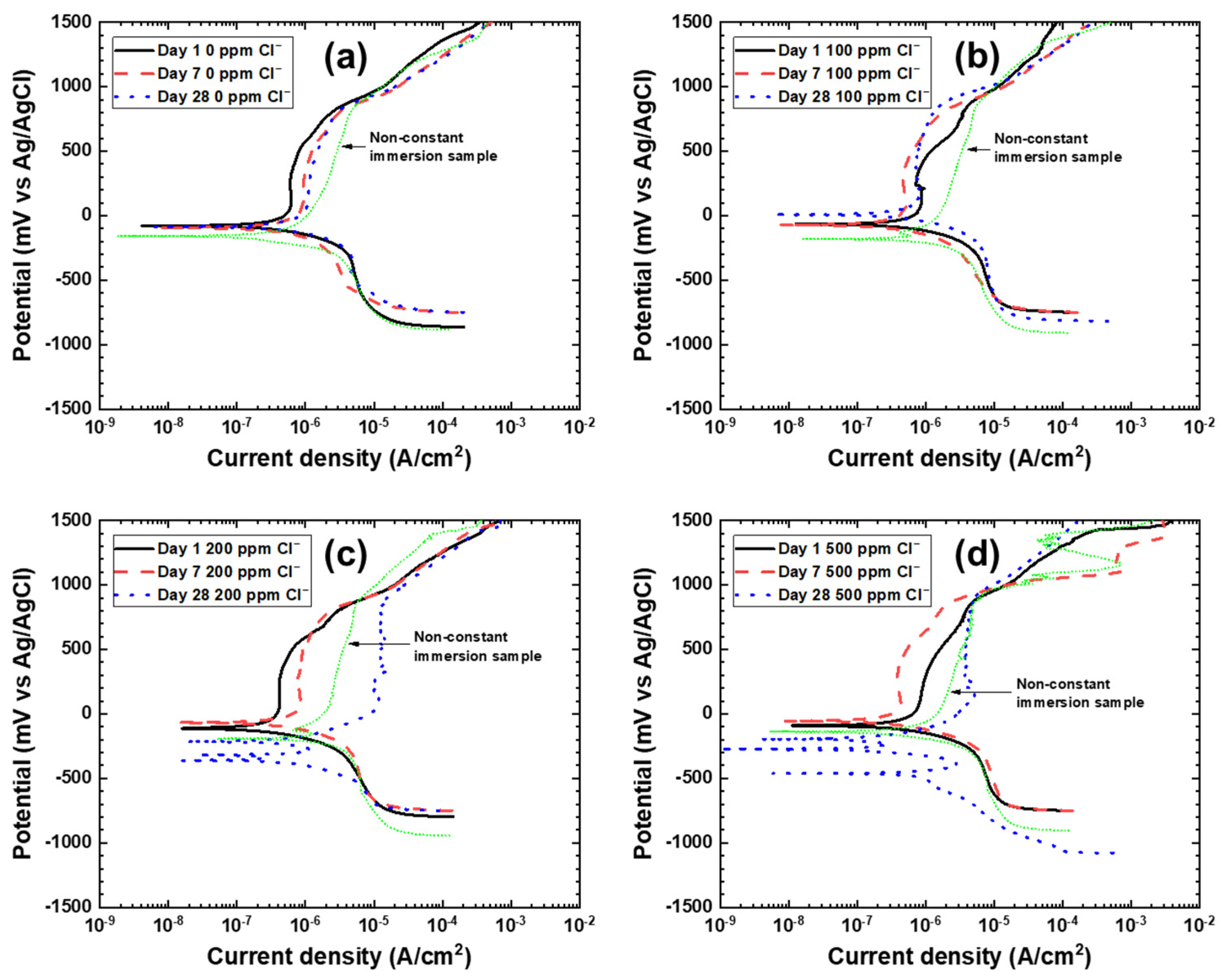
3.1. Potentiodynamic Polarization
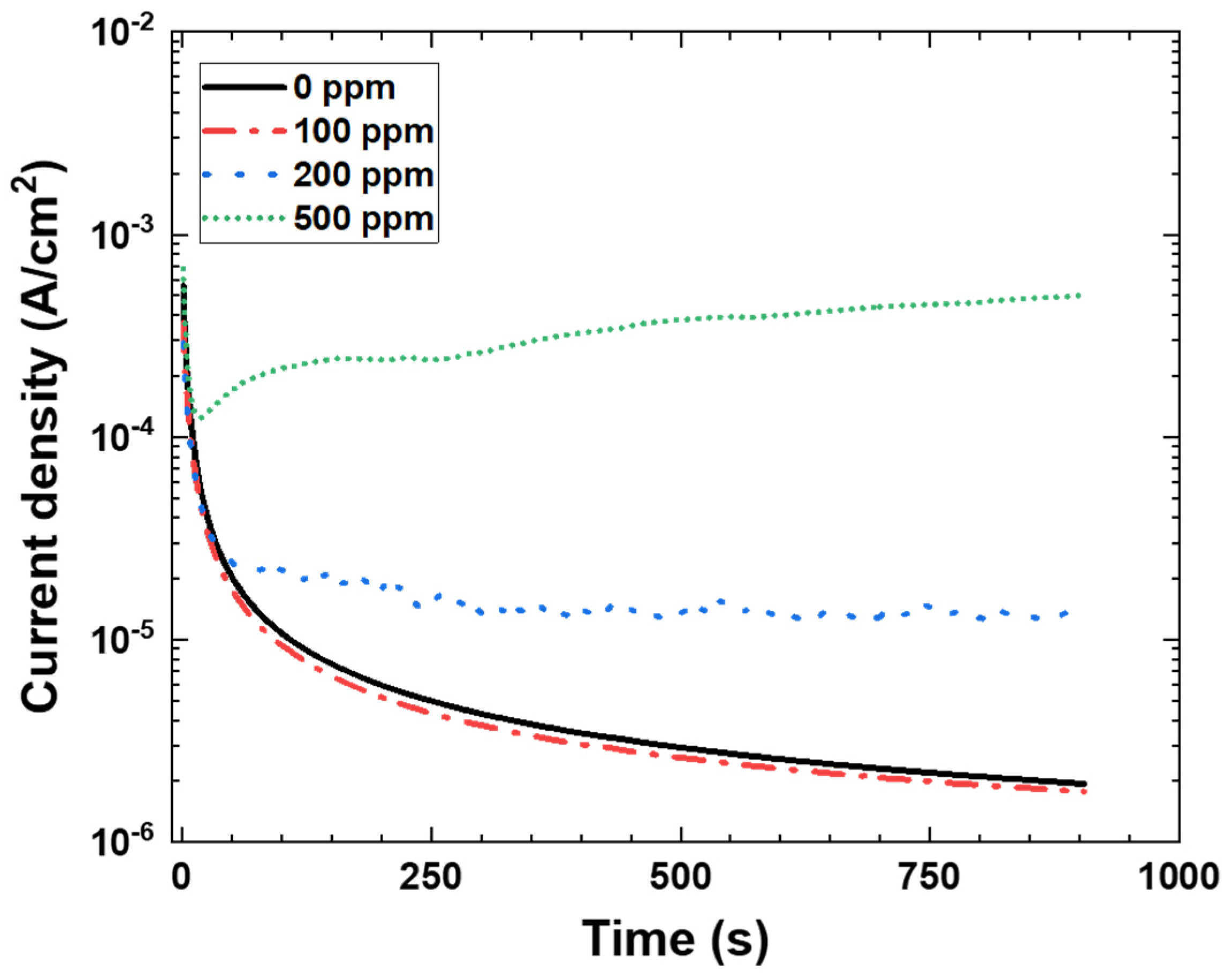
3.2. Potentiostatic Polarization
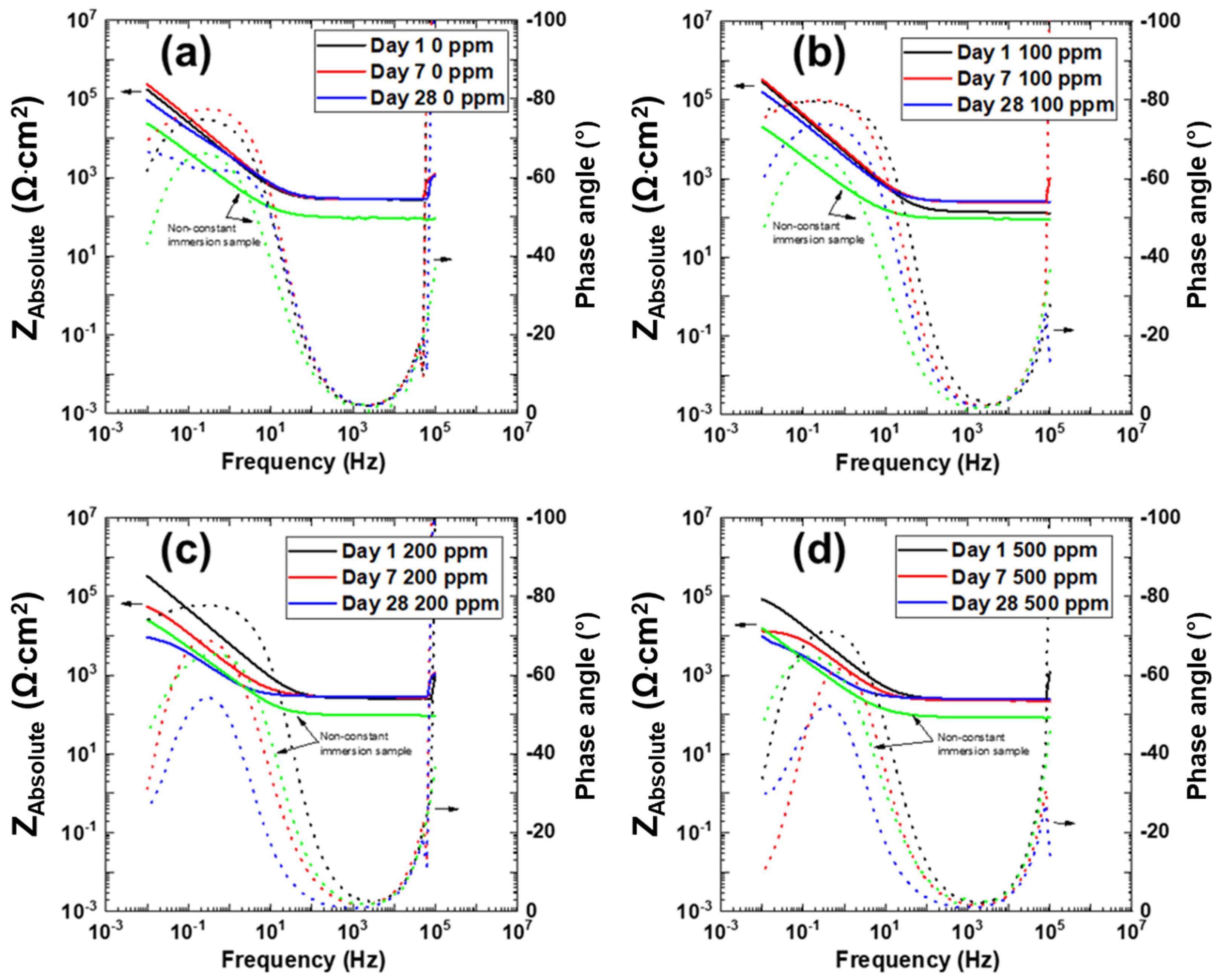
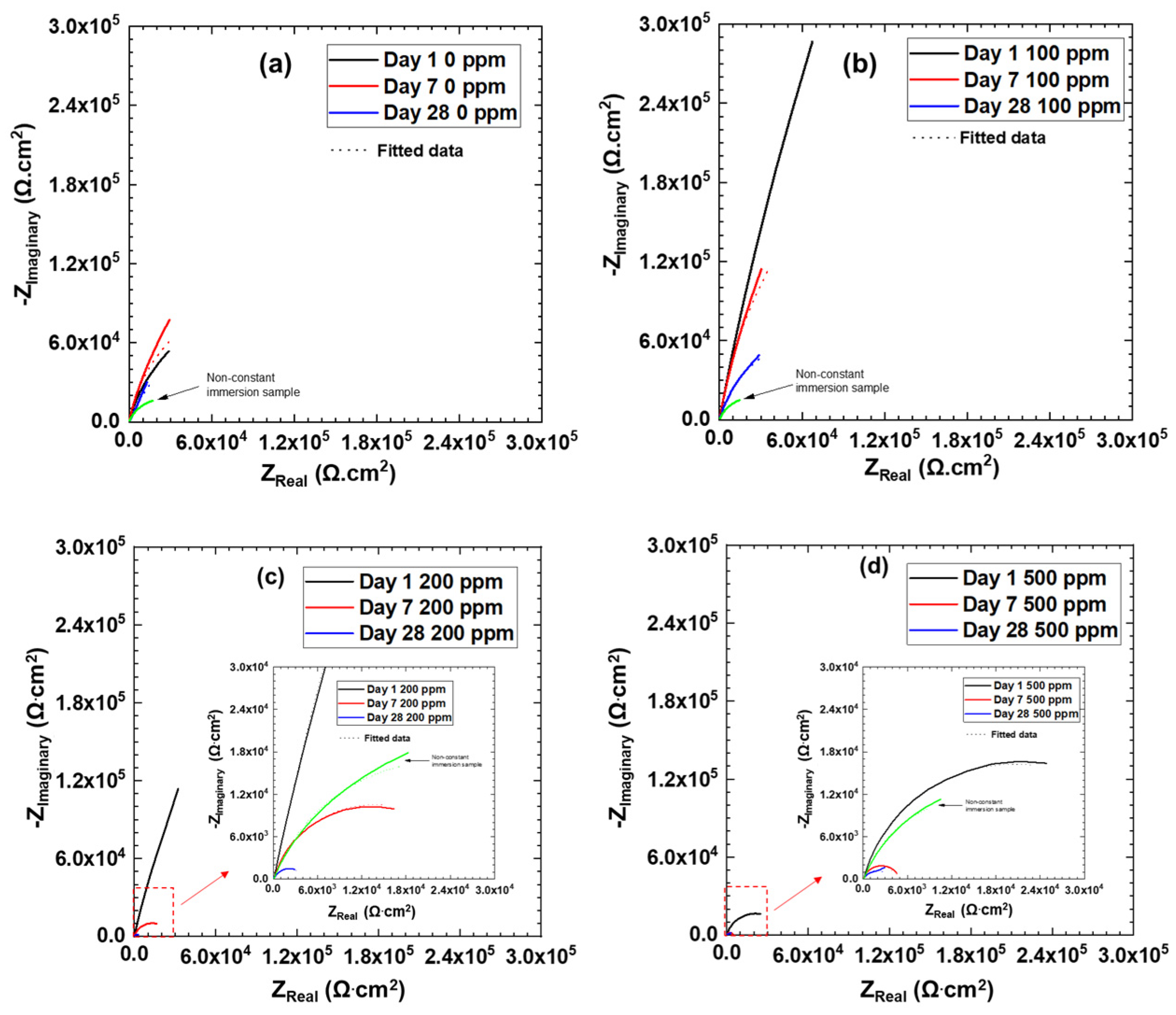
3.3. Electrochemical Impedance Spectroscopy
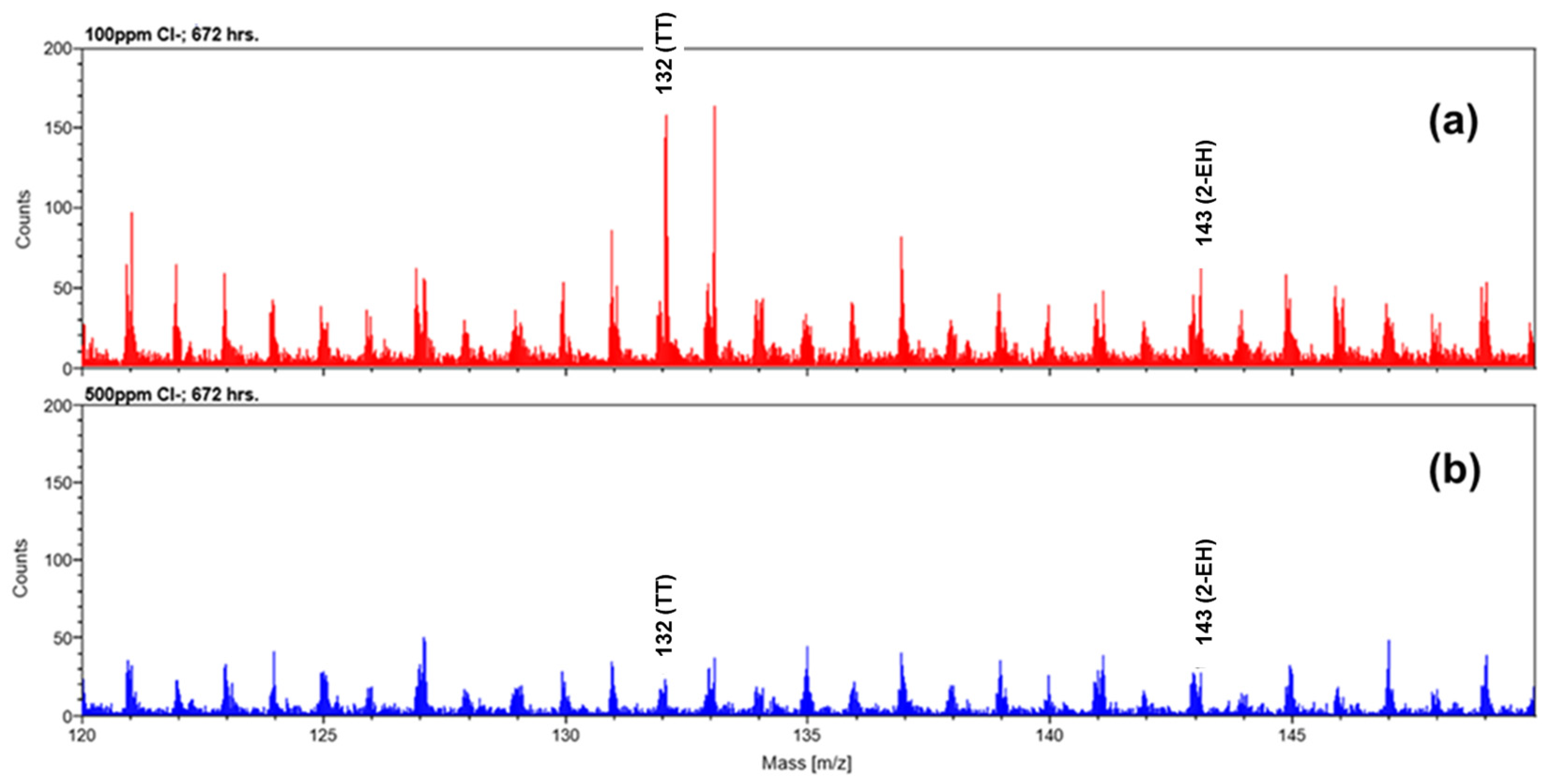
3.4. Surface Analysis of Corrosion Coupons
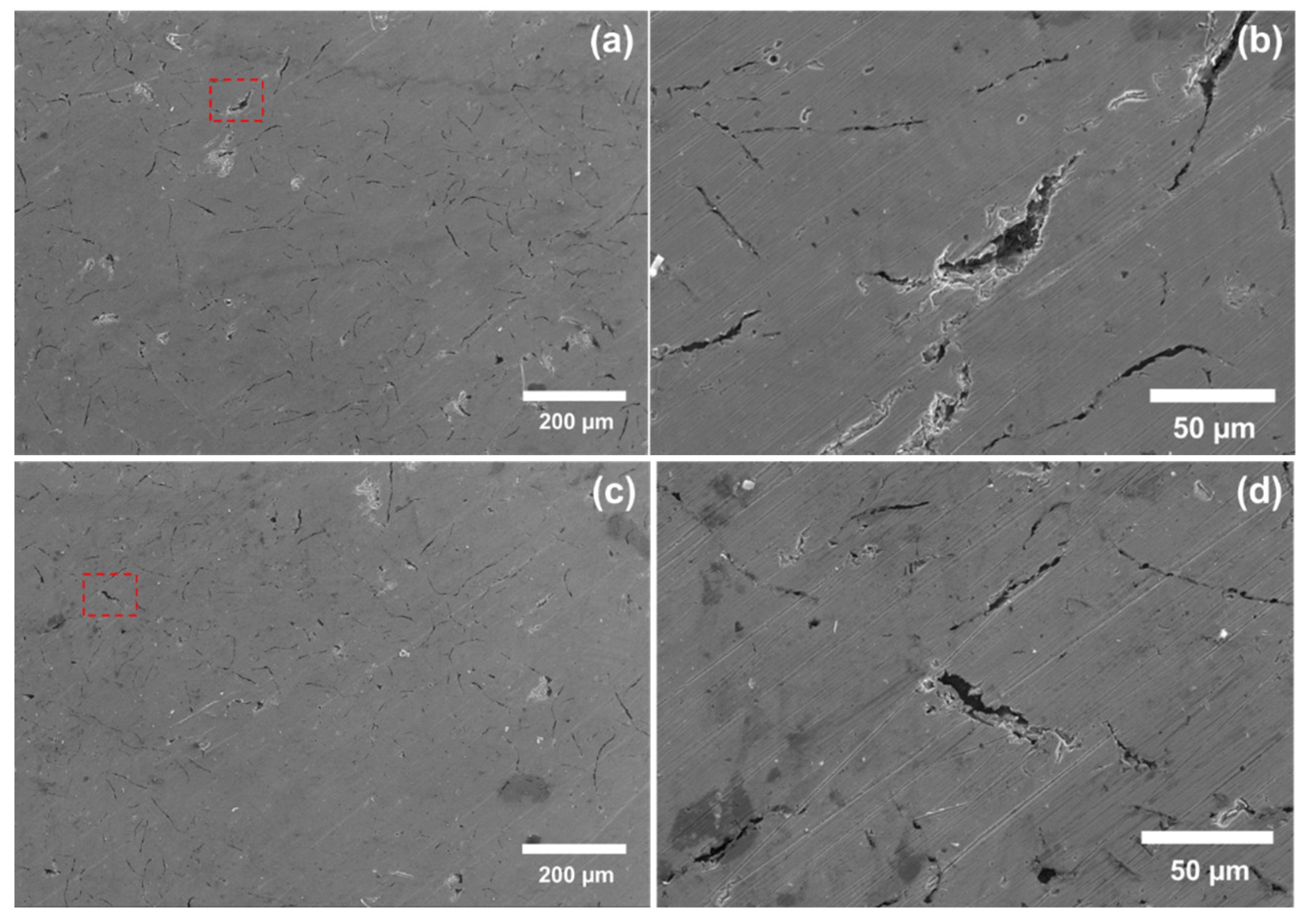
3.4.1. SEM Analysis of the Constant Immersion Coupons
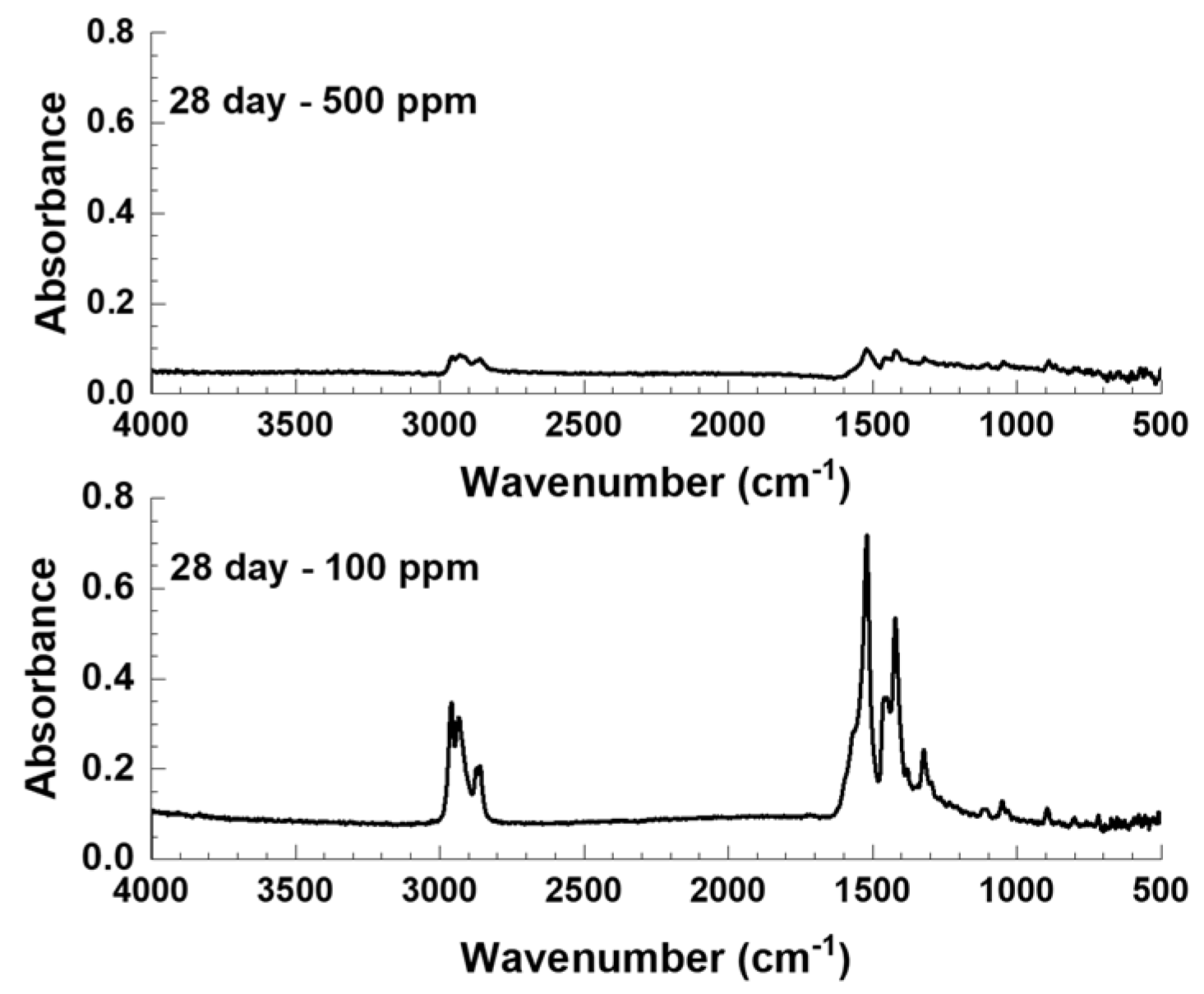
3.4.2. FTIR and Zygo Analysis
4. Discussion
4.1. Evolution of Corrosion Behavior
4.2. Inhibition Mechanism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pierce, D.; Haynes, A.; Hughes, J.; Graves, R.; Maziasz, P.; Muralidharan, G.; Shyam, A.; Wang, B.; England, R.; Daniel, C. High temperature materials for heavy duty diesel engines: Historical and future trends. Prog. Mater. Sci. 2019, 103, 109–179. [Google Scholar] [CrossRef]
- Reynaud, A. 3.02-Corrosion of cast irons. Shreir’s Corros. 2010, 3, 1737–1788. [Google Scholar]
- Hudgens, R.D. Comparison of conventional and organic acid technology (OAT) coolants in heavy duty diesel engine service. SAE Trans. 1999, 108, 82–91. [Google Scholar]
- Weir, T.W.; Ven, P.V. Review of organic acids as inhibitors in engine coolants. SAE Tech. Paper 1996, 37–47. [Google Scholar]
- Pellet, R.J.; Bartley, L.S.; Hunsicker, D.P. The role of carboxylate-based coolants in cast iron corrosion protection. SAE Trans. 2001, 110, 1342–1348. [Google Scholar]
- Darden, J.W.; Triebel, C.A.; Maes, J.P.; VanNeste, W. Monoacid/diacid combination as corrosion inhibitors in antifreeze formulations. SAE Trans. 1990, 99, 1664–1680. [Google Scholar]
- Maes, J.P.; VanNeste, W.A. Corrosion inhibited antifreeze formulations having monocarboxylic, triazole, and imidazole compounds. U.S. Patent 5,366,651, 22 November 1994. [Google Scholar]
- Yang, B.; Woyciesjes, P.; Gershun, A. Comparison of extended life coolant corrosion protection performance. SAE Tech. Paper 2017, 28. [Google Scholar] [CrossRef]
- Rajeshwari, V.; Kesavan, D.; Gopiraman, M.; Viswanathamurthi, P. Physicochemical studies of glucose, gellan gum, and hydroxypropyl cellulose - inhibition of cast iron corrosion. Carbohydr. Polym. 2013, 95, 288–294. [Google Scholar] [CrossRef]
- Vieira, D.E.L.; Sokol, D.; Smalenskaite, A.; Kareiva, A.; Ferreira, M.G.S.; Vieira, J.M.; Salak, A.N. Cast iron corrosion protection with chemically modified Mg-Al layered double hydroxides synthesized using a novel approach. Surf. Coat. Technol. 2019, 375, 158–163. [Google Scholar] [CrossRef]
- Finsgar, M.; Petovar, B.; Xhanari, K.; Maver, U. The corrosion inhibition of certain azoles on steel in chloride media: Electrochemistry and surface analysis. Corros. Sci. 2016, 111, 370–381. [Google Scholar] [CrossRef]
- Sahin, M.; Bilgic, S.; Yilmaz, H. The inhibition effects of some cyclic nitrogen compounds on the corrosion of the steel in NaCl mediums. Appl. Surf. Sci. 2002, 195, 1–7. [Google Scholar] [CrossRef]
- Hudgens, R.D.; Hercamp, R.D. A perspective on extended service intervals and long-life coolants for heavy duty engines. SAE Trans. 1996, 105, 1877–1887. [Google Scholar]
- Santambrogio, M.; Perrucci, G.; Trueba, M.; Trasatti, S.P.; Casaletto, M.P. Effect of major degradation products of ethylene glycol aqueous solutions on steel corrosion. Electrochim. Acta 2016, 203, 439–450. [Google Scholar] [CrossRef]
- ASTM A247-19. Standard Test Method for Evaluating the Microstructure of Graphite in Iron Castings; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]
- ASTM D1384-05. Standard Test Method for Corrosion Test for Engine Coolants in Glassware; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]
- Chilukuri, A. Corrosion Inhibition by Inorganic Cationic Inhibitors on the High Strength Aluminum Alloy. Ph.D Thesis, The Ohio State University, Columbus, OH, USA, 2012. Available online: https://etd.ohiolink.edu/apexprod/rws_etd/send_file/send?accession=osu1343784869&disposition=inline (accessed on 21 March 2021).
- Rammelt, U.; Koehler, S.; Reinhard, G. Electrochemical characterisation of the ability of dicarboxylic acid salts to the corrosion inhibition of mild steel in aqueous solutions. Corros. Sci. 2011, 53, 3515–3520. [Google Scholar] [CrossRef]
- Somers, A.; Hinton, B.R.W.; Bruin-Dickason, C.D.; Deacon, G.B. New, environmentally friendly, rare earth carboxylate corrosion inhibitors for mild steel. Corros. Sci. 2018, 139, 430–437. [Google Scholar] [CrossRef]
- Valek, L.; Martinez, S.; Rnardić, I.; Mikulić, D. The inhibition activity of ascorbic acid towards corrosion of steel in alkaline media containing chloride ions. Corros. Sci. 2008, 50, 2705–2709. [Google Scholar] [CrossRef]
- Lebrini, M.; Fontaine, G.; Gengembre, L.; Traisnel, M. Corrosion protection of galvanized steel and electroplating steel by decanoïc acid in aqueous solution: Electrochemical impedance spectroscopy, XPS and ATR-FTIR. Corros. Sci. 2009, 51, 1201–1206. [Google Scholar] [CrossRef]
- Deng, Q.; Ding, N.; Wei, X.; Cai, L.; He, X.; Long, Y.; Chen, G.; Chen, K. Identification of diverse 1,2,3-triazole connected benzyl glycoside-serine/threonine conjugates as potent corrosion inhibitors for mild steel in HCl. Corros. Sci. 2012, 64, 64–73. [Google Scholar] [CrossRef]
- Rammelt, U.; Koehler, S.; Reinhard, G. Synergistic effect of benzoate and benzotriazole on passivation of mild steel. Corros. Sci. 2008, 50, 1659–1663. [Google Scholar] [CrossRef]
- Rammelt, U.; Koehler, S.; Reinhard, G. EIS characterization of the inhibition of mild steel corrosion with carboxylates in neutral aqueous solution. Electrochim. Acta 2008, 53, 6968–6972. [Google Scholar] [CrossRef]
- Mercer, W.C. An investigation of carboxylic acids as corrosion inhibitors in engine coolant. ASTM STP 1993, 3, 44–62. [Google Scholar]
- Butler, G.; Mercer, A.D. Inhibitor formulations for engine coolants. Br. Corros. J. 1977, 12, 171–174. [Google Scholar] [CrossRef]
- Petrunin, M.; Maksaeva, L.; Gladkikh, N.; Makarychev, Y.; Maleeva, M.; Yurasova, T.; Nazarov, A. Thin benzotriazole films for inhibition of carbon steel corrosion in neutral electrolytes. Coatings 2020, 10, 362. [Google Scholar] [CrossRef]
- Finšgar, M. Electrochemical, 3D topography, XPS, and ToF-SIMS analyses of 4-methyl-2-phenylimidazole as a corrosion inhibitor for brass. Corrs. Sci. 2020, 169, 108362. [Google Scholar] [CrossRef]
- Coelho, L.B.; Cossement, D.; Olivier, M.G. Benzotriazole and cerium chloride as corrosion inhibitors for AA2024-T3: An EIS investigation supported by SVET and ToF-SIMS analysis. Corrs. Sci. 2018, 130, 177–189. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Carriere, C.; Seyeux, A.; Marcus, P.; Singh, A. Electrochemical, ToF-SIMS and computational studies of 4-amino-5-methyl-4H-1,2,4-triazole-3-thiol as a novel corrosion inhibitor for copper in 3.5% NaCl. J. Mol. Liq. 2019, 289, 111113. [Google Scholar] [CrossRef]
- Esmaily, M.; Malmberg, P.; Shahabi-Navid, M.; Svensson, J.E.; Johansson, L.G. A ToF-SIMS investigation of the corrosion behavior of Mg alloy AM50 in atmospheric environments. Appl. Surf. Sci. 2016, 360, 98–106. [Google Scholar] [CrossRef]
- Argade, G.R.; Chilukuri, A.; Perry, J.; Trobaugh, C.; Schafer, R.; Raisor, E.; Steenhoek, J.; Lugo, G.P. An investigation of cast iron corrosion behavior in chloride containing engine coolant environment. Mater. Sci. Technol. 2019, 737–741. [Google Scholar] [CrossRef]
- Chilukuri, A.; Argade, G.R.; Perry, J.; Trobaugh, C.; Schafer, R.; Raisor, E.; Steenhoek, J.; Lugo, G.P. A corrosion study of light metal cylinder head material in chloride containing engine coolant environment. Mater. Sci. Technol. 2019, 1255–1259. [Google Scholar] [CrossRef]
- Yan, Y.; Shi, H.; Wang, J.; Liu, F.; Han, E. Corrosion inhibition of galvanized steel in NaCl solution by tolytriazole. Acta Metall. Sin. 2019, 32, 471–480. [Google Scholar] [CrossRef]






| Nominal Chemistry | C | Si | Mn | S | P | Cr + Mo + Cu + Ni | Nb + Ti + V | Carbon Equivalent (C.E.) |
|---|---|---|---|---|---|---|---|---|
| Weight % | 3.0–3.5 | 1.5–2.5 | Max. 1.0 | Max. 0.20 | Max. 0.15 | Max. 2.5 | Max. 0.025 | Max. 4.25 |
| UTS (MPa) | Min. 295 | |||||||
| Chemical Name | Abbreviation | Chemical Formula | Structural Notification |
|---|---|---|---|
| Sebacate | SA | C10H16O4−2 | 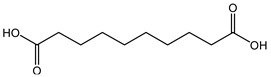 |
| 2-Ethylhexanoate | 2-EH | C8H15O2−1 | 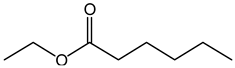 |
| Tolyltriazole | TTA | C7H7N3 | 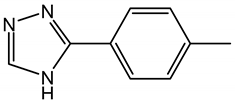 |
| Title | Exposure Time | Ecorr mV vs. Ag/AgCl | icorr µA/cm2 | Ebreakdown mV vs. Ag/AgCl |
|---|---|---|---|---|
| 0 ppm Cl− | Day 1 | −75.6 | 3.2 | 831.9 |
| Day 7 | −101.9 | 1.7 | 782.7 | |
| Day 28 | −84.9 | 3.6 | 730.0 | |
| 100 ppm Cl− | Day 1 | −72.2 | 4.4 | 854.8 |
| Day 7 | −72.2 | 1.6 | 798.8 | |
| Day 28 | 3.40 | 5.5 | 844.7 | |
| 200 ppm Cl− | Day 1 | −114.6 | 2.2 | 830.2 |
| Day 7 | −65.4 | 3.6 | 878.3 | |
| Day 28 | −200.1 | 2.9 | 875.4 | |
| 500 ppm Cl− | Day 1 | −91.7 | 4.1 | 818.3 |
| Day 7 | −52.6 | 4.7 | 703.7 | |
| Day 28 | −180.4 | 3.0 | 870.6 |
| Exposure Time | Cl– Concentration (ppm) | Charge Transfer Resistance Rct (Ω·cm2) | Constant Phase Element CPEdl-T (F cm−2 s−n) | Constant Phase Element CPEdl-P (n) | Double Layer CaPacitance Cdl (F·cm−2) |
|---|---|---|---|---|---|
| Day 1 | 0 | 208,930 | 1.60 × 10−4 | 0.86 | 2.83 × 10−4 |
| 100 | 4,653,000 | 4.0 × 10−5 | 0.89 | 7.6 × 10−5 | |
| 200 | 1,115,300 | 9.27 × 10−5 | 0.88 | 1.74 × 10−4 | |
| 500 | 41,063 | 2.2 × 10−4 | 0.85 | 3.24 × 10−4 | |
| Day 7 | 0 | 233,510 | 1.60 × 10−4 | 0.9 | 2.39 × 10−4 |
| 100 | 711,730 | 1.0 × 10−4 | 0.91 | 1.52 × 10−4 | |
| 200 | 27,254 | 3.4 × 10−4 | 0.84 | 5.2 × 10−4 | |
| 500 | 4495 | 4.0 × 10−4 | 0.87 | 4.37 × 10−4 | |
| Day 28 | 0 | 323,350 | 2.2 × 10−4 | 0.75 | 9.12 × 10−4 |
| 100 | 163,250 | 1.60 × 10−4 | 0.86 | 2.72 × 10−4 | |
| 200 | 4078 | 1.0 × 10−3 | 0.78 | 1.49 × 10−3 | |
| 500 | 3466 | 1.2 × 10−3 | 0.78 | 1.79 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argade, G.; Chilukuri, A.; Perry, J.; Viers, M.; Steenhoek, J.; Debusk, J.; Wang, C.; Trobaugh, C. Corrosion Behavior of Alloyed Cast Iron in Ethylene Glycol-Based Engine Coolants at Elevated Temperature. Coatings 2021, 11, 357. https://doi.org/10.3390/coatings11030357
Argade G, Chilukuri A, Perry J, Viers M, Steenhoek J, Debusk J, Wang C, Trobaugh C. Corrosion Behavior of Alloyed Cast Iron in Ethylene Glycol-Based Engine Coolants at Elevated Temperature. Coatings. 2021; 11(3):357. https://doi.org/10.3390/coatings11030357
Chicago/Turabian StyleArgade, Gaurav, Anusha Chilukuri, Justin Perry, Monica Viers, Jacob Steenhoek, Jacob Debusk, Chinpei Wang, and Corey Trobaugh. 2021. "Corrosion Behavior of Alloyed Cast Iron in Ethylene Glycol-Based Engine Coolants at Elevated Temperature" Coatings 11, no. 3: 357. https://doi.org/10.3390/coatings11030357
APA StyleArgade, G., Chilukuri, A., Perry, J., Viers, M., Steenhoek, J., Debusk, J., Wang, C., & Trobaugh, C. (2021). Corrosion Behavior of Alloyed Cast Iron in Ethylene Glycol-Based Engine Coolants at Elevated Temperature. Coatings, 11(3), 357. https://doi.org/10.3390/coatings11030357






