High Efficiency Fluorinated Oligo(ethylenesuccinamide) Coating for Stone
Abstract
:1. Introduction
2. Materials and Methods
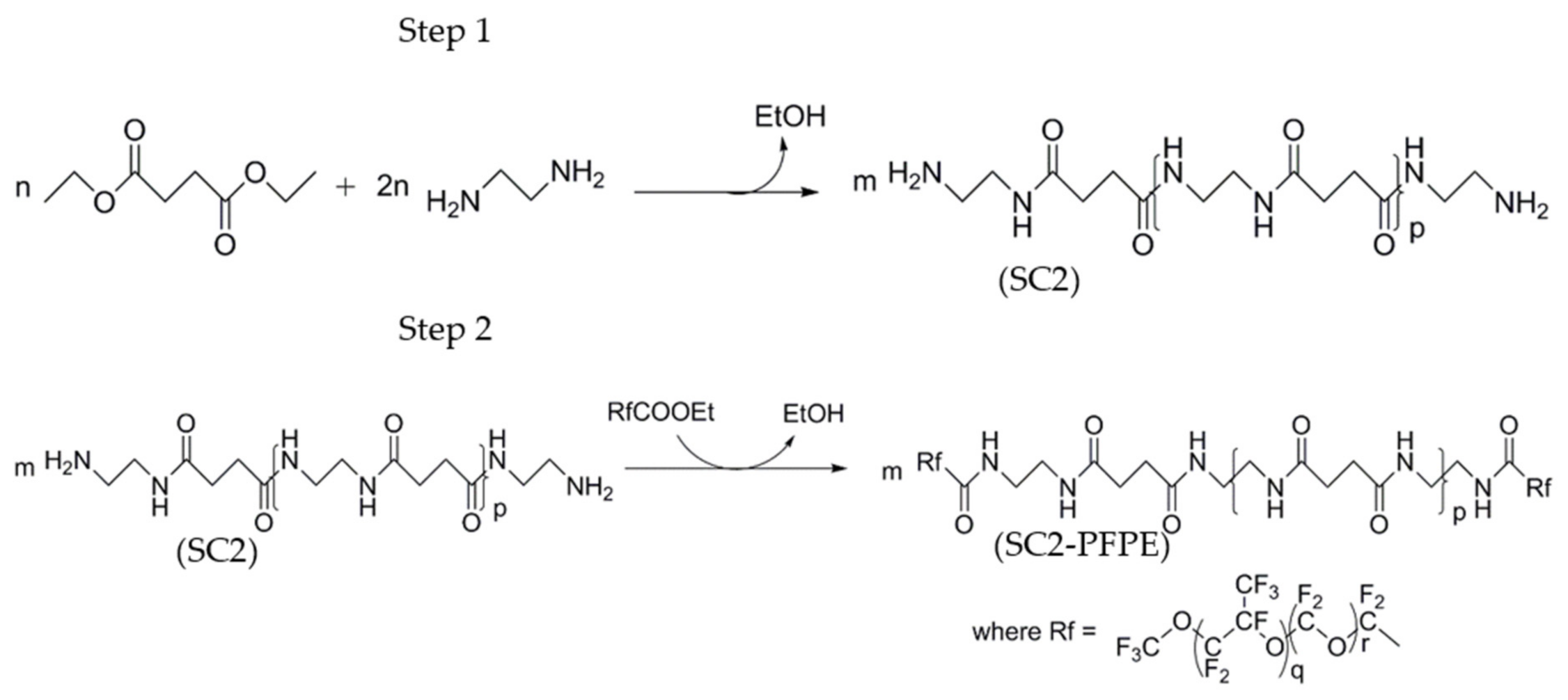
2.1. Materials and Coating Procedure
2.2. Coating Performance Evaluation
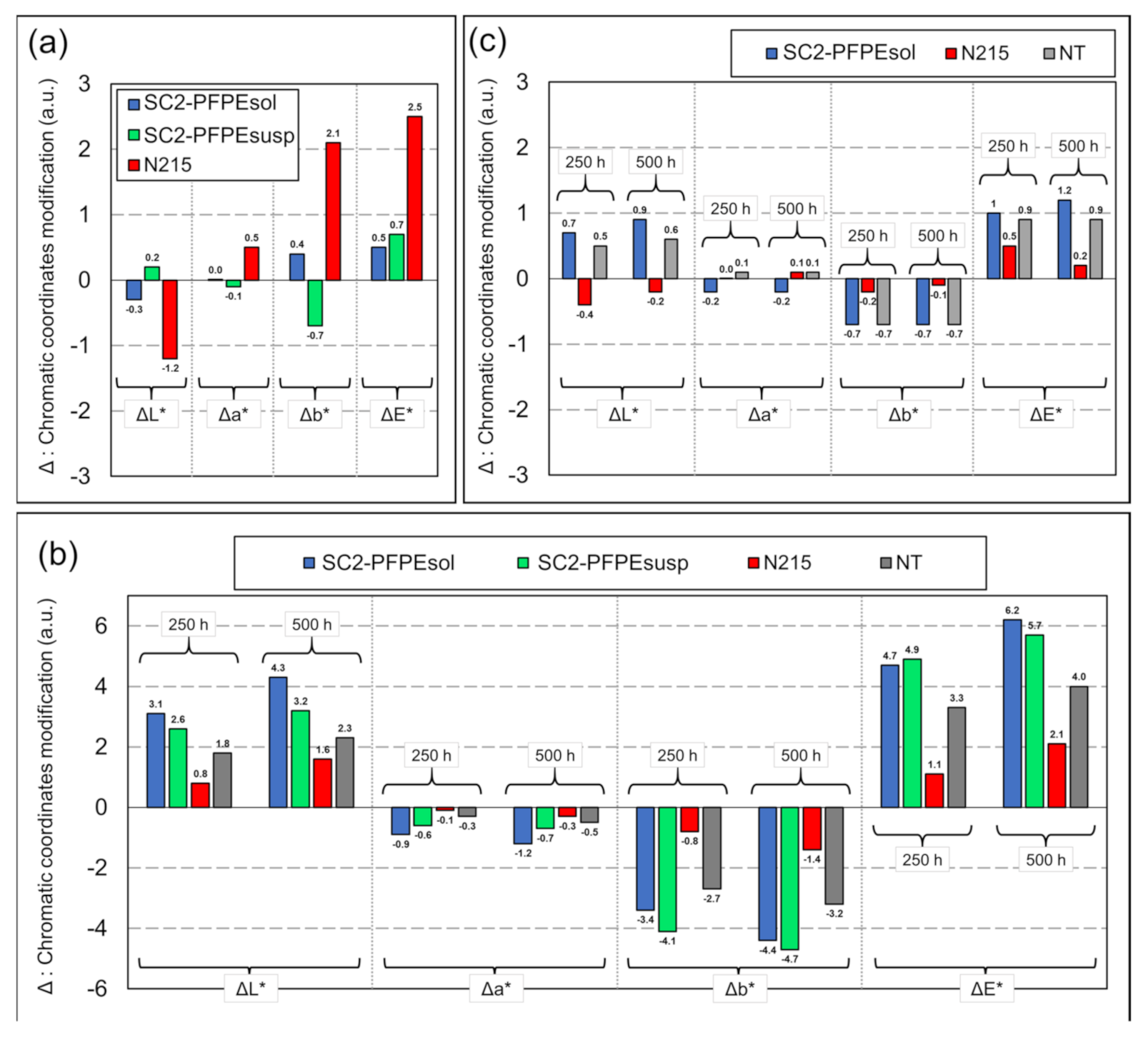
2.2.1. Photo-Stability
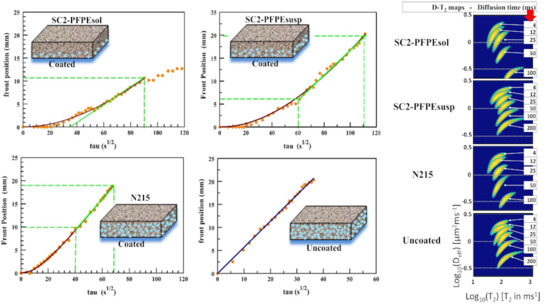
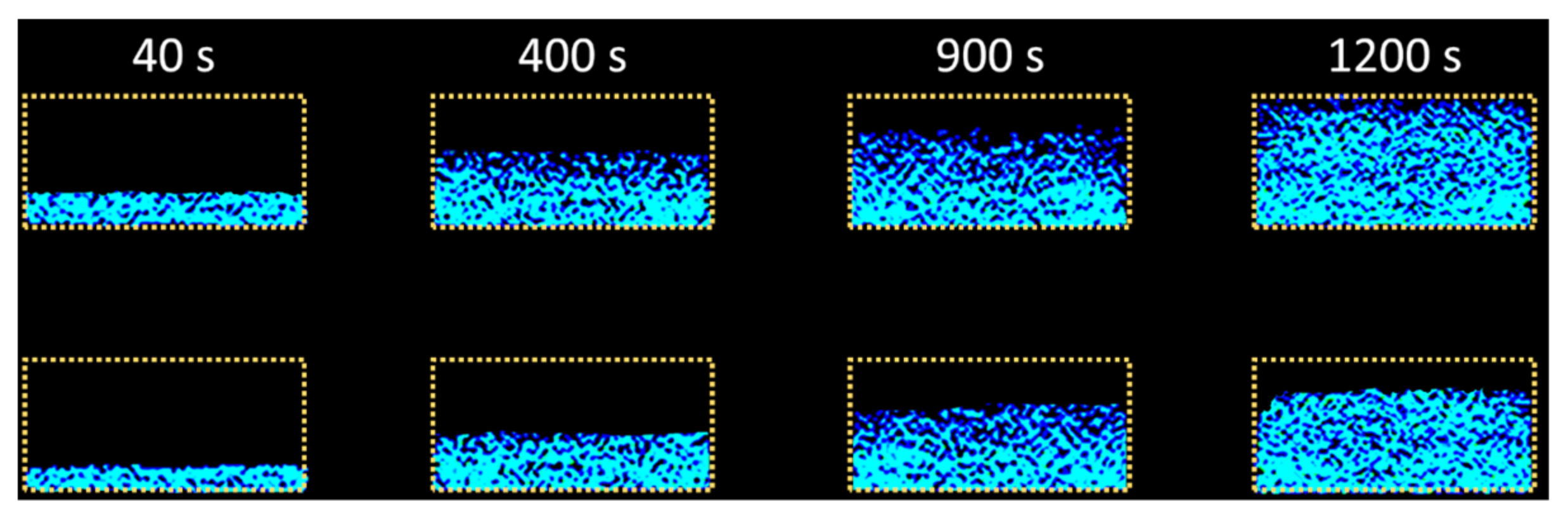
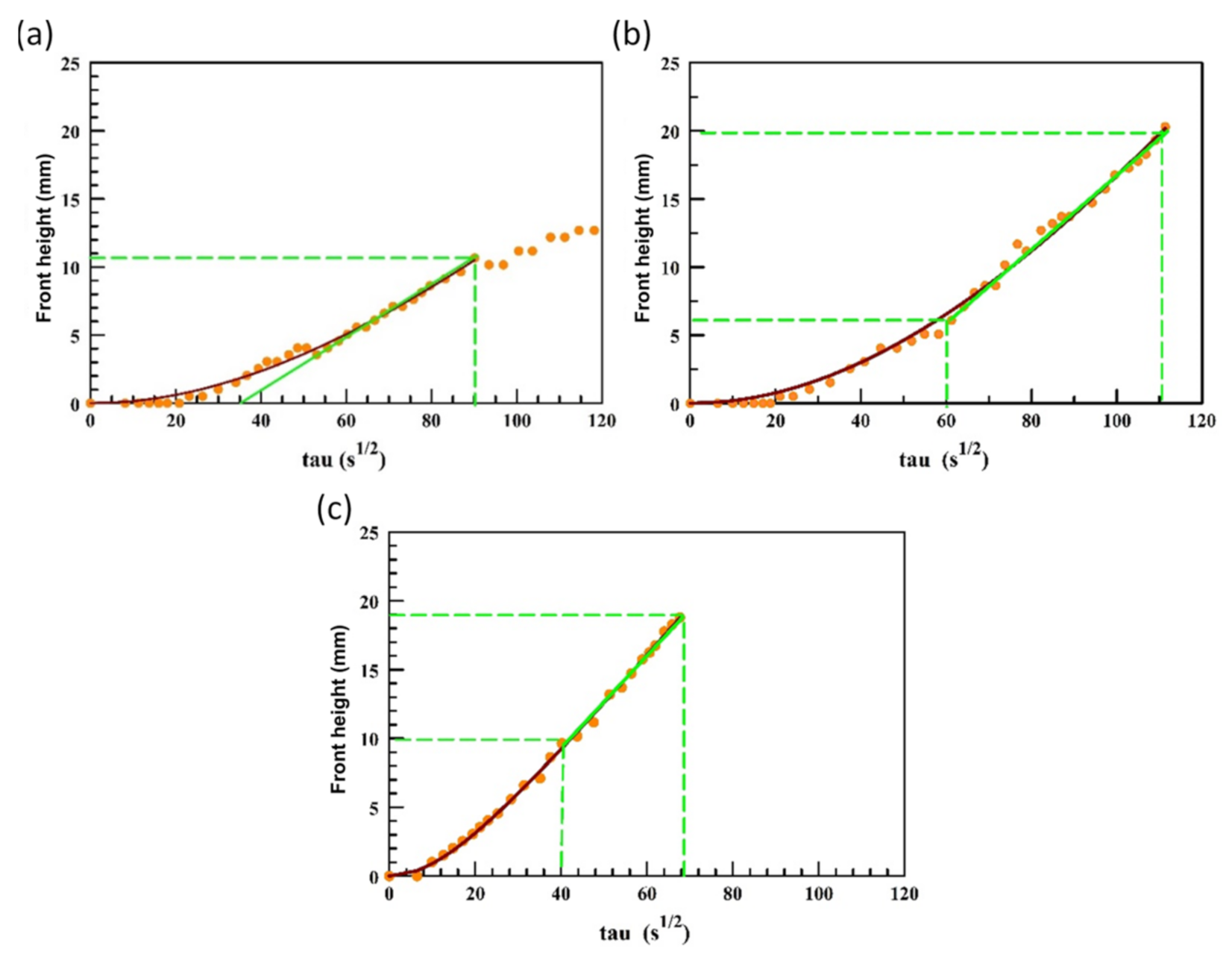
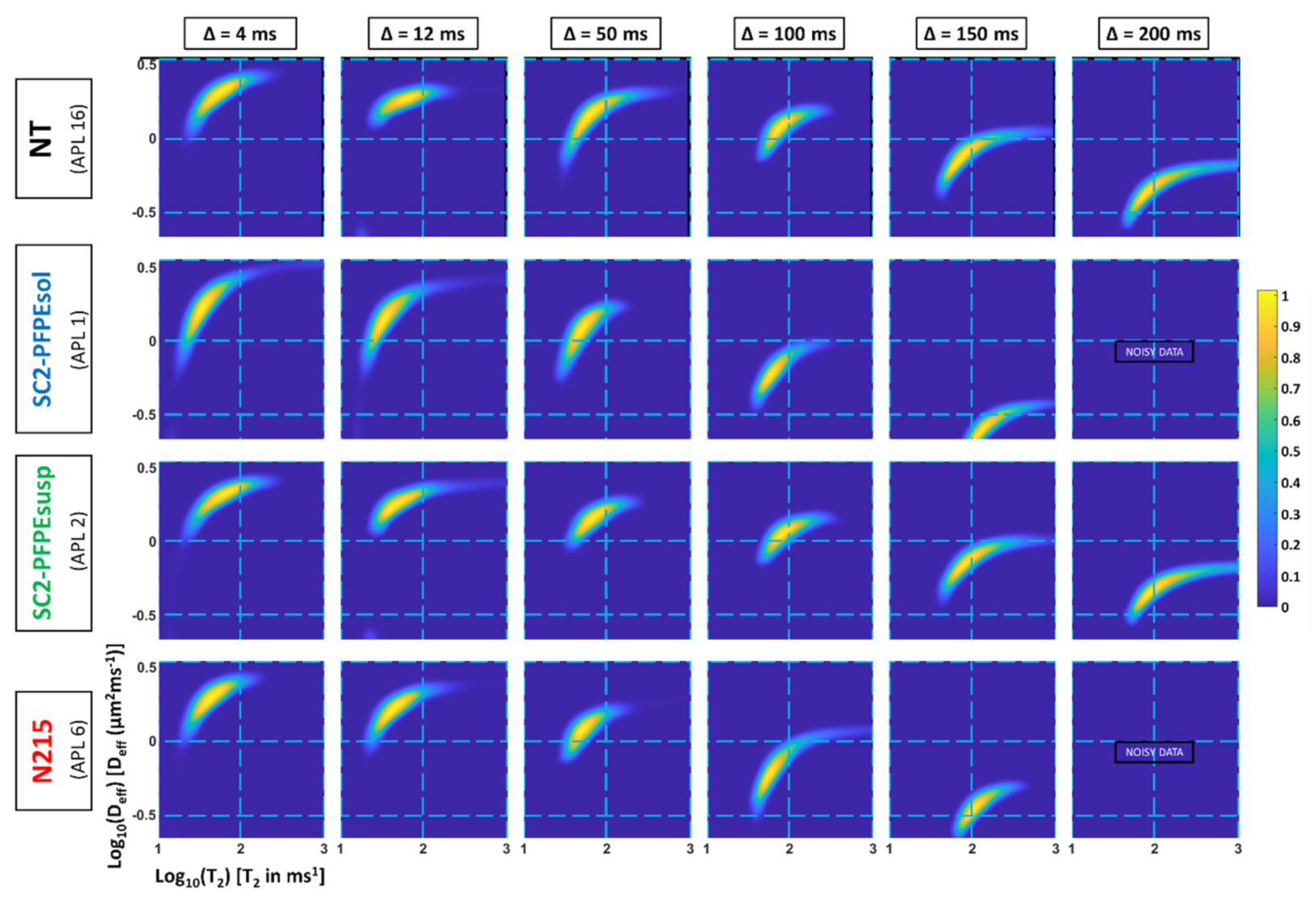
2.2.2. Sorptivity (MRIsorp)
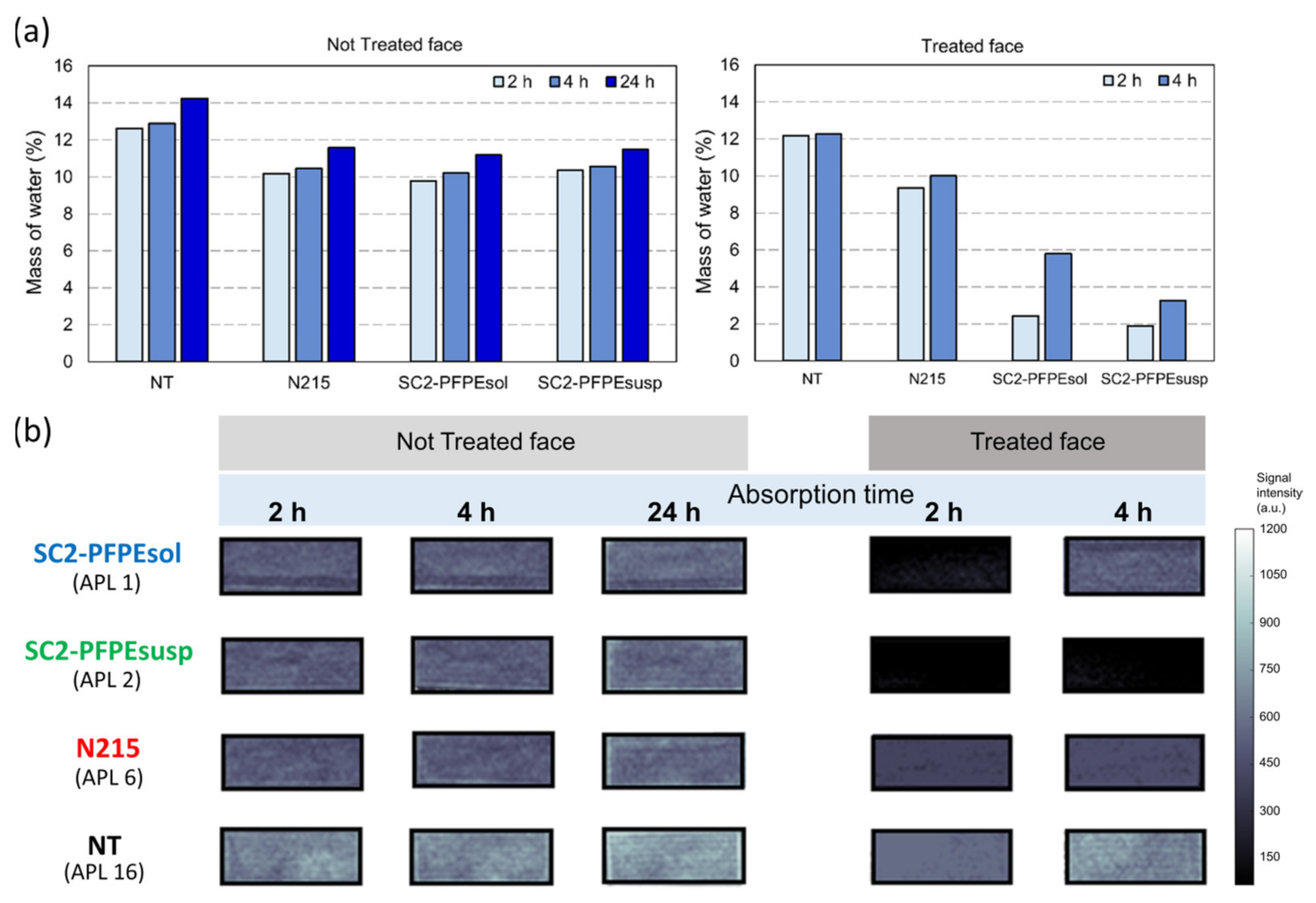
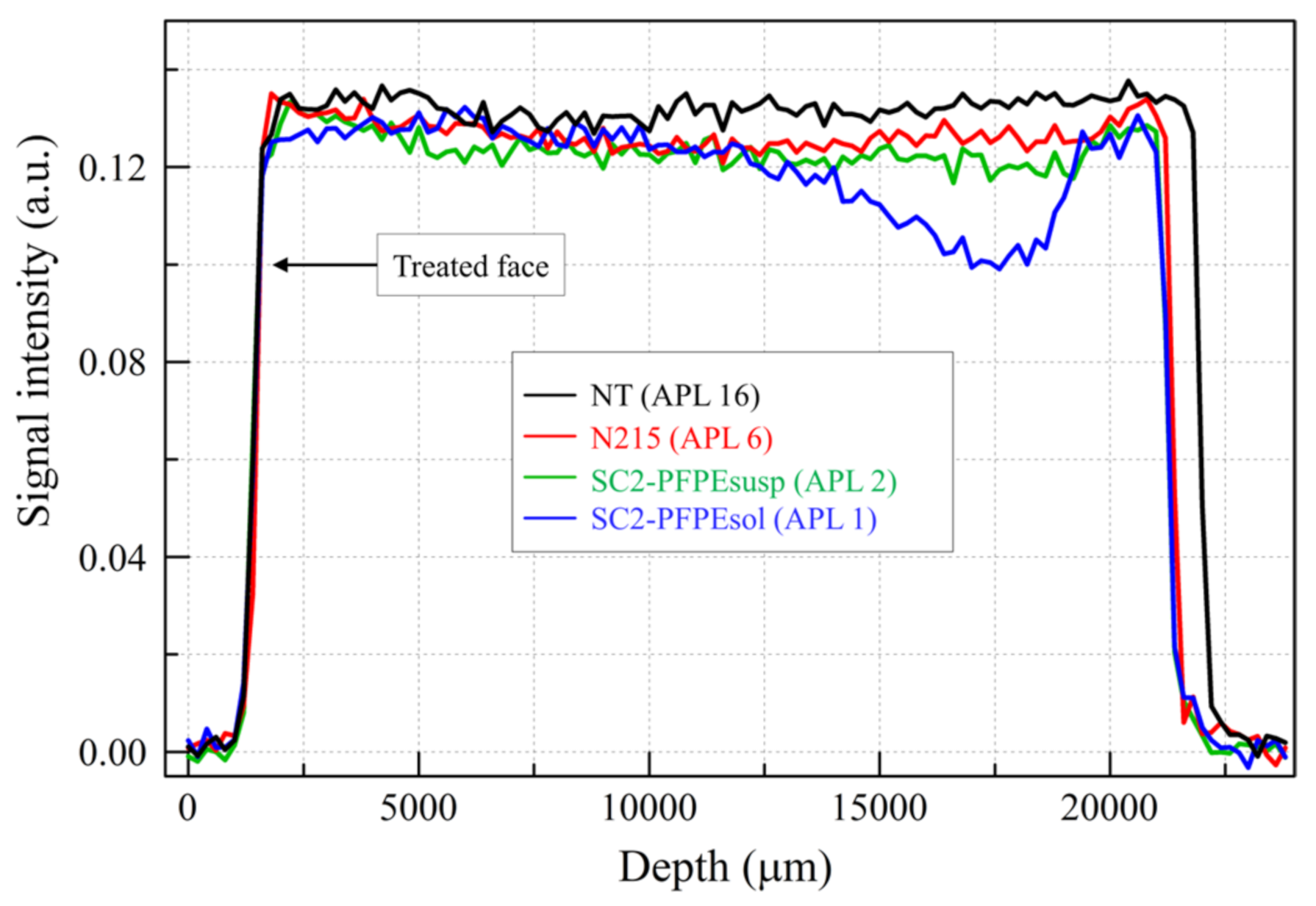
2.2.3. Indirect Visualization of the Coating Distribution (MRIvisual)
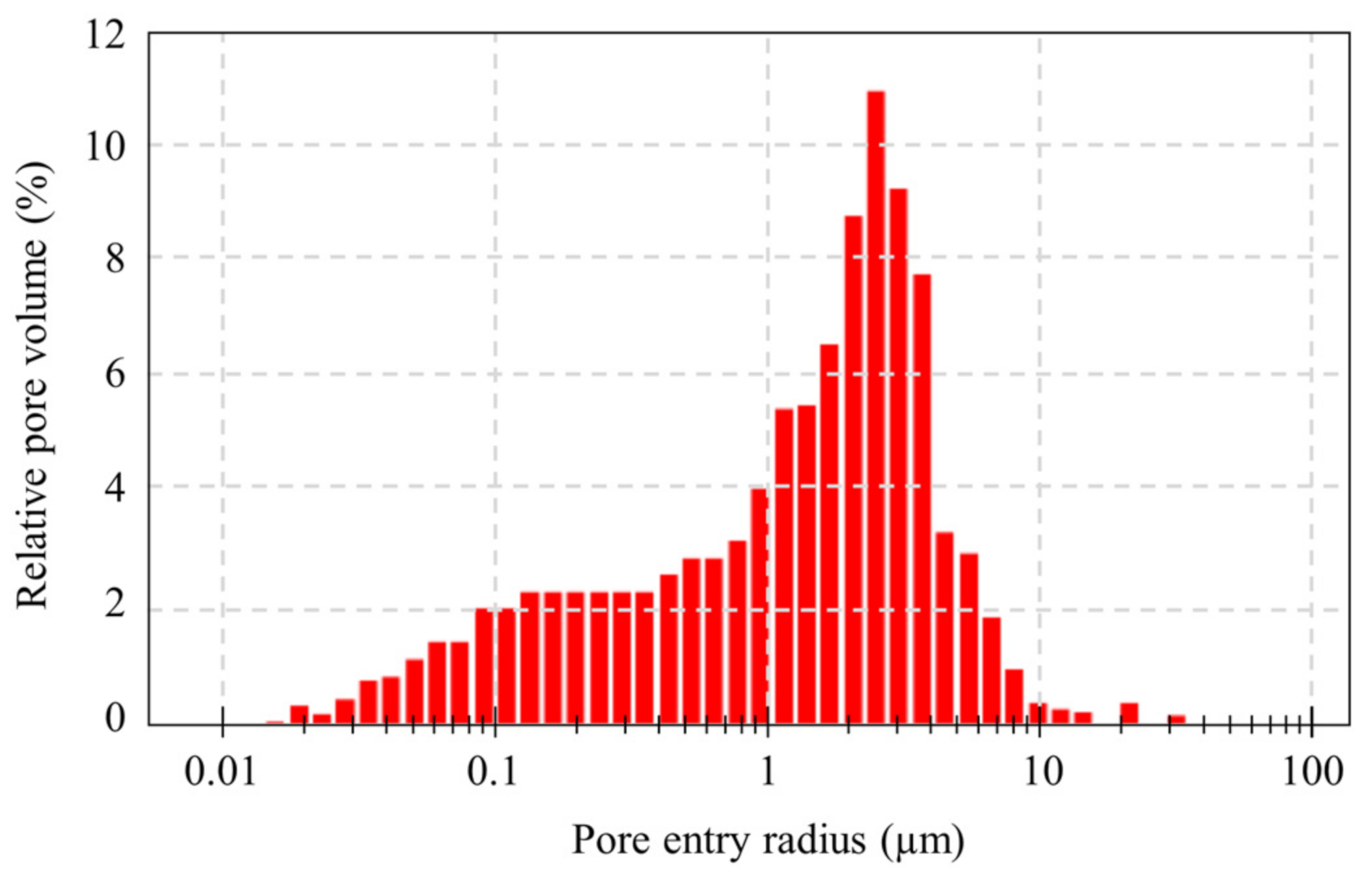
2.2.4. Porous Structure Modification
2.2.5. Wet-Dry Aging
3. Results and Discussion
3.1. Photo-Stability
3.2. Sorptivity
3.3. Porous Structure Modification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dümcke, C.; Gnedovsky, M. The Social and Economic Value of Cultural Heritage: Literature Review; European Expert Network on Culture (EENC) Paper; Interarts: Barcelona, Spain, 2013. [Google Scholar]
- Amoroso, G.G.; Fassina, V. Stone Decay and Conservation; Elsevier: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Doehne, E.; Price, C.A. Stone Conservation: An Overview of Current Research, 2nd ed.; The Getty Conservation Institute: Los Angeles, CA, USA, 2010. [Google Scholar]
- Siegesmund, S.; Weiss, T.; Vollbrecht, A. Natural Stone, Weathering Phenomena, Conservation Strategies and Case Studies; Geological Society Special Publications, No. 205; The Geological Society: London, UK, 2002. [Google Scholar]
- Striegel, M.F.; Guin, E.B.; Hallett, K.; Sandoval, D.; Swingle, R.; Knox, K.; Best, F.; Fornea, S. Air pollution, coatings, and cultural resources. Prog. Org. Coat. 2003, 48, 281–288. [Google Scholar] [CrossRef]
- Barca, D.; Comite, V.; Belfiore, C.M.; Bonazza, A.; La Russa, M.F.; Ruffolo, S.A.; Crisci, G.M.; Pezzino, A.; Sabbioni, C. Impact of air pollution in deterioration of carbonate building materials in Italian urban environments. Appl. Geochem. 2014, 48, 122–131. [Google Scholar] [CrossRef]
- Haneef, S.J.; Johnson, J.B.; Dickinson, C.; Thompson, G.E.; Wood, G.C. Effect of dry deposition of NOx and SO2 gaseous pollutants on the degradation of calcareous building stones. Atmos. Environ. A 1992, 26, 2963–2974. [Google Scholar] [CrossRef]
- Charola, A.E. Stone deterioration characterization for its conservation. Geonomos 2016, 24, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Hassine, M.A.; Beck, K.; Brunetaud, X.; Al-Mukhtar, M. Strain measurements during capillary water infiltration in porous limestones. Constr. Build. Mater. 2018, 175, 439–447. [Google Scholar] [CrossRef]
- Al-Omari, A.; Beck, K.; Brunetaud, X.; Török, Á.; Al-Mukhtar, M. Critical degree of saturation: A control factor of freeze–thaw damage of porous limestones at Castle of Chambord, France. Eng. Geol. 2015, 185, 71–80. [Google Scholar] [CrossRef]
- Cai, H.; Liu, X. Freeze-thaw durability of concrete: Ice formation process in pores. Cem. Concr. Res. 1998, 28, 1281–1287. [Google Scholar] [CrossRef]
- Chabas, A.; Jeannette, D. Weathering of marbles and granites in marine environment: Petrophysical properties and special role of atmospheric salts. Environ. Geol. 2001, 40, 359–368. [Google Scholar] [CrossRef]
- Cardell, C.; Delalieux, F.; Roumpopoulos, K.; Moropoulou, A.; Auger, F.; Van Grieken, R. Salt-induced decay in calcareous stone monuments and buildings in a marine environment in SW France. Constr. Build. Mater. 2003, 17, 165–179. [Google Scholar] [CrossRef]
- Rijniers, L.A.; Pel, L.; Huinink, H.P.; Kopinga, K. Salt crytallization as damage in porous building materials—A nuclear magnetic resonance study. Magn. Reson. Imaging 2005, 23, 273–276. [Google Scholar] [CrossRef]
- Horie, C.V. Materials for Conservation—Organic Consolidants, Adhesives and Coatings, 2nd ed.; Butterworth-Heinemann—Elsevier: Oxford, UK, 2010. [Google Scholar]
- Stefanidou, M.; Karozou, A. Testing the effectiveness of protective coatings on traditional bricks. Constr. Build. Mater. 2016, 111, 482–487. [Google Scholar] [CrossRef]
- Tsakalof, A.; Manoudis, P.; Karapanagiotis, I.; Chryssoulakis, I.; Panayiotou, C. Assessment of synthetic polymeric coatings for the protection and preservation of stone monuments. J. Cult. Herit. 2007, 8, 69–72. [Google Scholar] [CrossRef]
- Sadat-Shojai, A.; Ershad-Langroudi, M.A. Polymeric coatings for protection of historic monument: Opportunities and challenges. J. Appl. Polym. Sci. 2009, 112, 2535–2551. [Google Scholar] [CrossRef]
- Princi, E. Handbook of Polymers in Stone Conservation; Smithers Rapra Technology Ltd.: Shawbury, UK, 2014. [Google Scholar]
- Borgia, G.C.; Camaiti, M.; Cerri, F.; Fantazzini, P.; Piacenti, F. Hydrophobic treatments for stone conservation—Influence of the application method on penetration, distribution and efficiency. Stud. Conserv. 2003, 48, 217–226. [Google Scholar] [CrossRef]
- Borgia, G.C.; Bortolotti, V.; Camaiti, M.; Cerri, F.; Fantazzini, P.; Piacenti, F. Performance evolution of hydrophobic treatments for stone conservation investigated by MRI. Magn. Reson. Imaging 2001, 19, 513–516. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Favaro, M.; Mendichi, R.; Ossola, F.; Russo, U.; Simon, S.; Tomasin, P.; Vigato, P.A. Evaluation of polymers for conservation treatments of outdoor exposed stone monuments. Part I: Photo-oxidative weathering. Polym. Degrad. Stab. 2006, 91, 3083–3096. [Google Scholar] [CrossRef]
- Charola, A.E. Water-repellent treatments for building stones: A practical overview. APT Bull. 1995, 26, 10–17. [Google Scholar] [CrossRef]
- Piacenti, F. Chemistry for the conservation of the cultural heritage. Sci. Total Environ. 1994, 143, 113–120. [Google Scholar] [CrossRef]
- Stahl, T.; Mattern, D.; Brunn, H. Toxicology of perfluorinated compounds. Environ. Sci. Eur. 2011, 23, 38. [Google Scholar] [CrossRef] [Green Version]
- Matteoli, U.; Tiano, P.; Scala, A.; Camaiti, M.; Piacenti, F. Perfluoropolyether isobutylamide and isobutylester performance in the protection of stone. In Proceedings of the VI International Congress on Deterioration and Conservation of Stone, Torun, Poland, 12–14 September 1988; Ciabach, J., Ed.; Nicholas Copernicus University: Torun, Poland, 1988; pp. 509–516. [Google Scholar]
- Piacenti, F.; Camaiti, M. Synthesis and characterization of fluorinated polyetheric amides. J. Fluor. Chem. 1994, 68, 227–235. [Google Scholar] [CrossRef]
- Hénon, F.E.; Camaiti, M.; Burke, A.L.C.; Carbonell, R.G.; DeSimone, J.M.; Piacenti, F. Supercritical CO2 as a solvent for polymeric stone protective materials. J. Supercrit. Fluids 1999, 15, 173–179. [Google Scholar] [CrossRef]
- Licchelli, M.; Malagodi, M.; Weththimuni, M.L.; Zanchi, C. Water-repellent properties of fluoroelastomers on a very porous stone: Effect of the application procedure. Prog. Org. Coat. 2013, 76, 495–503. [Google Scholar] [CrossRef]
- Casazza, E.; Mariani, A.; Ricco, L.; Russo, S. Synthesis, characterization, and properties of a novel acrylic terpolymer with pendant perfluoropolyether segments. Polymer 2002, 43, 1207–1214. [Google Scholar] [CrossRef]
- Toniolo, L.; Poli, T.; Castelvetro, V.; Manariti, A.; Chiantore, O.; Lazzari, M. Tailoring new fluorinated acrylic copolymers as protective coatings for marble. J. Cult. Herit. 2002, 3, 309–316. [Google Scholar] [CrossRef]
- Torrisi, A. Evaluation of five fluorinated compounds as calcarenite protectives. J. Cult. Herit. 2008, 9, 135–145. [Google Scholar] [CrossRef]
- Sabatini, V.; Cattò, C.; Cappelletti, G.; Cappitelli, F.; Antenucci, S.; Farina, H.; Ortenzi, M.A.; Camazzola, S.; Di Silvestro, G. Protective features, durability and biodegration study of acrylic and methacrylic fluorinated polymer coatings for marble protection. Prog. Org. Coat. 2018, 114, 47–57. [Google Scholar] [CrossRef]
- Chatzigrigoriou, A.; Manoudis, P.N.; Karapanagiotis, I. Fabrication of water repellent coatings using waterborne resins for the protection of the cultural heritage. Macromol. Symp. 2013, 331–332, 158–165. [Google Scholar] [CrossRef]
- Camaiti, M.; Brizi, L.; Bortolotti, V.; Papacchini, A.; Salvini, A.; Fantazzini, P. An environmental friendly fluorinated oligoamide for producing nonwetting coatings with high performance on porous surfaces. ACS Appl. Mater. Interfaces 2017, 42, 37279–37288. [Google Scholar] [CrossRef]
- Cao, Y.; Salvini, A.; Camaiti, M. Oligoamide grafted with perfluoropolyether blocks: A potential protective coating for stone materials. Prog. Org. Coat. 2017, 111, 164–174. [Google Scholar] [CrossRef]
- Cao, Y.; Salvini, A.; Camaiti, M. Facile design of “sticky” near superamphiphobic surfaces on highly porous substrate. Mater. Des. 2018, 153, 139–152. [Google Scholar] [CrossRef]
- Cao, Y.; Salvini, A.; Camaiti, M. Superhydrophobic fluorinated oligomers as protective agents for outdoor stone artworks. J. Cult. Herit. 2020, 44, 90–97. [Google Scholar] [CrossRef]
- Cnudde, V.; Dierick, M.; Vlassenbroeck, J.; Masschaele, B.; Lehmann, E.; Jacobs, P.; Van Hoorebeke, L. High-speed neutron radiography for monitoring the water absorption by capillarity in porous materials. Nucl. Instrum. Methods Phys. Res. B 2008, 266, 155–163. [Google Scholar] [CrossRef]
- Nizovtsev, M.I.; Stankus, S.V.; Sterlyagov, A.N.; Terekhov, V.I.; Khairulin, R.A. Experimental determination of the diffusitives of moisture in porous materials in capillary and sorption moistening. J. Eng. Phys. Thermophys. 2005, 78, 68–74. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Zhang, Y.; Lv, H.; Fu, P.; Li, A. In situ characterisation of the depth and mass of water intrusion in unsaturated cement pastes via X-ray computed tomography. Adv. Cem. Res. 2017, 29, 91–100. [Google Scholar] [CrossRef]
- Yang, L.; Gao, D.; Zhang, Y.; Tang, J.; Li, Y. Relationship between sorptivity and capillary coefficient for water absorption of cement-based materials: Theory analysis and experiment. R. Soc. Open Sci. 2019, 6, 190112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopinga, K.; Pel, L. One-dimensional scanning of moisture in porous materials with NMR. Rev. Sci. Instrum. 1994, 65, 3673–3681. [Google Scholar] [CrossRef] [Green Version]
- Ceballos-Ruano, J.S.; Kupka, T.; Nicoll, D.W.; Benson, J.W.; Ioannidis, M.A.; Hansson, C.; Pintar, M.M. Nuclear magnetic resonance monitoring of capillary imbibition and diffusion of water into hardened white cement paste. J. Appl. Phys. 2002, 91, 6588–6594. [Google Scholar] [CrossRef]
- Blümich, B.; Casanova, F.; Perlo, J. Mobile Single-sided NMR. Prog. Nucl. Magn. Reson. Spectrosc. 2008, 52, 197–269. [Google Scholar] [CrossRef]
- Borgia, G.C.; Camaiti, M.; Cerri, F.; Fantazzini, P.; Piacenti, F. Study of water penetration in rock materials by nuclear magnetic resonance tomography: Hydrophobic treatment effects. J. Cult. Herit. 2000, 1, 127–132. [Google Scholar] [CrossRef]
- Camaiti, M.; Casieri, C.; De Luca, F.; Fantazzini, P.; Terenzi, C. The use of portable single-sided relaxometry and laboratory imaging NMR devices in stone conservation. Stud. Conserv. 2007, 52, 37–49. [Google Scholar] [CrossRef]
- Washburn, K.E.; Callaghan, P.T. Tracking Pore to Pore Exchange Using Relaxation Exchange Spectroscopy. Phys. Rev. Lett. 2010, 97, 175502. [Google Scholar] [CrossRef]
- Gombia, M.; Bortolotti, V.; Brown, R.J.S.; Camaiti, M.; Fantazzini, P. Models of water imbibition in untreated and treated porous media validated by quantitative magnetic resonance imaging. J. Appl. Phys. 2008, 103, 094913. [Google Scholar] [CrossRef]
- Camaiti, M.; Bortolotti, V.; Fantazzini, P. Stone porosity, wettability changes and other features detected by MRI and NMR relaxometry: A more than 15-year study. Magn. Reson. Chem. 2015, 53, 34–47. [Google Scholar] [CrossRef]
- Song, Y.-Q. Magnetic resonance of porous media (MRPM): A perspective. J. Magn. Reson. 2013, 229, 12–24. [Google Scholar] [CrossRef]
- Borgia, G.C.; Brown, R.J.S.; Fantazzini, P.; Mesini, E.; Valdre, G. Diffusion-weighted spatial information from 1H relaxation in restricted geometries. Il Nuovo Cimento D 1992, 14, 745–759. [Google Scholar] [CrossRef]
- Brizi, L.; Camaiti, M.; Bortolotti, V.; Fantazzini, P.; Blümich, B.; Haber-Pohlmeier, S. One and two-dimensional NMR to evaluate the performance of consolidants in porous media with a wide range of pore sizes: Applications to cultural heritage. Microp. Mesopor. Mat. 2018, 269, 186–190. [Google Scholar] [CrossRef]
- Camaiti, M.; Bugani, S.; Bernardi, E.; Morselli, L.; Matteini, M. Effects of atmospheric NOX on biocalcarenite coated with different conservation products. Appl. Geochem. 2007, 22, 1248–1254. [Google Scholar] [CrossRef]
- UNI-EN 15801-2010. In Conservation of Cultural Property—Test Methods. Determination of Water Absorption by Capillarity; Ente Nazionale Italiano di Unificazione: Milan, Italy, 2010.
- UNI-EN 15886-2010. In Conservation of Cultural Property—Test Methods. Colour Measurement of Surfaces; Ente Nazionale Italiano di Unificazione: Milan, Italy, 2010.
- Bortolotti, V.; Camaiti, M.; Casieri, C.; De Luca, F.; Fantazzini, P.; Terenzi, C. Water absorbtion kinetics in different wettability conditions studied at pore and sample scale in porous media by portable single-sided and laboratory imaging devices. J. Magn. Reson. 2006, 181, 287–295. [Google Scholar] [CrossRef]
- Mitra, P.P.; Sen, P.N.; Schwartz, L.M.; Le Doussal, P. Diffusion propagator as a probe of the structure of porous-media. Phys. Rev. Lett. 1992, 68, 3555–3558. [Google Scholar] [CrossRef]
- Sen, P.N. Time-dependent diffusion coefficient as a probe of geometry. Concepts Magn. Reson. Part A 2004, 23A, 1–21. [Google Scholar] [CrossRef]
- Bortolotti, V.; Brizi, L.; Fantazzini, P.; Landi, G.; Zama, F. Upen2DTool: A Uniform PENalty Matlab tool for inversion of 2D NMR relaxation data. SoftwareX 2019, 10, 100302. [Google Scholar] [CrossRef]
- Sharkey, W.H.; Mochel, W.E. Mechanism of the photoöxidation of amides. J. Am. Chem. Soc. 1959, 81, 3000–3005. [Google Scholar] [CrossRef]
- Zdravkov, B.D.; Čermák, J.J.; Šefara, M.; Janků, J. Pore classification in the characterization of porous materials: A perspective. Cent. Eur. J. Chem. 2007, 5, 385–395. [Google Scholar]
- Hürlimann, M.D.; Venkataramanan, L.; Flaum, C. The diffusion–spin relaxation time distribution function as an experimental probe to characterize fluid mixtures in porous media. J. Chem. Phys. 2002, 117, 10223. [Google Scholar] [CrossRef]




| Specimen # | Coating | Applied Coating Mass (g/m2) 1 | Investigation |
|---|---|---|---|
| APL1 | SC2-PFPEsol | 16.4 | NMR |
| APL15 | 12.4 | UV | |
| APL4 | 14.0 | reference | |
| APL2 | SC2-PFPEsusp | 16.3 | NMR |
| APL18 | 12.8 | UV | |
| APL8 | 16.0 | reference | |
| APL6 | N215 | 13.6 | NMR |
| APL21 | 11.2 | UV | |
| APL14 | 10.4 | reference | |
| APL16 | NT 2 | / | NMR |
| APL12 | / | UV | |
| APL17 | / | reference | |
| APL26 | / | reference |
| Aging Methodology | Abbreviation |
|---|---|
| 3 initial water capillary absorption tests up to 1 h | Abs |
| 6 water capillary absorption tests after UV irradiation up to 1 h | UV |
| 2 water capillary absorption tests up to 7 days for MRI then 2 saturation under vacuum tests and 4 water capillary absorption tests up to 1 h | MR |
| Specimen # | Treatment | WIE (%) at | |||||
|---|---|---|---|---|---|---|---|
| t = 0 | t = 250 h | t = 500 h | |||||
| 30 min | 1 h | 30 min | 1 h | 30 min | 1 h | ||
| APL15 | SC2-PFPEsol | 95.5 ± 0.4 | 94.5 ± 0.6 | 92.6 ± 1.2 | 90.9 ± 1.2 | 92.6 ± 0.4 | 91.7 ± 0.6 |
| APL18 | SC2-PFPE susp | 94.6 ± 1.0 | 94.7 ± 0.9 | 93.8 ± 0.5 | 93.4 ± 0.9 | 92.1 ± 0.3 | 90.9 ± 1.0 |
| APL21 | N215 | 35.2 ± 19.5 | 29.9 ± 17.0 | 13.8 ± 5.0 | 10.5 ± 4.5 | 45.1 ± 14.4 | 47.5 ± 20.1 |
| APL12 | NT | 8.6 ± 3.2 | 6.5 ± 2.2 | −7.9 ± 10.5 | −11.4 ± 9.3 | −32.1 ± 5.6 | −26.7 ± 1.1 |
| Specimen # | Treatment | WIE (%) at | ||||
|---|---|---|---|---|---|---|
| Wet-Dry Cycles | t = 0 | t = 3 Years | ||||
| 30 min | 1 h | 30 min | 1 h | |||
| APL1 | SC2-PFPEsol | Abs + MR | 95.2 ± 0.7 | 93.3 ± 0.9 | 64.0 ± 0.9 | 57.0 ± 0.9 |
| APL15 | Abs + UV | 95.5 ± 0.4 | 94.5 ± 0.6 | 68.8 ± 1.8 | 63.6 ± 0.3 | |
| APL4 | Abs | 90.9 ± 2.2 | 88.3 ± 1.9 | 89.3 ± 2.2 | 85.1 ± 3.3 | |
| APL2 | SC2-PFPEsusp | Abs + MR | 96.9 ± 0.1 | 96.5 ± 0.2 | 51.7 ± 1.7 | 45.6 ± 0.9 |
| APL18 | Abs + UV | 94.6 ± 1.0 | 94.7 ± 0.9 | 80.9 ± 3.9 | 76.9 ± 3.0 | |
| APL8 | Abs | 97.0 ± 0.2 | 96.5 ± 0.3 | 96.3 ± 0.6 | 95.3 ± 0.7 | |
| APL 6 | N215 | Abs + MR | 70.7 ± 6.3 | 64.3 ± 4.8 | 25.2 ± 9.9 | 20.0 ± 9.7 |
| APL21 | Abs + UV | 35.2 ± 19.5 | 29.9 ± 17.0 | 48.9 ± 6.4 | 48.3 ± 5.0 | |
| APL14 | Abs | 53.5 ± 4.6 | 48.1 ± 4.2 | 40.1 ± 1.5 | 35.9 ± 2.6 | |
| APL16 APL12 | NT | Abs + MR | 0.0 ± 4.7 | −0.8 ± 2.7 | −82.1 ± 12.8 | −46.8 ± 1.3 |
| APL17 | Abs + UV | 8.6 ± 3.2 | 6.5 ± 2.2 | −40.4 ± 8.8 | −28.8 ± 2.3 | |
| APL26 | Abs | 7.2 ± 3.2 | −2.5 ± 1.6 | −31.8 ± 1.9 | −6.4 ± 0.9 | |
| Abs | 13.5 ± 4.5 | 10.1 ± 4.0 | −27.5 ± 3.5 | −11.2 ± 1.2 | ||
| Specimen | Coating | Untreated Face | Treated Face | |||
|---|---|---|---|---|---|---|
| S (mm/s1/2) | S (mm/s1/2) * | t0eff (s) | S’ (mm/s1/2) | t’ (s) | ||
| APL 1 | SC2-PFPEsol | 0.33 | 0.20 | 537 | 0.29 | 9166 |
| APL 2 | SC2-PFPEsusp | 0.36 | 0.28 | 440 | 0.46 | 14,533 |
| APL 6 | N215 | 0.41 | 0.32 | 99 | 0.37 | 371 |
| APL 16 | NT | 0.57 | - | |||
| – | WIE at 30 min (%) | Color Change ΔE | Sorptivity (mm/s1/2) | S/V (m−1) × 105 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Coating | Specimen | Wet-Dry Cycles (Aging) | After Coating | After UV Aging (500 h) | After Aging (3 Years) | After Coating | After UV Aging (500 h) | From Coated Face | At 15 mm from the Coated Face |
| SC2-PFPEsol | APL1 | Abs + MR | 95.2 | 64.0 | 0.20 | 1.6 | |||
| APL15 | Abs + UV | 95.5 | 92.6 | 68.8 | 6.2 | ||||
| APL4 | Abs | 90.9 | 89.3 | 0.5 | |||||
| SC2-PFPEsusp | APL2 | Abs + MR | 96.9 | 51.7 | 0.28 | 1.3 | |||
| APL18 | Abs + UV | 94.6 | 92.1 | 80.9 | 5.7 | ||||
| APL8 | Abs | 97.0 | 96.3 | 0.7 | |||||
| N215 | APL6 | Abs + MR | 70.7 | 25.2 | 0.32 | 1.8 | |||
| APL21 | Abs + UV | 35.2 | 45.1 | 48.9 | 2.1 | ||||
| APL14 | Abs | 53.5 | 40.1 | 2.5 | |||||
| NT | APL16 | Abs + MR | 0.0 | −82.1 | 0.57 | 1.2 | |||
| APL12 | Abs + UV | 8.6 | −32.1 | −40.4 | 4.0 | ||||
| APL17 | Abs | 7.2 | −31.8 | - | |||||
| APL26 | Abs | 13.5 | −27.5 | - | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camaiti, M.; Bortolotti, V.; Cao, Y.; Papacchini, A.; Salvini, A.; Brizi, L. High Efficiency Fluorinated Oligo(ethylenesuccinamide) Coating for Stone. Coatings 2021, 11, 452. https://doi.org/10.3390/coatings11040452
Camaiti M, Bortolotti V, Cao Y, Papacchini A, Salvini A, Brizi L. High Efficiency Fluorinated Oligo(ethylenesuccinamide) Coating for Stone. Coatings. 2021; 11(4):452. https://doi.org/10.3390/coatings11040452
Chicago/Turabian StyleCamaiti, Mara, Villiam Bortolotti, Yijian Cao, Alessandra Papacchini, Antonella Salvini, and Leonardo Brizi. 2021. "High Efficiency Fluorinated Oligo(ethylenesuccinamide) Coating for Stone" Coatings 11, no. 4: 452. https://doi.org/10.3390/coatings11040452
APA StyleCamaiti, M., Bortolotti, V., Cao, Y., Papacchini, A., Salvini, A., & Brizi, L. (2021). High Efficiency Fluorinated Oligo(ethylenesuccinamide) Coating for Stone. Coatings, 11(4), 452. https://doi.org/10.3390/coatings11040452