Microstructure and Mechanical Properties of ZrN, ZrCN and ZrC Coatings Grown by Chemical Vapor Deposition
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
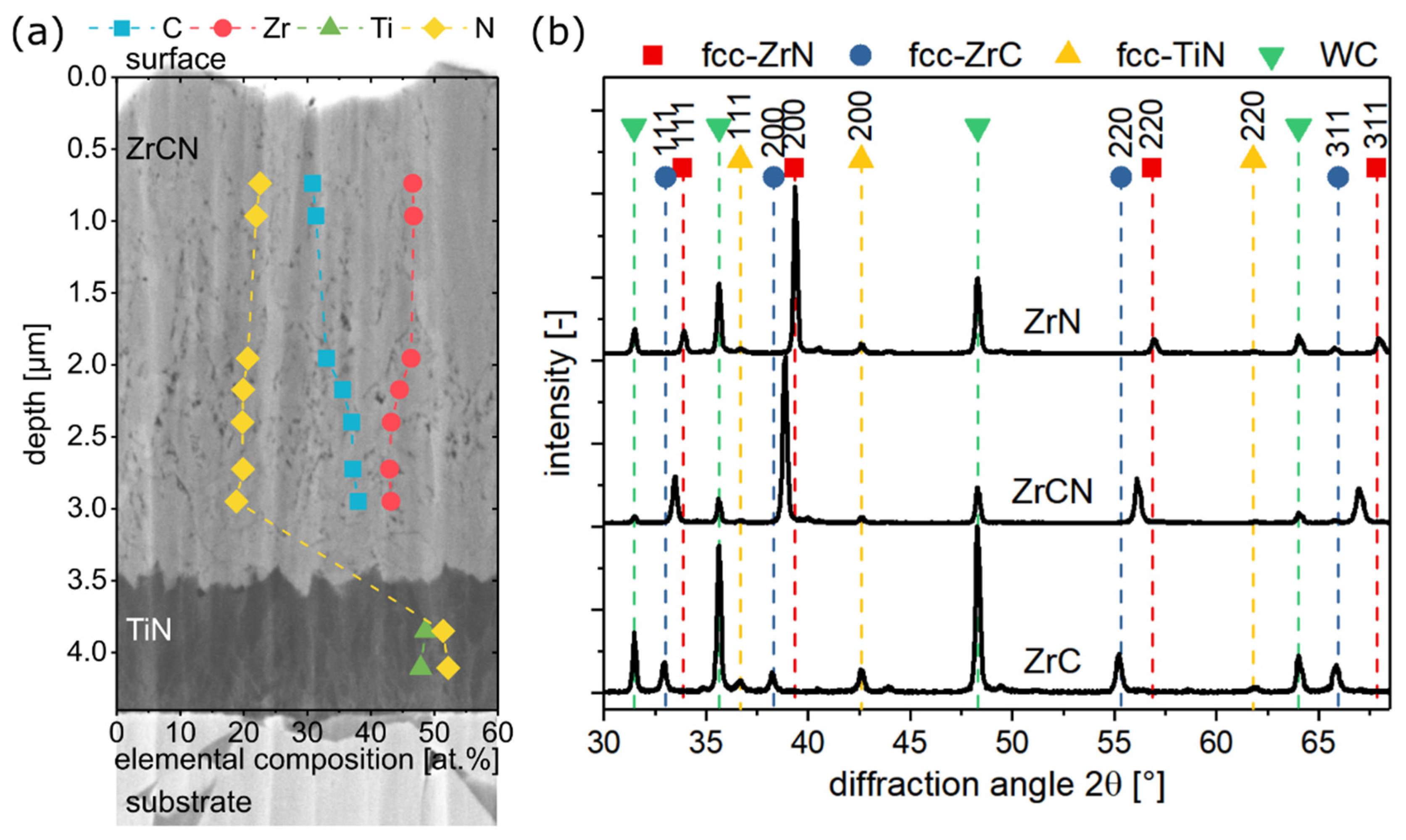
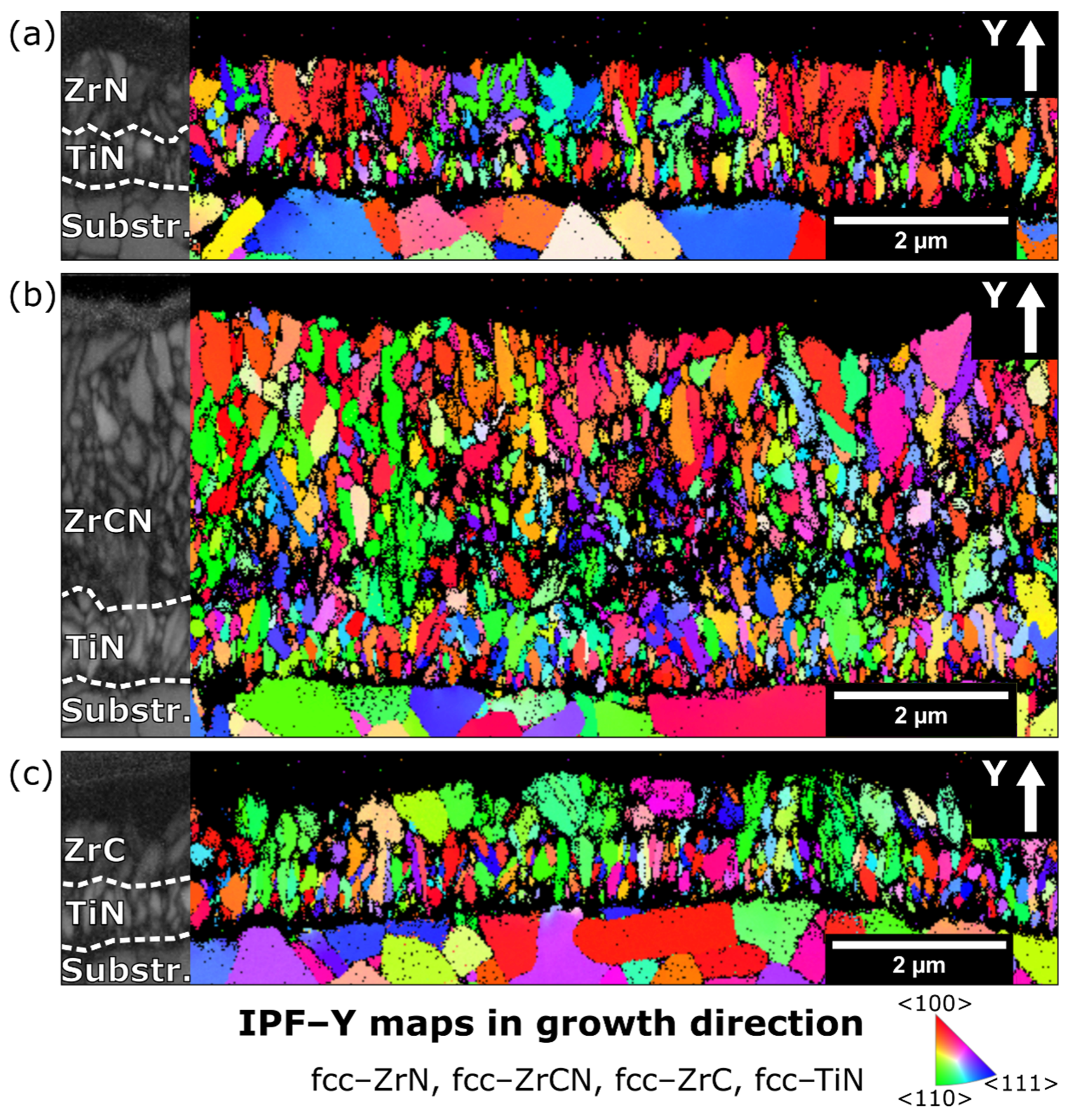
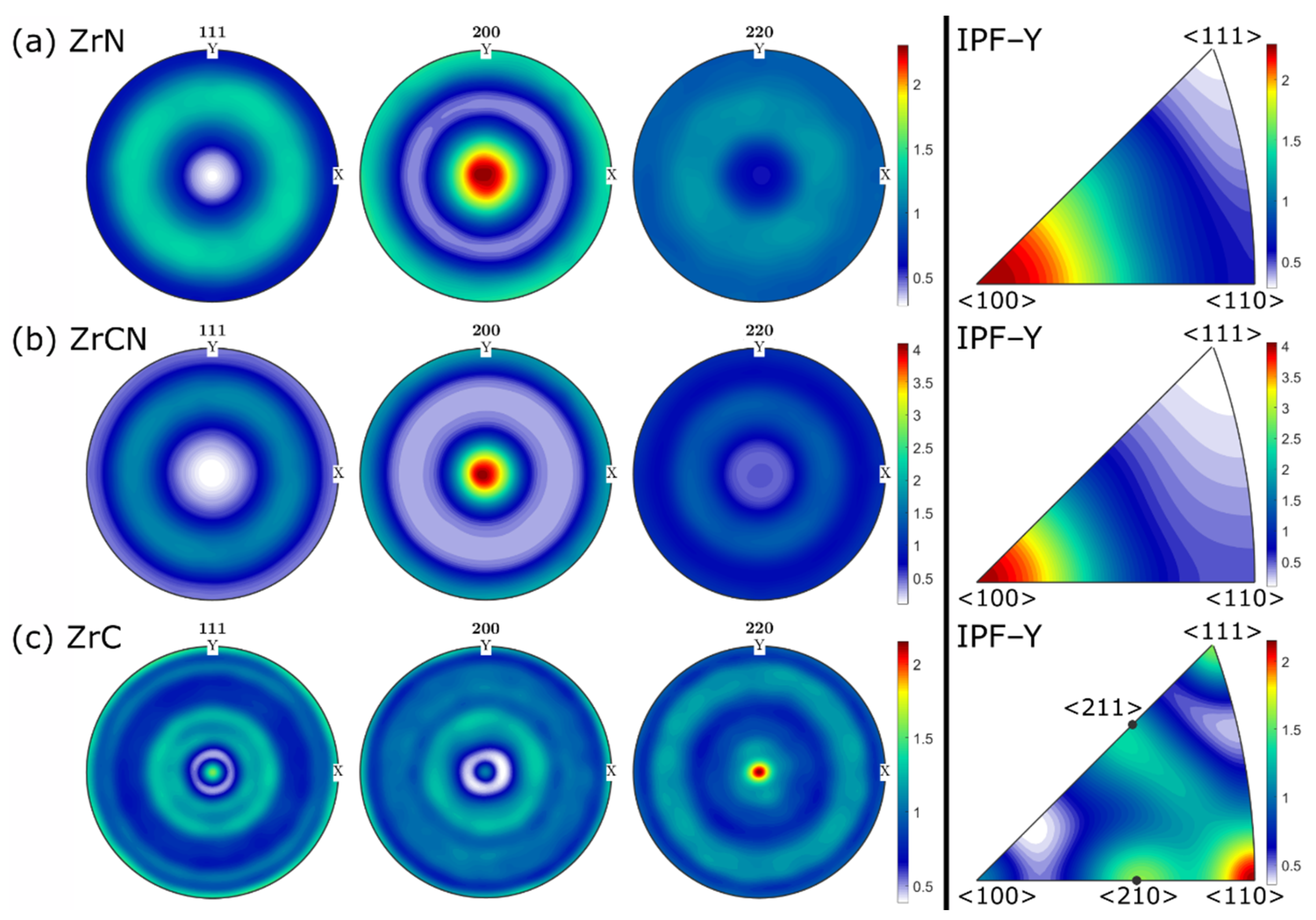
3.1. Chemical Composition and Microstructure
3.2. Residual Stress and Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitterer, C. PVD and CVD Hard Coatings. In Comprehensive Hard Materials; Sarin, V.K., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 2, pp. 449–467. ISBN 9780080965284. [Google Scholar]
- Kainz, C.; Schalk, N.; Tkadletz, M.; Mitterer, C.; Czettl, C. Microstructure and mechanical properties of CVD TiN/TiBN multilayer coatings. Surf. Coat. Technol. 2019, 370, 311–319. [Google Scholar] [CrossRef]
- Schintlmeister, W.; Wallgram, W.; Kanz, J.; Gigl, K. Cutting tool materials coated by chemical vapour deposition. Wear 1984, 100, 153–169. [Google Scholar] [CrossRef]
- Kainz, C.; Schalk, N.; Tkadletz, M.; Mitterer, C.; Czettl, C. The effect of B and C addition on microstructure and mechanical properties of TiN hard coatings grown by chemical vapor deposition. Thin Solid Film. 2019, 688, 137283. [Google Scholar] [CrossRef]
- Wagner, J.; Mitterer, C.; Penoy, M.; Michotte, C.; Wallgram, W.; Kathrein, M. The effect of deposition temperature on microstructure and properties of thermal CVD TiN coatings. Int. J. Refract. Met. Hard Mater. 2008, 26, 120–126. [Google Scholar] [CrossRef]
- Rie, K.-T.; Wöhle, J. Plasma-CVD of TiCN and ZrCN films on light metals. Surf. Coat. Technol. 1999, 112, 226–229. [Google Scholar] [CrossRef]
- Rie, K.-T.; Gebauer, A.; Wöhle, J. Plasma assisted CVD for low temperature coatings to improve the wear and corrosion resistance. Surf. Coat. Technol. 1996, 86-87, 498–506. [Google Scholar] [CrossRef]
- Haubner, R.; Rauchenwald, E.; Lessiak, M.; Pitonak, R.; Weissenbacher, R. Novel High-Performance CVD Coatings for Machining Applications. Powder Met. Prog. 2018, 18, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-H.; Kuo, K.-L.; Yu, G.-P. Oxidation behavior and corrosion resistance of vacuum annealed ZrN-coated stainless steel. Surf. Coat. Technol. 2019, 358, 308–319. [Google Scholar] [CrossRef]
- Garcia, J.; Moreno, M.F.; Östby, J.; Persson, J.; Pinto, H.C. Design of coated cemented carbides with improved comb crack resistance. In Proceedings of the 19th Plansee Semin, Reutte, Austria, 29 May–2 June 2017. [Google Scholar]
- Ul-Hamid, A. Microstructure, properties and applications of Zr-carbide, Zr-nitride and Zr-carbonitride coatings: A review. Mater. Adv. 2020, 1, 1012–1037. [Google Scholar] [CrossRef]
- El Azhari, I.; Garcia, J.; Zamanzade, M.; Soldera, F.; Pauly, C.; Llanes, L.; Mücklich, F. Investigations on micro-mechanical properties of polycrystalline Ti(C,N) and Zr(C,N) coatings. Acta Mater. 2018, 149, 364–376. [Google Scholar] [CrossRef] [Green Version]
- El Azhari, I.; García, J.; Soldera, F.; Suarez, S.; Jiménez-Piqué, E.; Mücklich, F.; Llanes, L. Contact damage investigation of CVD carbonitride hard coatings deposited on cemented carbides. Int. J. Refract. Met. Hard Mater. 2020, 86, 105050. [Google Scholar] [CrossRef]
- Russell, W.C. Experimental Design Approach to Development of a CVD ZrN Coating. Le J. Phys. Colloq. IV 1995, 5, C5–C127. [Google Scholar] [CrossRef]
- Long, Y.; Javed, A.; Chen, J.; Chen, Z.-K.; Xiong, X. Phase composition, microstructure and mechanical properties of ZrC coatings produced by chemical vapor deposition. Ceram. Int. 2014, 40, 707–713. [Google Scholar] [CrossRef]
- Sundgren, J.-E.; Hentzell, H.G. A review of the present state of art in hard coatings grown from the vapor phase. J. Vac. Sci. Technol. A Vac. Surf. Film. 1986, 4, 2259–2279. [Google Scholar] [CrossRef]
- El Azhari, I.; Barrirero, J.; García, J.; Soldera, F.; Llanes, L.; Mücklich, F. Atom Probe Tomography investigations on grain boundary segregation in polycrystalline Ti(C,N) and Zr(C,N) CVD coatings. Scr. Mater. 2019, 162, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Kudapa, S.; Narasimhan, K.; Boppana, P.; Russell, W.C. Characterization and properties of MTCVD TiCN and MTCVD ZrCN coatings. Surf. Coat. Technol. 1999, 120-121, 259–264. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, L.; Cheng, L.; Wang, Y. Morphologies and growth mechanisms of zirconium carbide films by chemical vapor deposition. J. Coat. Technol. Res. 2008, 6, 269–273. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Liu, J.; Zhang, L.; Cheng, L. Deposition Mechanism for Chemical Vapor Deposition of Zirconium Carbide Coatings. J. Am. Ceram. Soc. 2008, 91, 1249–1252. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, L.; Ma, B.; Gao, S.; Feng, W.; Liu, Y.; Zhang, L. Calculation and synthesis of ZrC by CVD from ZrCl4–C3H6–H2–Ar system with high H2 percentage. Appl. Surf. Sci. 2015, 332, 591–598. [Google Scholar] [CrossRef]
- Rauchenwald, E.; Lessiak, M.; Weissenbacher, R.; Haubner, R. Chemical vapor deposition of ZrN using in situ produced ZrCl4 as a precursor. Ceram. Int. 2019, 45, 9410–9414. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, S.H. Comparative studies of TiN and Ti1−xAlxN by plasma-assisted chemical vapor deposition using a TiCl4/AlCl3/N2/H2/Ar gas mixture. Thin Solid Film. 1996, 283, 165–170. [Google Scholar] [CrossRef]
- Kawata, K.; Sugimura, H.; Takai, O. Effects of chlorine on tribological properties of TiN films prepared by pulsed d.c. plasma-enhanced chemical vapor deposition. Thin Solid Film. 2002, 407, 38–44. [Google Scholar] [CrossRef]
- Rie, K.-T.; Gebauer, A.; Woehle, J. Investigation of PA-CVD of TiN: Relations between process parameters, spectroscopic measurements and layer properties. Surf. Coat. Technol. 1993, 60, 385–388. [Google Scholar] [CrossRef]
- Anderbouhr, S.; Ghetta, V.; Blanquet, E.; Chabrol, C.; Schuster, F.; Bernard, C.; Madar, R. LPCVD and PACVD (Ti,Al)N films: Morphology and mechanical properties. Surf. Coat. Technol. 1999, 115, 103–110. [Google Scholar] [CrossRef]
- Stylianou, R.; Velic, D.; Daves, W.; Ecker, W.; Stark, A.; Schell, N.; Tkadletz, M.; Schalk, N.; Czettl, C.; Mitterer, C. Stress relaxation through thermal crack formation in CVD TiCN coatings grown on WC-Co with different Co contents. Int. J. Refract. Met. Hard Mater. 2020, 86, 105102. [Google Scholar] [CrossRef]
- Gassner, M.; Schalk, N.; Tkadletz, M.; Czettl, C.; Mitterer, C. Thermal crack network on CVD TiCN/α-Al2O3 coated cemented carbide cutting tools. Int. J. Refract. Met. Hard Mater. 2019, 81, 1–6. [Google Scholar] [CrossRef]
- ISO 1832:2017(en); Indexable Inserts for Cutting Tools—Designation; International Organization for Standardization: Geneva, Switzerland, 2017.
- Bachmann, F.; Hielscher, R.; Schaeben, H. Texture Analysis with MTEX—Free and Open Source Software Toolbox. Solid State Phenom. 2010, 160, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Abadias, G.; Chason, E.; Keckes, J.; Sebastiani, M.; Thompson, G.B.; Barthel, E.; Doll, G.L.; Murray, C.E.; Stoessel, C.H.; Martinu, L. Review Article: Stress in thin films and coatings: Current status, challenges, and prospects. J. Vac. Sci. Technol. A Vac. Surf. Film. 2018, 36, 020801. [Google Scholar] [CrossRef] [Green Version]
- Hill, R. The Elastic Behaviour of a Crystalline Aggregate. Proc. Phys. Soc. Sect. A 1952, 65, 349–354. [Google Scholar] [CrossRef]
- Ivashchenko, V.I.; Turchi, P.E.A.; Shevchenko, V.I. First-principles study of elastic and stability properties of ZrC–ZrN and ZrC–TiC alloys. J. Phys. Condens. Matter 2009, 21, 395503. [Google Scholar] [CrossRef]
- Krasnenko, V.; Brik, M.G. First-principles calculations of the structural, elastic and electronic properties of MNxC1−x (M = Ti, Zr, Hf; 0 ≤ x ≤ 1) carbonitrides at ambient and elevated hydrostatic pressure. Solid State Sci. 2014, 28, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Chen, X.-J.; Struzhkin, V.V.; Cohen, R.E. Trends in elasticity and electronic structure of transition-metal nitrides and carbides from first principles. Phys. Rev. B 2005, 71, 214103. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-J.; Struzhkin, V.V.; Wu, Z.; Somayazulu, M.; Qian, J.; Kung, S.; Christensen, A.N.; Zhao, Y.; Cohen, R.E.; Mao, H.-K.; et al. Hard superconducting nitrides. Proc. Natl. Acad. Sci. USA 2005, 102, 3198–3201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Czettl, C.; Mitterer, C.; Muhle, U.; Rafaja, D.; Puchner, S.; Hutter, H.; Penoy, M.; Michotte, C.; Kathrein, M. CO addition in low-pressure chemical vapour deposition of medium-temperature TiCxN1-x based hard coatings. Surf. Coat. Technol. 2011, 206, 1691–1697. [Google Scholar] [CrossRef]
- Larsson, A.; Ruppi, S. Microstructure and properties of Ti(C,N) coatings produced by moderate temperature chemical vapour deposition. Thin Solid Film. 2002, 402, 203–210. [Google Scholar] [CrossRef]
- Bonetti, R.S.; Wiprächtiger, H.; Mohn, E. CVD of titanium carbonitride at moderate temperature: Properties and applications. Met. Powder Rep. 1990, 45, 837–840. [Google Scholar] [CrossRef]
- Sabitzer, C.; Steinkellner, C.; Koller, C.M.; Polcik, P.; Rachbauer, R.; Mayrhofer, P.H. Diffusion behavior of C, Cr, and Fe in arc evaporated TiN- and CrN-based coatings and their influence on thermal stability and hardness. Surf. Coat. Technol. 2015, 275, 185–192. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Dreiling, I.; Haug, A.; Holzschuh, H.; Chassé, T. Raman spectroscopy as a tool to study cubic Ti–C–N CVD coatings. Surf. Coat. Technol. 2009, 204, 1008–1012. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Chemical Vapor Deposition (CVD), 2nd ed.; Noyes Publications: Park Ridge, IL, USA, 1999; ISBN 0815514328. [Google Scholar]
- Thompson, C.V. Structure Evolution during Processing of Polycrystalline Films. Annu. Rev. Mater. Sci. 2000, 30, 159–190. [Google Scholar] [CrossRef]
- Park, J.H.; Jung, C.H.; Kim, D.J.; Park, J.Y. Effect of H2 dilution gas on the growth of ZrC during low pressure chemical vapor deposition in the ZrCl4–CH4–Ar system. Surf. Coat. Technol. 2008, 203, 87–90. [Google Scholar] [CrossRef]
- Sun, W.; Xiong, X.; Huang, B.Y.; Li, G.D.; Zhang, H.B.; Xiao, P.; Chen, Z.K.; Zheng, X.L. Microstructural Control of Zirconium Carbide Coating Prepared by Chemical Vapor Deposition. ECS Trans. 2009, 25, 291–299. [Google Scholar] [CrossRef]
- Wang, S.-L.; Li, K.-Z.; Li, H.-J.; Zhang, Y.-L.; Wang, Y.-J. Effects of microstructures on the ablation behaviors of ZrC deposited by CVD. Surf. Coat. Technol. 2014, 240, 450–455. [Google Scholar] [CrossRef]
- Imai, Y.; Mukaida, M.; Watanabe, A.; Tsunoda, T. Formation energies of two-dimensional nuclei randomly-generated on (001), (110), and (111) planes of a face-centered-cubic crystal. Thin Solid Film. 1997, 300, 305–313. [Google Scholar] [CrossRef]
- Stylianou, R.; Velic, D.; Daves, W.; Ecker, W.; Tkadletz, M.; Schalk, N.; Czettl, C.; Mitterer, C. Thermal crack formation in TiCN/α-Al2O3 bilayer coatings grown by thermal CVD on WC-Co substrates with varied Co content. Surf. Coat. Technol. 2020, 392, 125687. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Refractory Carbides and Nitrides: Properties, Characteristics, Processing and Applications, 1st ed.; Noyes Publications: New York, NY, USA, 1996; ISBN 9780815513926. [Google Scholar]
- Gao, C.; Zhao, Z.; Li, X. Modeling of thermal stresses in elastic multilayer coating systems. J. Appl. Phys. 2015, 117, 055305. [Google Scholar] [CrossRef]
- Pister, K.S. Flexural Vibration of Thin Laminated Plates. J. Acoust. Soc. Am. 1959, 31, 233–234. [Google Scholar] [CrossRef]
- Reissner, E.; Stavsky, Y. Bending and Stretching of Certain Types of Heterogeneous Aeolotropic Elastic Plates. J. Appl. Mech. 1961, 28, 402–408. [Google Scholar] [CrossRef]
- Chair of Strucural and Functional Ceramics, Classical Laminate Theory (CLT). Available online: https://www.isfk.at/de/1428/ (accessed on 2 November 2020).
- Yang, Q.; Lengauer, W.; Koch, T.; Scheerer, M.; Smid, I. Hardness and elastic properties of Ti(CxN1−x), Zr(CxN1−x) and Hf(CxN1−x). J. Alloys Compd. 2000, 309, L5–L9. [Google Scholar] [CrossRef]
- Jhi, S.-H.; Ihm, J.; Louie, S.G.; Cohen, M.L. Electronic mechanism of hardness enhancement in transition-metal carbonitrides. Nat. Cell Biol. 1999, 399, 132–134. [Google Scholar] [CrossRef]
- Ivashchenko, V.I.; Turchi, P.E.A.; Gonis, A.; Ivashchenko, L.A.; Skrynskii, P.L. Electronic origin of elastic properties of titanium carbonitride alloys. Met. Mater. Trans. A 2006, 37, 3391–3396. [Google Scholar] [CrossRef]
- Kováčik, J. Correlation between Young’s modulus and porosity in porous materials. J. Mater. Sci. Lett. 1999, 18, 1007–1010. [Google Scholar] [CrossRef]
- Bouzakis, K.-D.; Hadjiyiannis, S.; Skordaris, G.; Mirisidis, I.; Michailidis, N.; Efstathiou, K.; Pavlidou, E.; Erkens, G.; Cremer, R.; Rambadt, S.; et al. The effect of coating thickness, mechanical strength and hardness properties on the milling performance of PVD coated cemented carbides inserts. Surf. Coat. Technol. 2004, 177, 657–664. [Google Scholar] [CrossRef]


| Sample | Time [min] | Temperature [°C] | Pressure [kPa] | H2 (Balance) [vol.%] | N2 [vol.%] | CH3 CN[vol.%] | CH4 [vol.%] |
|---|---|---|---|---|---|---|---|
| ZrN | 260 | 1000 | 16 | 76 | 24 | - | - |
| ZrCN | 260 | 1000 | 10 | 99.9 | - | 2.2 × 10−5 | - |
| ZrC | 260 | 1000 | 16 | 94 | - | - | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frank, F.; Tkadletz, M.; Czettl, C.; Schalk, N. Microstructure and Mechanical Properties of ZrN, ZrCN and ZrC Coatings Grown by Chemical Vapor Deposition. Coatings 2021, 11, 491. https://doi.org/10.3390/coatings11050491
Frank F, Tkadletz M, Czettl C, Schalk N. Microstructure and Mechanical Properties of ZrN, ZrCN and ZrC Coatings Grown by Chemical Vapor Deposition. Coatings. 2021; 11(5):491. https://doi.org/10.3390/coatings11050491
Chicago/Turabian StyleFrank, Florian, Michael Tkadletz, Christoph Czettl, and Nina Schalk. 2021. "Microstructure and Mechanical Properties of ZrN, ZrCN and ZrC Coatings Grown by Chemical Vapor Deposition" Coatings 11, no. 5: 491. https://doi.org/10.3390/coatings11050491
APA StyleFrank, F., Tkadletz, M., Czettl, C., & Schalk, N. (2021). Microstructure and Mechanical Properties of ZrN, ZrCN and ZrC Coatings Grown by Chemical Vapor Deposition. Coatings, 11(5), 491. https://doi.org/10.3390/coatings11050491





