Synthesis of Cubic Aluminum Nitride (AlN) Coatings through Suspension Plasma Spray (SPS) Technology
Abstract
:1. Introduction
1.1. Background
1.2. Synthesis Methods of AlN
1.3. Non-Wetting Properties
1.4. Research Objectives
2. Materials and Methods
2.1. Materials and Reagents
- (a)
- Substrates: 12.7 mm × 2.0 mm Titanium disks, Ti (McMaster-Carr, Chicago, IL, USA) of composition Ti (88.10–90.92%), Al (5.50–6.75%), C (0.08%), Fe (0.40% Max), V (3.5–4.5%), and 0–0.30% others; Molybdenum disks, Mo (99%, American element, USA); AISI 1144 Carbon steel disks (McMaster-Carr, Chicago, IL, USA) of mainly Fe with inclusions of composition C (0.40–0.48%), Mn (1.35–1.65%), p (0.04% Max) and S (0.24–0.33%).
- (b)
- Suspension liquid carriers: Hexadecane (99% C16C34, Sigma Aldrich, St. Louis, MO, USA), ethylene glycol (99.8% C2H6O2, Sigma Aldrich, St. Louis, MO, USA), mineral oil (light oil, C16H10N2Na2O7S2, Sigma Aldrich, St. Louis, MO, USA) and anhydrous ethyl alcohol (C2H5OH, commercial alcohol).
- (c)
- Powders: Pure aluminum metal powder (99.9% Al, Atlantic equipment engineers) with particle size range (1–35 µm), urea grains (CH₄N₂O, Sigma-V1428), Melamine (99% C3N6H6, Alfa Aesar, Fisher Scientific, Tewksbury, MA, USA).
2.2. Preparation of Substrates
2.3. Suspension Feedstock for SPS Injection
2.4. Synthesis of Cubic AlN Coatings by SPS
2.5. Materials Characterization
2.5.1. X-ray Diffraction (XRD) Analysis
2.5.2. Optical Microscopy (OM)
2.5.3. Scanning Electron Microscopy (SEM)
2.5.4. Surface Roughness Test and the Coating Thickness Measurements
2.6. Materials Testing
- (a)
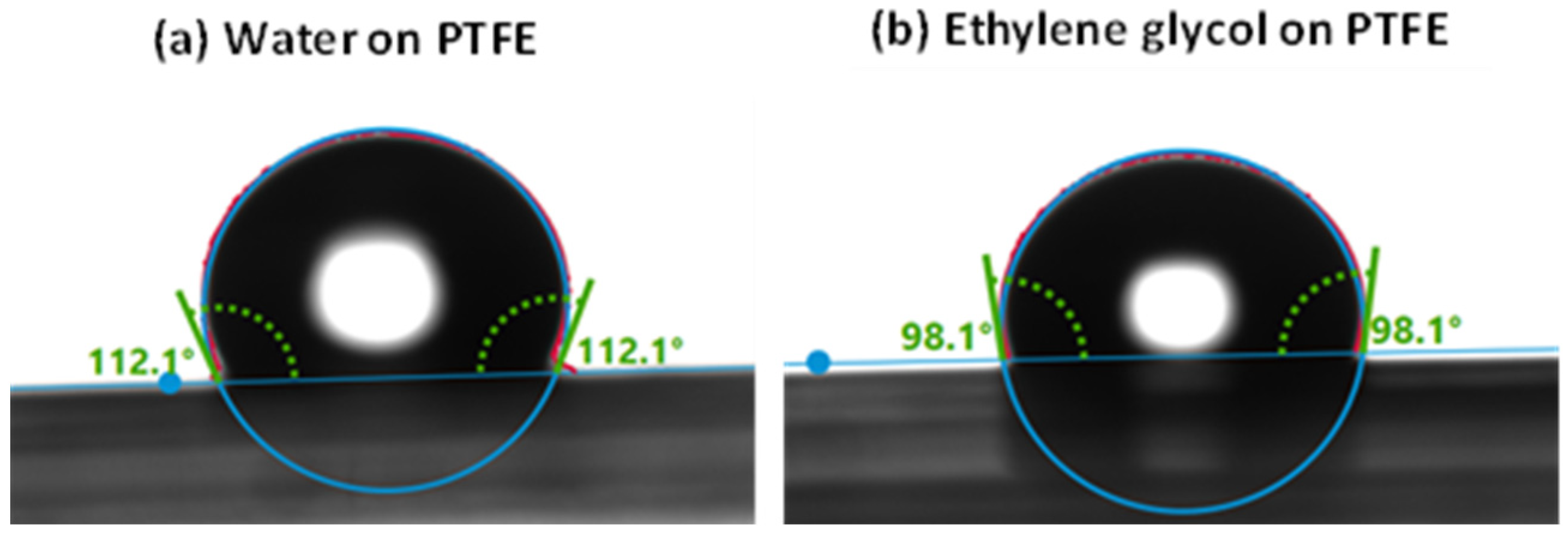
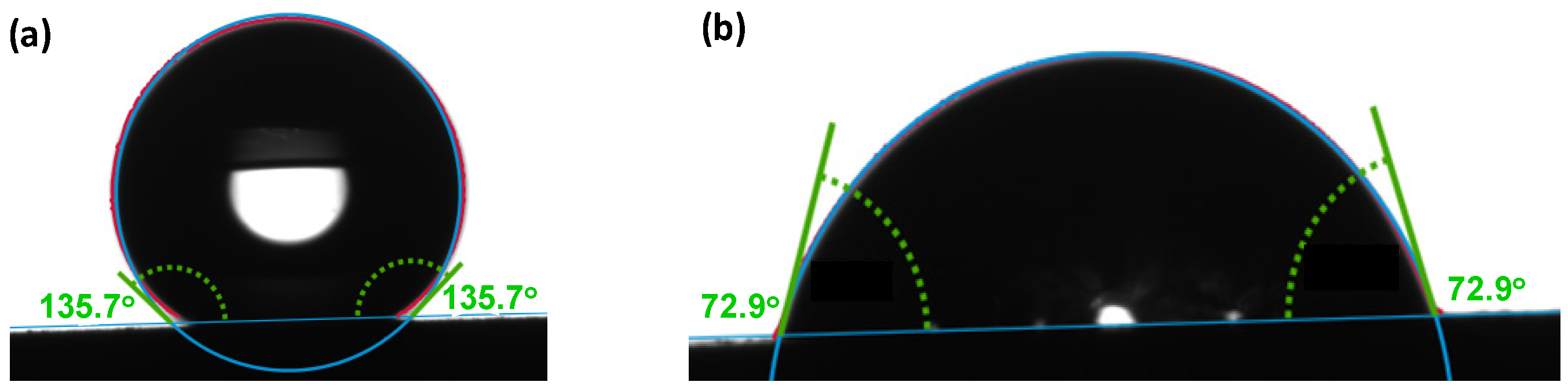
- Wetting test: Wettability of the samples was studied using a drop shape analyzer, the Krüss Advance goniometer model DSA25E (KRÜSS, Hamburg, Germany). In order to validate the analysis method, the static contact angle was calibrated at ambient conditions by depositing a droplet of water or ethylene glycol on the surface of Teflon (polytetrafluoroethylene, PTFE). From the manufacturer’s database, the contact angle of water on PTFE is known to be 113.7°, which we validated to be 112.1° as indicated in Figure 1a, while that of ethylene glycol was found to be 98.1° as shown in Figure 1b against 94.9° of the database [59]. A liquid on a solid surface with contact angle below 90° is considered to be a wetting liquid, while a contact angle above 90° indicates non-wetting properties [60]. Superhydrophobic surfaces with the ‘Lotus Effect’ that exhibit an extremely high contact angle with water (>150°) have been broadly investigated and can be considered as a perfect example in anti-corrosion applications [61].
- (b)
- Corrosion test: The fresh coatings synthesized by SPS technology were tested for corrosion resistance by contacting them directly with a molten Al-alloy containing 5% magnesium (Al-5 wt% Mg) for 2 h at 850 °C. OM was used to observe the surface properties of the coatings to investigate their corrosion resistance when in contact with molten Al-Mg alloy.
3. Results
3.1. FactSage™ Modelling for the In Situ Synthesis of AlN in Plasma
3.2. Materials Characterization of AlN Coatings
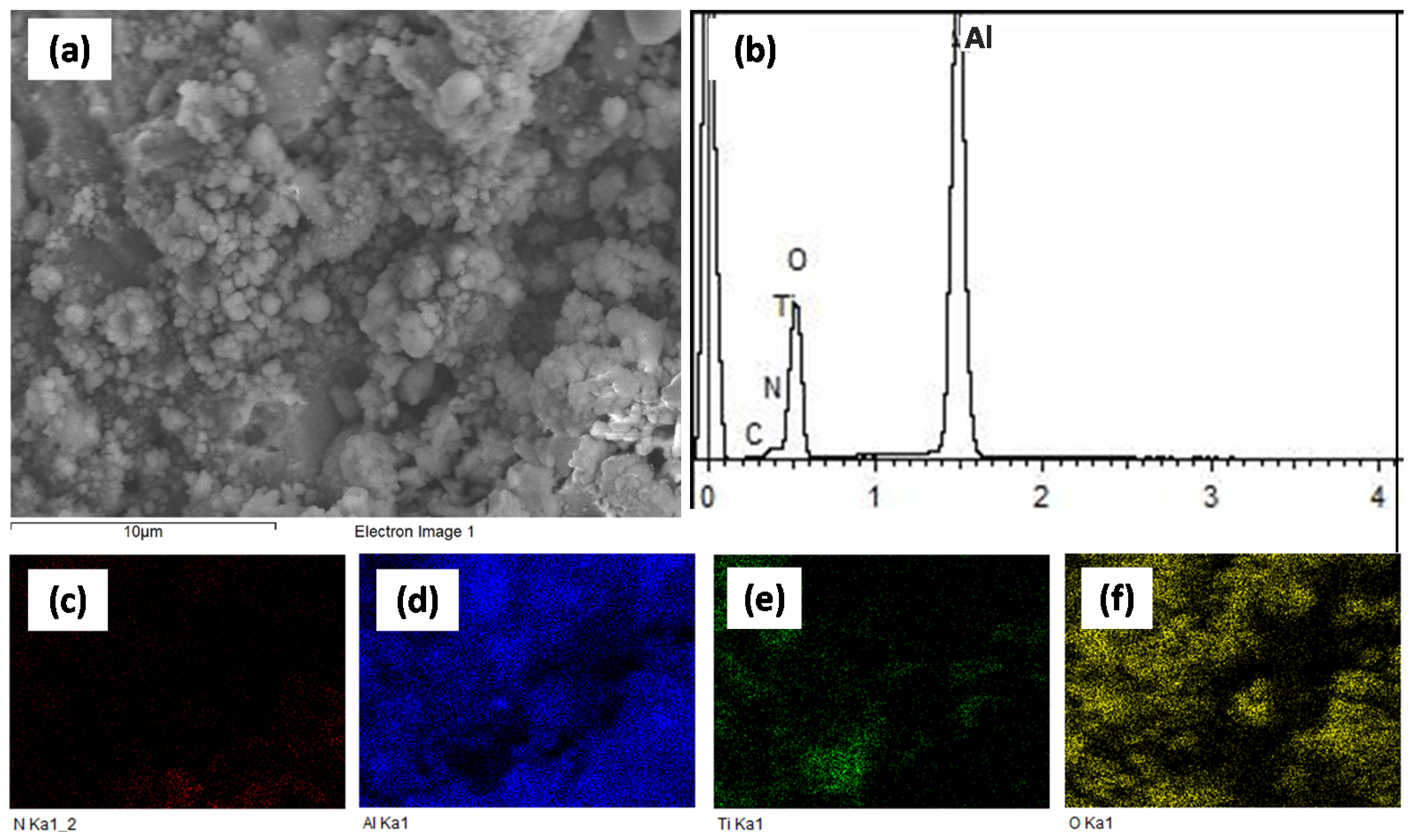
3.2.1. SEM Analysis
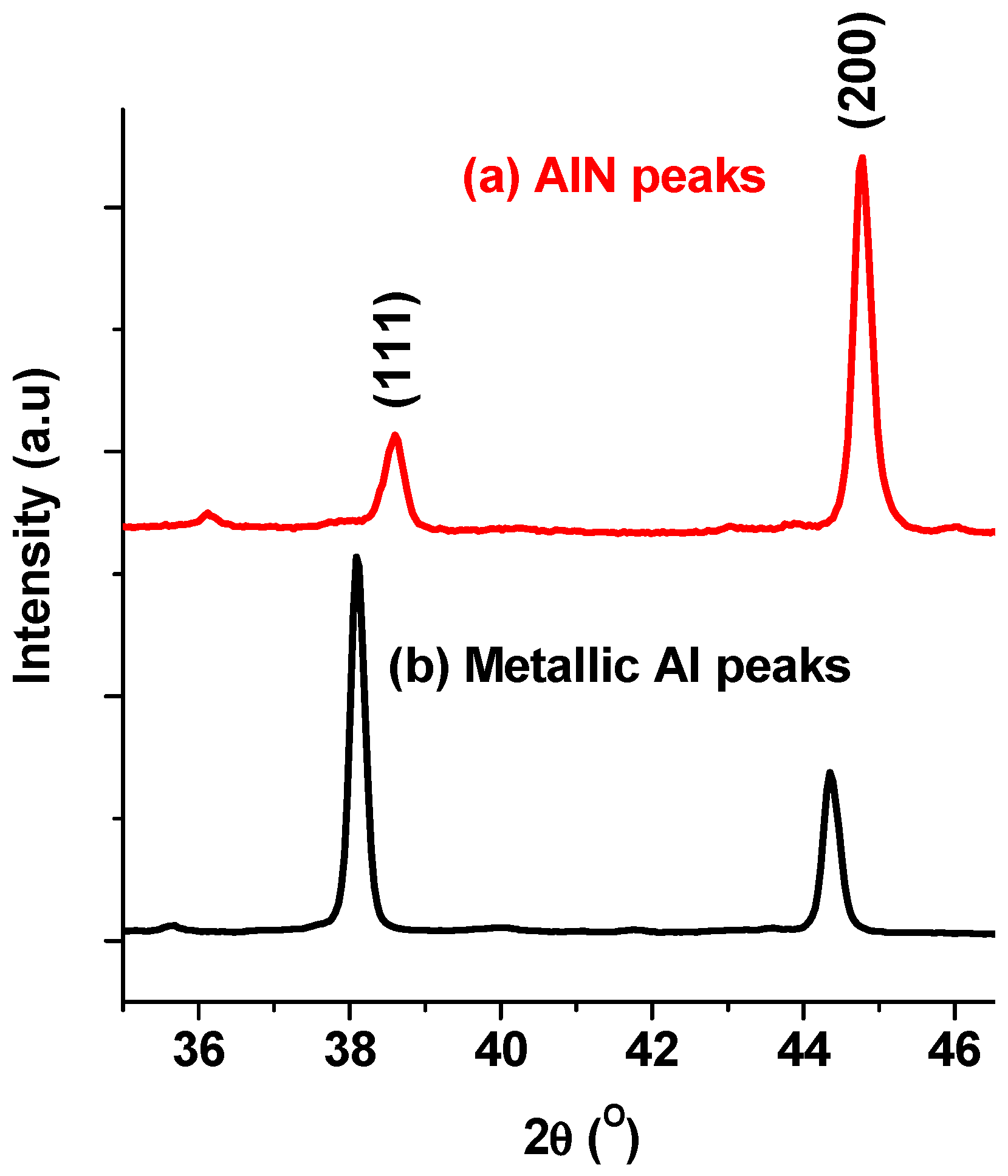
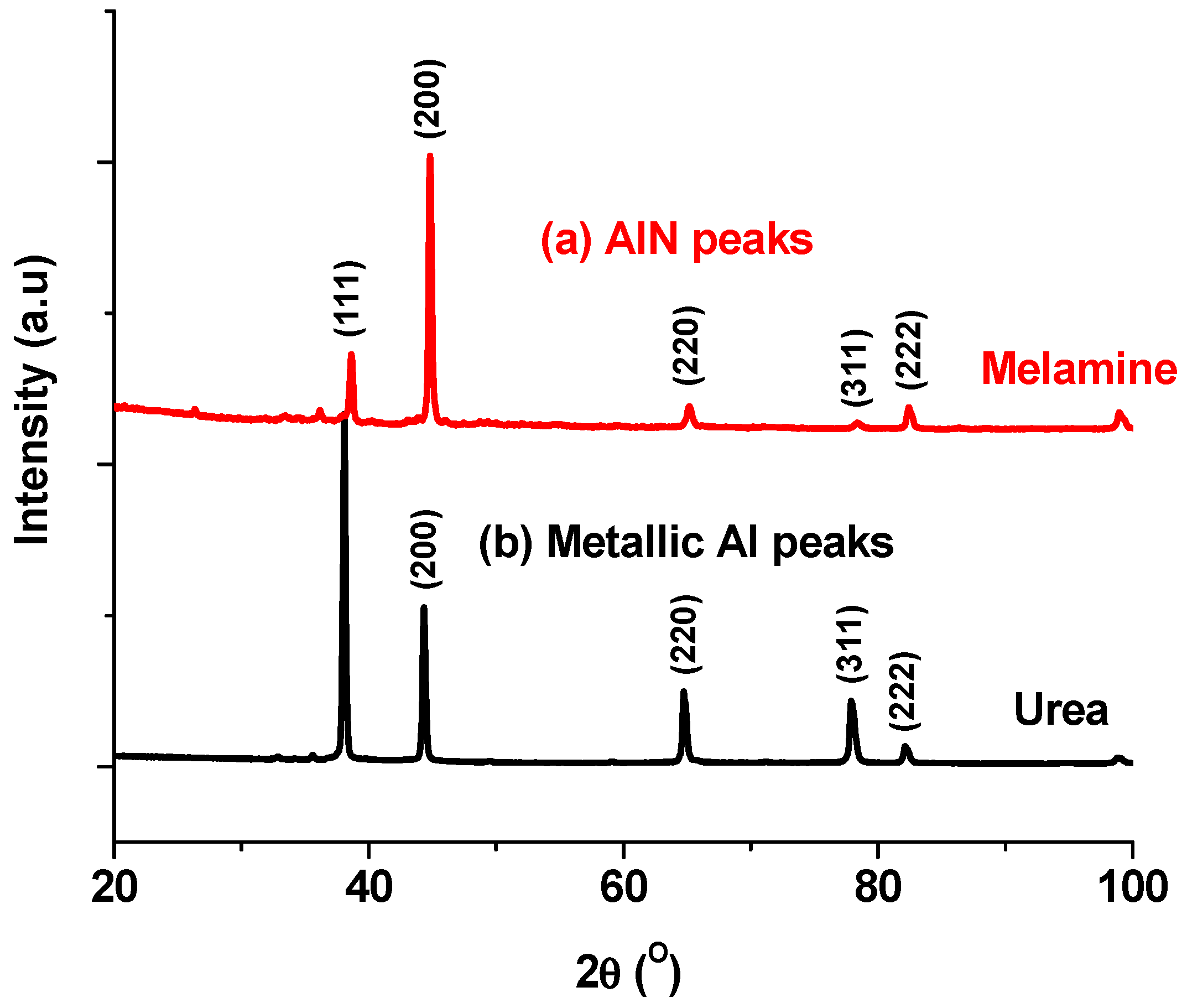
3.2.2. XRD Analysis
4. Discussion
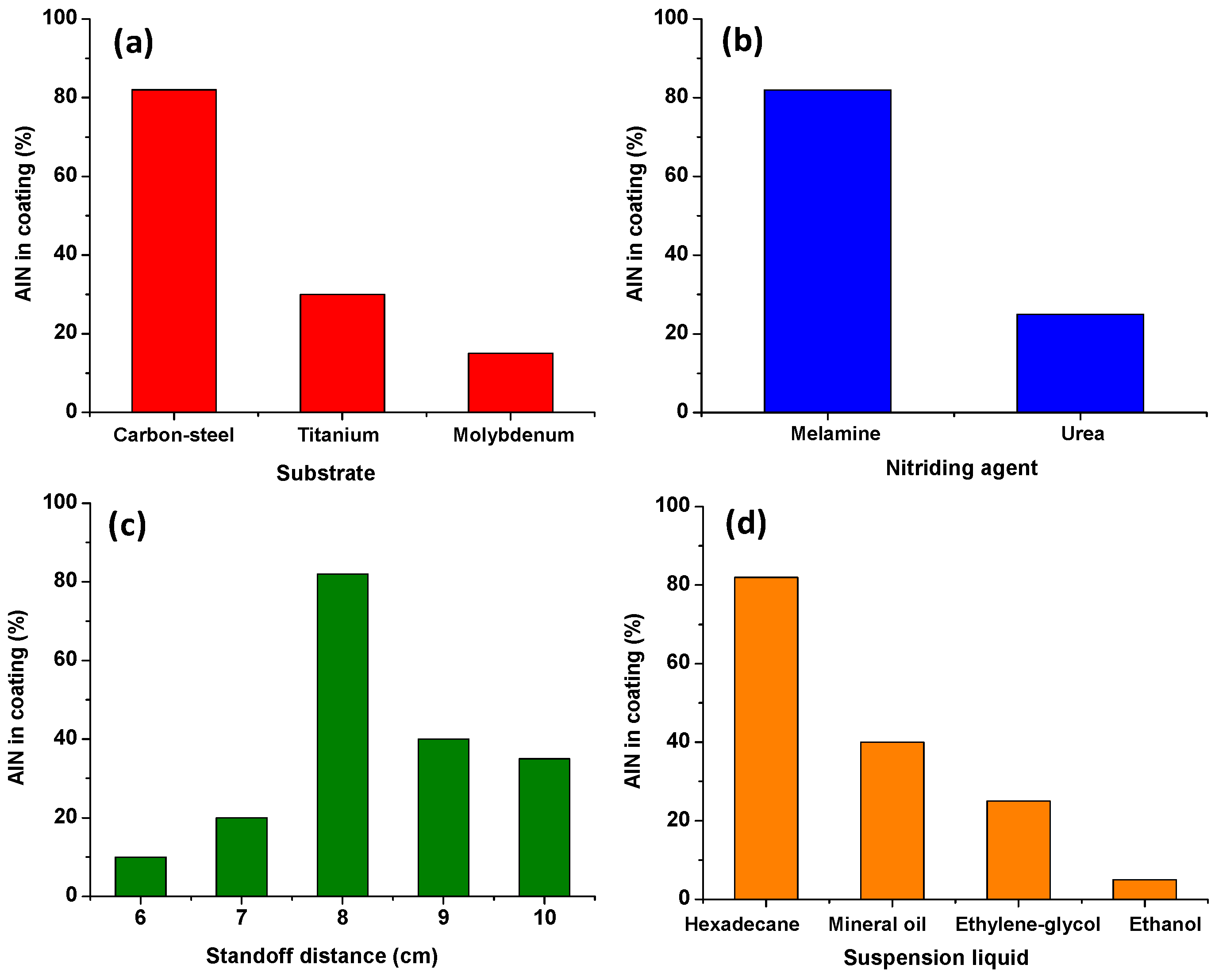
4.1. Effect of Nitriding Agent on AlN Formation
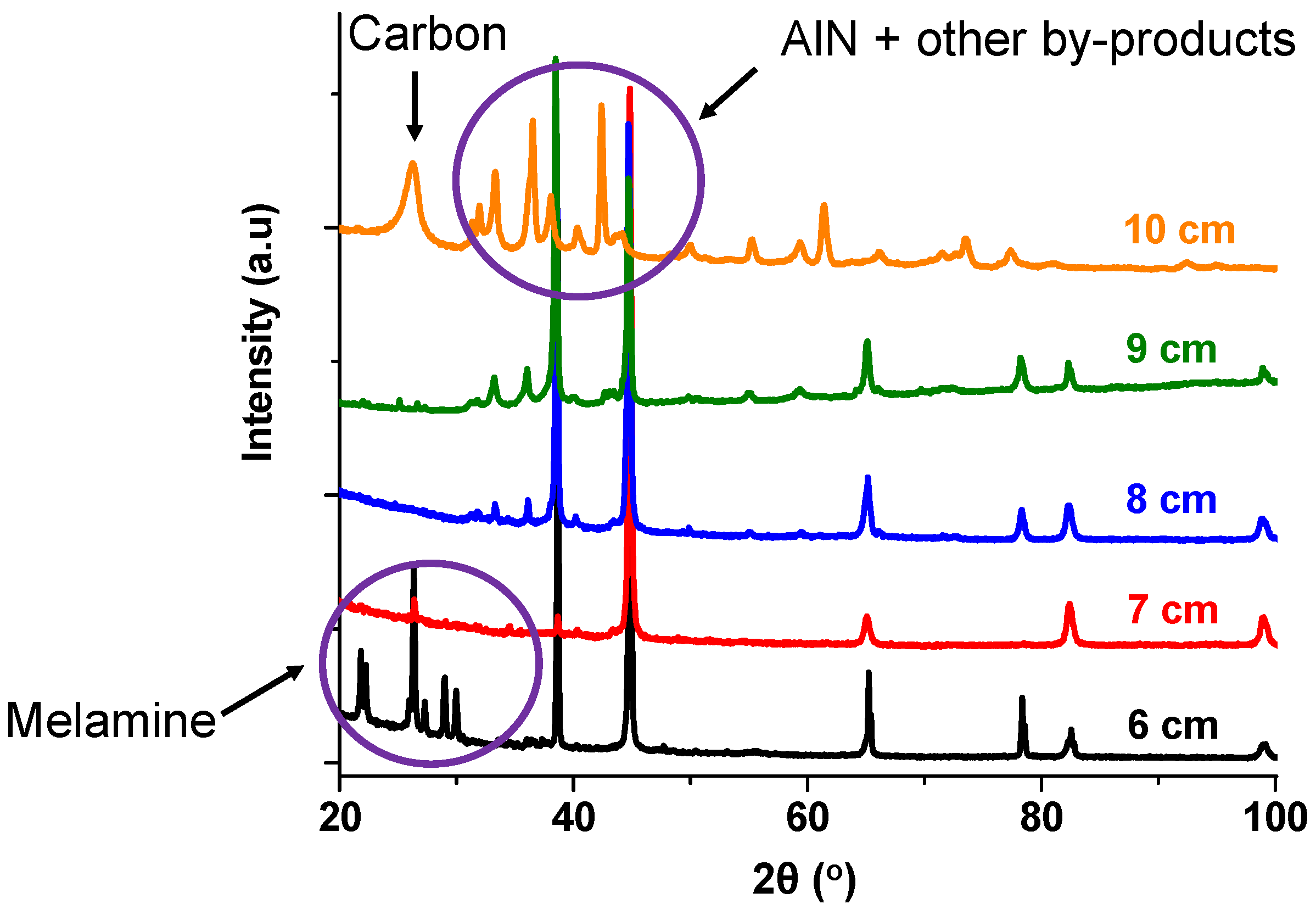
4.2. Effect of Standoff Distance on AlN Formation
- (i)
- Long standoff distance: where after the nitridation, AlN failed to stick to the substrate, which was too far because either the AlN decomposed before reaching the substrate, or the AlN condensed and reached the substrate after hardening.
- (ii)
- Short standoff distance: characterized by low residence time in the reactor because the materials did not have enough time to react and produce AlN.
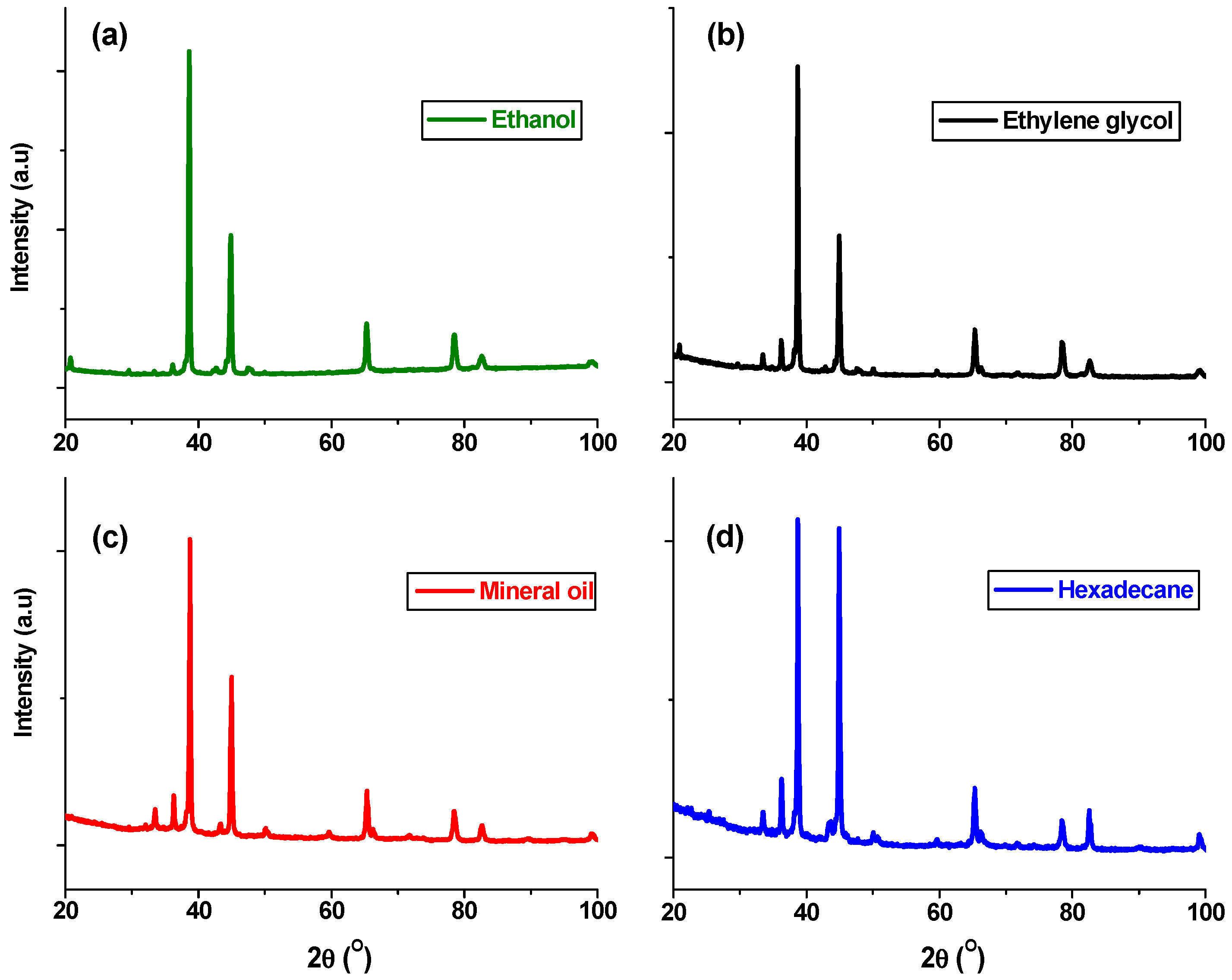
4.3. Effect of Suspension Liquids on AlN Formation
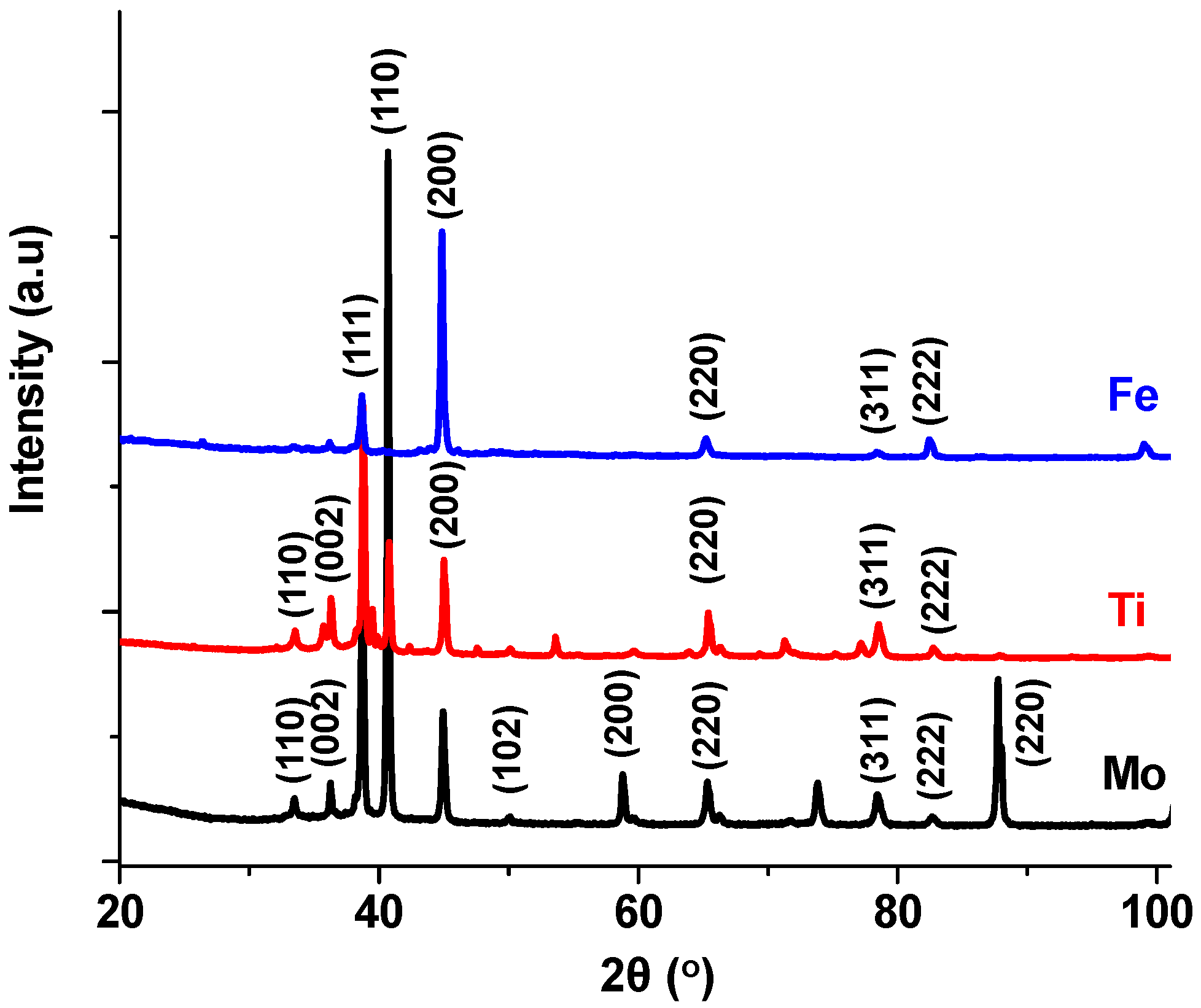
4.4. Effect of Substrate on AlN Formation
- (a)
- The surface of the Mo substrate was exposed and detectable by XRD analysis because very little of the coating was formed.
- (b)
- Ti reacted with Al to produce several TiAl alloys. In addition, the AlN produced on the Ti took after the hexagonal (hcp) crystal structure of the substrates.
- (c)
- Carbon steel had the highest amount of cubic AlN coating, which is typical of the cubic (fcc) crystal structure of carbon steel substrate at high temperature.
- (d)
- Prolonged deposition periods led to the substrates overheating, consequently resulting in less AlN deposition and enhancing surface carbon formation.
4.5. Materials Testing
4.5.1. Roughness and Thickness Measurements
4.5.2. The Wetting Test
4.5.3. Corrosion Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, G.; Foster, L.M. Aluminum nitride, a refractory for aluminum to 2000 °C. J. Am. Chem. Soc. 1959, 42, 53–59. [Google Scholar]
- Yan, M.; Fan, Z. Durability of materials in molten aluminum alloys. J. Mater. Sci. 2001, 36, 285–295. [Google Scholar] [CrossRef]
- Iqbal, A.; Mohd-Yasin, F. Reactive sputtering of aluminum nitride (002) thin films for piezoelectric applications: A review. Sensors 2018, 18, 1797. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.M.; Park, D.W. Preparation of AlN fine powder by thermal plasma processing. Thin Solid Films 1998, 316, 189–194. [Google Scholar] [CrossRef]
- Sung, M.-C.; Kuo, Y.-M.; Hsieh, L.-T.; Tsai, C.-H. Two-stage plasma nitridation approach for rapidly synthesizing aluminum nitride powders. J. Mater. Res. 2017, 32, 1279–1286. [Google Scholar] [CrossRef]
- Yaddanapudi, K. First-principles study of structural phase transformation and dynamical stability of cubic AlN semiconductors. AIP Adv. 2018, 8, 125006. [Google Scholar] [CrossRef] [Green Version]
- Kim, K. Plasma synthesis and characterization of nanocrystalline aluminum nitride particles by aluminum plasma jet discharge. J. Cryst. Growth 2005, 283, 540–546. [Google Scholar] [CrossRef]
- Shahien, M.; Yamada, M.; Yasui, T.; Fukumoto, M. Cubic aluminum nitride coating through atmospheric reactive plasma nitriding. J. Therm. Spray Technol. 2010, 19, 635–641. [Google Scholar] [CrossRef]
- Kudyakova, V.S.; Shishkin, R.A.; Elagin, A.A.; Baranov, M.V.; Beketov, A.R. Aluminium nitride cubic modifications synthesis methods and its features. Review. J. Eur. Ceram. Soc. 2017, 37, 1143–1156. [Google Scholar] [CrossRef]
- Jackson, T.B.; Virkar, A.V.; More, K.L.; Dinwiddie, R.B., Jr.; Cutler, R.A. High-thermal-conductivity aluminum nitride ceramics: The effect of thermodynamic, kinetic, and microstructural factors. J. Am. Ceram. Soc. 1997, 80, 1421–1435. [Google Scholar] [CrossRef]
- Da Cruz, A.C.; Munz, R.J. Review on the vapour-phase synthesis of aluminum nitride powder using thermal plasmas. KONA Powder Part. J. 1999, 17, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-H.; Choi, S.; Park, D.-W. Effects of NH3 flow rate on the thermal plasma synthesis of AlN nanoparticles. J. Korean Phys. Soc. 2013, 63, 1864–1870. [Google Scholar] [CrossRef]
- Choi, S.; Im, H.; Kim, J. Synthesis of AlN particles by chemical route for theral interface material. Adv. Mater. Lett. 2017, 8, 939–943. [Google Scholar]
- Ahn, J.B.; Kim, D.S.; Kim, Y.K.; Lee, J.G. Synthesis of AlN particles by microwave-assisted urea route. Appl. Mech. Mater. 2016, 851, 191–195. [Google Scholar] [CrossRef]
- Grigoriu, C.; Hirai, M.; Nishiura, K.; Jiang, W.; Yatsui, K. Synthesis of nanosized aluminum nitride powders by pulsed laser ablation. J. Am. Ceram. Soc. 2000, 83, 2631–2633. [Google Scholar] [CrossRef]
- Iwata, M.; Adachi, K.; Furukawa, S.; Amakawa, T. Synthesis of purified AlN nano powder by transferred type arc plasma. J. Phys. D Appl. Phys. 2004, 37, 1041–1047. [Google Scholar] [CrossRef]
- Yamada, M.; Yasui, T.; Fukumoto, M.; Takahashi, K. Nitridation of aluminum particles and formation process of aluminum nitride coatings by reactive RF plasma spraying. Thin Solid Films 2007, 515, 4166–4171. [Google Scholar] [CrossRef]
- Shahien, M.; Yamada, M.; Yasui, T.; Fukumoto, M. Controlling of nitriding process on reactive plasma spraying of Al particles. IOP Conf. Ser. Mater. Sci. Eng. 2011, 18, 202006. [Google Scholar] [CrossRef]
- Choudhary, R.K.; Mishra, S.C.; Mishra, P.; Limaye, P.K.; Singh, K. Mechanical and tribological properties of crystalline aluminum nitride coatings deposited on stainless steel by magnetron sputtering. J. Nucl. Mater. 2015, 466, 69–79. [Google Scholar] [CrossRef]
- Belkerk, B.E.; Bensalem, S.; Soussou, A.; Carette, M.; Al Brithen, H.; Djouadi, M.A.; Scudeller, Y. Substrate-dependent thermal conductivity of aluminum nitride thin-films processed at low temperature. Appl. Phys. Lett. 2014, 105, 221905. [Google Scholar] [CrossRef]
- Belkerk, B.E.; Soussou, A.; Carette, M.; Djouadi, M.A.; Scudeller, Y. Structural-dependent thermal conductivity of aluminium nitride produced by reactive direct current magnetron sputtering. Appl. Phys. Lett. 2012, 101, 1–5. [Google Scholar] [CrossRef]
- Shahien, M.; Yamada, M.; Yasui, T.; Fukumoto, M. Synthesis of cubic aluminum nitride coating from Al2O3 powder in reactive plasma spray process. Mater. Trans. 2013, 54, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Musa, I.; Qamhieh, N.; Said, K.; Mahmoud, S.T.; Alawadhi, H. Fabrication and characterization of aluminum nitride nanoparticles by RF magnetron sputtering and inert gas condensation technique. Coatings 2020, 10, 411. [Google Scholar] [CrossRef] [Green Version]
- Using, D.; Precursors, A.; Chloride, H. Aluminum nitride nanofilms by atomic layer deposition using alternative precursors hydrazinium chloride and triisobutylaluminum. Coatings 2020, 10, 195. [Google Scholar]
- Kim, H.H.; Lee, Y.S.; Chung, D.C.; Kim, B.J. Studies on preparation and characterization of aluminum nitride-coated carbon fibers and thermal conductivity of epoxy matrix composites. Coatings 2017, 7, 121. [Google Scholar] [CrossRef] [Green Version]
- Kolaklieva, L.; Chitanov, V.; Szekeres, A.; Antonova, K.; Terziyska, P.; Fogarassy, Z.; Petrik, P.; Mihailescu, I.N.; Duta, L. Pulsed laser deposition of aluminum nitride films: Correlation between mechanical, optical, and structural properties. Coatings 2019, 9, 195. [Google Scholar] [CrossRef] [Green Version]
- Metel, A.; Grigoriev, S.; Volosova, M.; Melnik, Y.; Mustafaev, E. Synthesis of aluminum nitride coatings assisted by fast argon atoms in a magnetron sputtering system with a separate input of argon and nitrogen. Surf. Coat. Technol. 2020, 398, 126078. [Google Scholar] [CrossRef]
- Gálvez, M.E.; Frei, A.; Meier, F.; Steinfeld, A. Production of AlN by carbothermal and methanothermal reduction of Al2O3 in a N2 flow using concentrated thermal radiation. Ind. Eng. Chem. Res. 2009, 48, 528–533. [Google Scholar] [CrossRef]
- Wu, N.C.; Tsai, M.S.; Wang, M.C.; Liu, H.S. Morphology and formation mechanism of aluminum nitride nanocrystals synthesized by chemical vapor deposition. J. Cryst. Growth 2000, 208, 189–196. [Google Scholar] [CrossRef]
- Jung, W.S.; Ahn, S.K. Synthesis of aluminium nitride by the reaction of aluminium sulfide with ammonia. Mater. Lett. 2000, 43, 53–56. [Google Scholar] [CrossRef]
- Chaundhuri, M.G. Feasibility study of synthesis of nanostructured aluminum nitride through sol-gel route. Int. J. Eng. Res. Appl. 2016, 6, 20–22. [Google Scholar]
- Li, P.; Xi, S.; Zhou, J. Phase transformation and gas-solid reaction of Al2O3 during high-energy ball milling in N2 atmosphere. Ceram. Int. 2009, 35, 247–251. [Google Scholar] [CrossRef]
- Rounaghi, S.A.; Kiani Rashid, A.R.; Eshghi, H.; Vahdati Khaki, J. Formation of nanocrystalline h-AlN during mechanochemical decomposition of melamine in the presence of metallic aluminum. J. Solid State Chem. 2012, 190, 8–11. [Google Scholar] [CrossRef]
- Li, C.H.; Kao, L.H.; Chen, M.J.; Wang, Y.F.; Tsai, C.H. Rapid preparation of aluminum nitride powders by using microwave plasma. J. Alloys Compd. 2012, 542, 78–84. [Google Scholar] [CrossRef]
- Li, L.; Hao, X.; Yu, N.; Cui, D.; Xu, X.; Jiang, M. Low-temperature solvent thermal synthesis of cubic AlN. J. Cryst. Growth 2003, 258, 268–271. [Google Scholar] [CrossRef]
- Kato, T.; Sugawara, K. Low-Temperature Synthesis of Aluminum Nitride by Addition of Ammonium Chloride. ACS Omega 2019, 4, 14714–14720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahien, M.; Yamada, M.; Yasui, T.; Fukumoto, M. In situ fabrication of AlN coating by reactive plasma spraying of Al/AlN powder. Coatings 2011, 1, 88–107. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.I.; Choi, S.C.; Kim, J.H.; Cho, W.S.; Hwang, K.T.; Han, K.S. Synthesis and characterization of high-purity aluminum nitride nanopowder by RF induction thermal plasma. Ceram. Int. 2014, 40, 8117–8123. [Google Scholar] [CrossRef]
- Ageorges, H.; Megy, S.; Chang, K.; Baronnet, J.M.; Williams, J.K.; Chapman, C. Synthesis of aluminum nitride in transferred arc plasma furnaces. Plasma Chem. Plasma Process. 1993, 13, 613–632. [Google Scholar] [CrossRef]
- Venkatramani, N. Industrial plasma torches and applications. Curr. Sci. 2002, 83, 254–262. [Google Scholar] [CrossRef]
- Shahien, M.; Yamada, M.; Fukumoto, M. Influence of Transient Liquid Phase Promoting Additives upon Reactive Plasma Spraying of AlN Coatings and Its Properties. Adv. Eng. Mater. 2018, 20, 1–14. [Google Scholar] [CrossRef]
- Qiu, Y.; Gao, L. Nitridation reaction of aluminum powder in flowing ammonia. J. Eur. Ceram. Soc. 2003, 23, 2015–2022. [Google Scholar] [CrossRef]
- Sun, J.F.; Wang, M.Z.; Zhao, Y.C.; Li, X.P.; Liang, B.Y. Synthesis of titanium nitride powders by reactive ball milling of titanium and urea. J. Alloys Compd. 2009, 482, 29–31. [Google Scholar] [CrossRef]
- Shatnawi, M.; Al-Mansi, W.; Arafa, I. Formation of Si-C-N ceramics from melamine-carbosilazane single source precursors. J. Solid State Chem. 2008, 181, 150–157. [Google Scholar] [CrossRef]
- Rounaghi, S.A.; Vanpoucke, D.E.P.; Eshghi, H.; Scudino, S.; Esmaeili, E.; Oswald, S.; Eckert, J. A combined experimental and theoretical investigation of the Al-Melamine reactive milling system: A mechanistic study towards AlN-based ceramics. J. Alloys Compd. 2017, 729, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Klaus Bretterbauer, C.S. Melamine derivatives—A review on synthesis and application. Curr. Org. Synth. 2012, 9, 342–356. [Google Scholar] [CrossRef]
- Rounaghi, S.A.; Eshghi, H.; Scudino, S.; Esmaeili, E.; Kiani-Rashid, A.R.; Eckert, J. Mechanochemical reaction of Al and melamine: A potential approach towards the: In situ synthesis of aluminum nitride-carbon nanotube nanocomposites. Phys. Chem. Chem. Phys. 2019, 21, 22121–22131. [Google Scholar] [CrossRef]
- Gitzhofer, F.; Bouyer, E. Suspension Plasma Spray. U.S. Patent 5,609,921, 26 August 1997. [Google Scholar]
- Yamada, M.; Nakamura, H.; Yasui, T.; Fukumoto, M.; Takahashi, K. Influence of substrate materials upon fabrication of aluminum nitride coatings by reactive RF plasma spraying. Mater. Trans. 2006, 47, 1671–1676. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.X.; Kang, L.; Wu, P.H.; Yang, S.Z.; Ji, Z.M. Fabrication of AlN thin films on different substrates at ambient temperature. Supercond. Sci. Technol. 2002, 15, 1781–1783. [Google Scholar] [CrossRef]
- Nogi, K.; Tomsia, A.; Eustathopoulos, N.; Mortensen, A.; Riman, R.; Milosevic, O.; Ohara, S.; Naito, M. Wettability of Solid by Liquid at High Temperature; Technical Report No. 2001MB037; Osaka University: Osaka, Japan, 2004. [Google Scholar]
- Sobczak, N.; Sobczak, J.; Asthana, R.; Purgert, R. The mystery of molten metal. China Foundry 2010, 7, 425–437. [Google Scholar]
- Fujii, H.; Nakae, H.; Okada, K. Interfacial reaction wetting in the boron nitride/molten aluminum system. Acta Metall. Mater. 1993, 41, 2963–2971. [Google Scholar] [CrossRef]
- Fujii, H.; Nakae, H. Equilibrium contact in the magnesium system oxide/aluminium. Acta Mater. 1996, 44, 3567–3573. [Google Scholar] [CrossRef]
- Mutale, C.T.; Weirauch, D.A.; Cramb, A.W. Determination of interfacial properties between AlN and aluminum beneath salt at high temperature. Mater. Sci. Eng. A 2008, 495, 60–64. [Google Scholar] [CrossRef]
- Cai, Y.; Coyle, T.W.; Azimi, G.; Mostaghimi, J. Superhydrophobic ceramic coatings by solution precursor plasma spray. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Barandehfard, F.; Aluha, J.; Hekmat-Ardakan, A.; Gitzhofer, F. Improving corrosion resistance of aluminosilicate refractories towards molten Al-Mg alloy using non-wetting additives: A short review. Materials 2020, 13, 4078. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Hild, F. Surface Energy of Plastics. Available online: https://www.tstar.com/blog/bid/33845/surface-energy-of-plastics (accessed on 14 December 2020).
- Majeed, M.H. Static contact angle and large water droplet thickness measurements with the change of water temperature. Coll. Eng. J. 2014, 1717, 114–128. [Google Scholar]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Fujishima, A. Superhydrophobic surfaces developed by mimicking hierarchical surface morphology of lotus leaf. Molecules 2014, 19, 4256–4283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rounaghi, S.A.; Eshghi, H.; Scudino, S.; Vyalikh, A.; Vanpoucke, D.E.P.; Gruner, W.; Oswald, S.; Kiani Rashid, A.R.; Samadi Khoshkhoo, M.; Scheler, U.; et al. Mechanochemical route to the synthesis of nanostructured Aluminium nitride. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mayer, I.; Schneider, S. ICDD Grant-in-Aid. Powder Diffr. J. 1987, 2. Available online: https://www.icdd.com/powder-diffraction-journal-volume-2/ (accessed on 31 March 2021).
- Rizolli, C.; Salamakha, S.P.; Sologub, O.L.; Bocelli, J.G. X-ray investigation of the Al–B–N ternary system: Isothermal section at 1500 °C: Crystal structure of the Al0. 185B6CN0. 256 compound. J. Alloys Compd. 2002, 343, 135–141. [Google Scholar] [CrossRef]
- Von Stackelberg, M.; Spiess, K.F. Die struktur des aluminiumcarbonitrids Al5C3N. Z. für Phys. Chem. 1935, 175, 140. Available online: https://www.degruyter.com/document/doi/10.1515/zpch-1935-17509/html (accessed on 31 March 2021). [CrossRef]
- Vollstadt, H.; Ito, E.; Akaishi, M.; Akimoto, S.; Fukunaga, O. High pressure synthesis of rocksalt type of AlN. Proc. Jpn. Acad. Ser. B 1990, 62, 7. Available online: https://www.jstage.jst.go.jp/article/pjab1977/66/1/66_1_7/_pdf (accessed on 31 March 2021). [CrossRef] [Green Version]
- Haglund, J.; Fernandez, F.; Grimvall, G.; Korling, M. Theory of bonding in transition-metal carbides and nitrides. Phys. Rev. B Condens. Matter Mater. Phys. 1993, 48, 11685. Available online: https://journals.aps.org/prb/abstract/10.1103/PhysRevB.48.11685 (accessed on 31 March 2021). [CrossRef] [PubMed]
- Huang, T.C.; Parrish, W.; Masciocchi, Ν.; Wang, P.W. Derivation of d-values from digitized X-ray and synchrotron diffraction data. Adv. X Ray Anal. 1990, 33, 295. [Google Scholar] [CrossRef]
- Brauer, G. Crystal structure of intermetallic alloys of aluminium with titanium, zirconium, thorium, niobium and tantalum. Naturewissenschaften 1938, 26, 14. [Google Scholar] [CrossRef]
- Zoriasatin, S.; Azarkharman, F.; Sebt, S.A.; Akhavan, M. Magnetic anisotropies in FeCo fine particles. J. Magn. Magn. Mater. 2006, 300, 525–531. [Google Scholar] [CrossRef]
- Elagin, A.A.; Beketov, A.R.; Baranov, M.V.; Shishkin, R.A. Aluminum nitride. Preparation methods (Review). Refract. Ind. Ceram. 2013, 53, 395–403. [Google Scholar] [CrossRef]
- Zhao, H.; Lei, M.; Chen, X.; Tang, W. Facile route to metal nitrides through melamine and metal oxides. J. Mater. Chem. 2006, 16, 4407–4412. [Google Scholar] [CrossRef]
- Engineering ToolBox. Thermal Conductivity of Metals, Metallic Elements and Alloys. 2005. Available online: https://www.engineeringtoolbox.com/thermal-conductivity-metals-d_858.html (accessed on 31 March 2021).
- Yang, Y.S.; Cho, T.P. Effect of annealing temperature on the water contact angle of PVD hard coatings. Materials 2013, 6, 3373–3386. [Google Scholar] [CrossRef]
- Engineering ToolBox. Coefficients of Linear Thermal Expansion. 2003. Available online: https://www.engineeringtoolbox.com/linear-expansion-coefficients-d_95.html (accessed on 31 March 2021).








| Properties | Values |
|---|---|
| Thermal expansion coefficient | α = 4.3 × 10−6 K−1 |
| Thermal conductivity | k = 319 W·m−1·K−1 |
| Crystalline structure | Hexagonal or cubic |
| Hardness | 1400 Hv |
| Electrical resistivity | 1013 Ω·cm |
| Band gap | 6.2 eV |
| Method | Precursors | Coating Substrate | Reference |
|---|---|---|---|
| RF plasma spray | Al powder + N2 | stainless steel, quartz plate | [17] |
| DC plasma spray | Al powder + NH4Cl | mild steel (SS400) | [18] |
| Atmospheric plasma spraying (APS) | Al powder + N2 + H2 | soft steel (SS400) | [8] |
| Magnetron sputtering | Al-disc + Ar + N2 | stainless steel (SS) 304L | [19] |
| LT reactive magnetron sputtering | Al powder + Ar + N2 | crystal Si, amorphous SiN | [20] |
| Reactive DC magnetron sputtering | Al powder + Ar + N2 | crystal Si, amorphous SiN | [21] |
| Reactive plasma spray | Al2O3 + N2 | mild steel (SS400) | [22] |
| RF magnetron sputtering | AlN + Ar | glass, mica, and silicon | [23] |
| Atomic layer deposition | Al(C4H9)3 + N2H5Cl + C12H27Al | silicon | [24] |
| Wet-thermal treatment (WTT) | NH3 + N2 + AlCl3 | carbon fiber | [25] |
| Pulsed laser deposition | AlN + N2 | Si (100) | [26] |
| Magnetron sputtering system | Al + N2 | silicon nitride | [27] |
| Method | Precursors | Reference |
|---|---|---|
| Carbothermal reduction | Al2O3 + N2 + C/CH4 | [28] |
| CVD | AlCl3 + NH3 + N2 | [29] |
| Reaction 500–1000 °C for 5 h | Al2S3 + NH3 | [30] |
| Sol-gel | C9H21O3Al + dextrose | [31] |
| Mechanochemical | Al2O3 powder + N2 | [32] |
| Mechanochemical | Al powder + Melamine | [33] |
| Transferred Arc-plasma | Al powder + N2 + NH3 | [16] |
| Microwave plasma | Al powder + NH4Cl + N2 + H2 | [34] |
| Solvent thermal synthesis | AlCl3 + NaN3 | [35] |
| Low-temperature synthesis | Al + NH4Cl | [36] |
| Reactive plasma spray | Al + AlN powders | [37] |
| Process Condition | Parameters | Value |
|---|---|---|
| Plasma system | Power system | 40 kW |
| Plasma torch | Tekna PL-50 (3.2MHz) | |
| Plasma gas flow rate (SLPM) | Sheath gas | Ar: 52, H2: 5.5, N2: 40 |
| Central gas | Ar: 22 | |
| Atomization gas | Ar: 10 | |
| Reactor pressure | Initial pressure | Below 2 kPa |
| Working Pressure | 16 kPa | |
| Suspension flow rate | Flow | 2.0 mL·min−1 |
| Standoff distance | Distance | 8.0 cm |
| Substrate | Ti, Mo, Carbon steel | 12.7 mm × 2.0 mm |
Suspension composition:
| Suspension liquid: Ethanol, Ethylene glycol, Hexadecane, light mineral oil | 100 mL |
| Precursor concentration | 5, 10, 20 g in 100 mL | |
| Al powder | 1.0 g | |
| Melamine | 9.0 g | |
| Urea | 13 g | |
| Concentration (precursor: liquid) | e.g., 10 g: 100 mL | |
| Number of loops | Deposition cycles | 200 |
| Substrates | Elements (Atomic-%) | ||
|---|---|---|---|
| Al | N | O | |
| Carbon steel | 41 | 35 | 24 |
| Titanium | 55 | 30 | 15 |
| Molybdenum | 50 | 22 | 28 |
| Synthesis Parameters | Phase Concentration in the Coatings (%) | ||||||
|---|---|---|---|---|---|---|---|
| Cubic-AlN | hcp-AlN | Al | Al4C3 | Others | |||
| Substrates | Carbon steel | 80 | 2 | 10 | 4.5 | 3.5% amorphous | |
| Ti | 17 | 13 | 50 | - | 18% Ti3Al | 2% TiAl3 | |
| Mo | 15 | - | 62 | - | 23% Mo | ||
| Suspension liquids | Ethylene glycol | 13 | 12 | 70 | - | 1% Al2O3 | 4% Fe5C2 |
| Ethanol | - | 5 | 85 | 10% carbon | - | ||
| Hexadecane | 80 | 2 | 10 | 4.5 | 3.5% amorphous | ||
| Light mineral oil | 22 | 16 | 60 | - | 2% FeN | ||
| Standoff distance | 6 cm | 10 | - | 28 | - | 60% Melamine | 2% amorphous |
| 7 cm | 20 | - | 5 | - | 5% Carbon | 70% Fe | |
| 8 cm | 80 | 2 | 10 | 4.5 | 3.5% amorphous | ||
| 9 cm | 27 | 15 | 46 | 8 | 2% Fe | 2% Fe3C | |
| 10 cm | - | 35 | 2 | 15 | 40% Carbon | 8% Fe3O4 | |
| Nitriding agent | Urea | 25 | - | 65 | - | 10% TiAl | - |
| Melamine | 80 | 2 | 10 | 4.5 | 3.5% amorphous | ||
| Substrates | Ra Values (µm) | Coating Thickness (µm) | |
|---|---|---|---|
| Before Coating | After Coating | ||
| Carbon steel | 0.543 ± 0.02 | 2.703 ± 0.4 | ~20–40 |
| Titanium | 0.388 ± 0.03 | 2.215 ± 0.3 | ~20–30 |
| Molybdenum | 0.515 ± 0.03 | 4.034 ± 0.6 | ~15–20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barandehfard, F.; Aluha, J.; Gitzhofer, F. Synthesis of Cubic Aluminum Nitride (AlN) Coatings through Suspension Plasma Spray (SPS) Technology. Coatings 2021, 11, 500. https://doi.org/10.3390/coatings11050500
Barandehfard F, Aluha J, Gitzhofer F. Synthesis of Cubic Aluminum Nitride (AlN) Coatings through Suspension Plasma Spray (SPS) Technology. Coatings. 2021; 11(5):500. https://doi.org/10.3390/coatings11050500
Chicago/Turabian StyleBarandehfard, Faranak, James Aluha, and François Gitzhofer. 2021. "Synthesis of Cubic Aluminum Nitride (AlN) Coatings through Suspension Plasma Spray (SPS) Technology" Coatings 11, no. 5: 500. https://doi.org/10.3390/coatings11050500





