Layer-by-Layer Self-Assembly Composite Coatings for Improved Corrosion and Wear Resistance of Mg Alloy for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
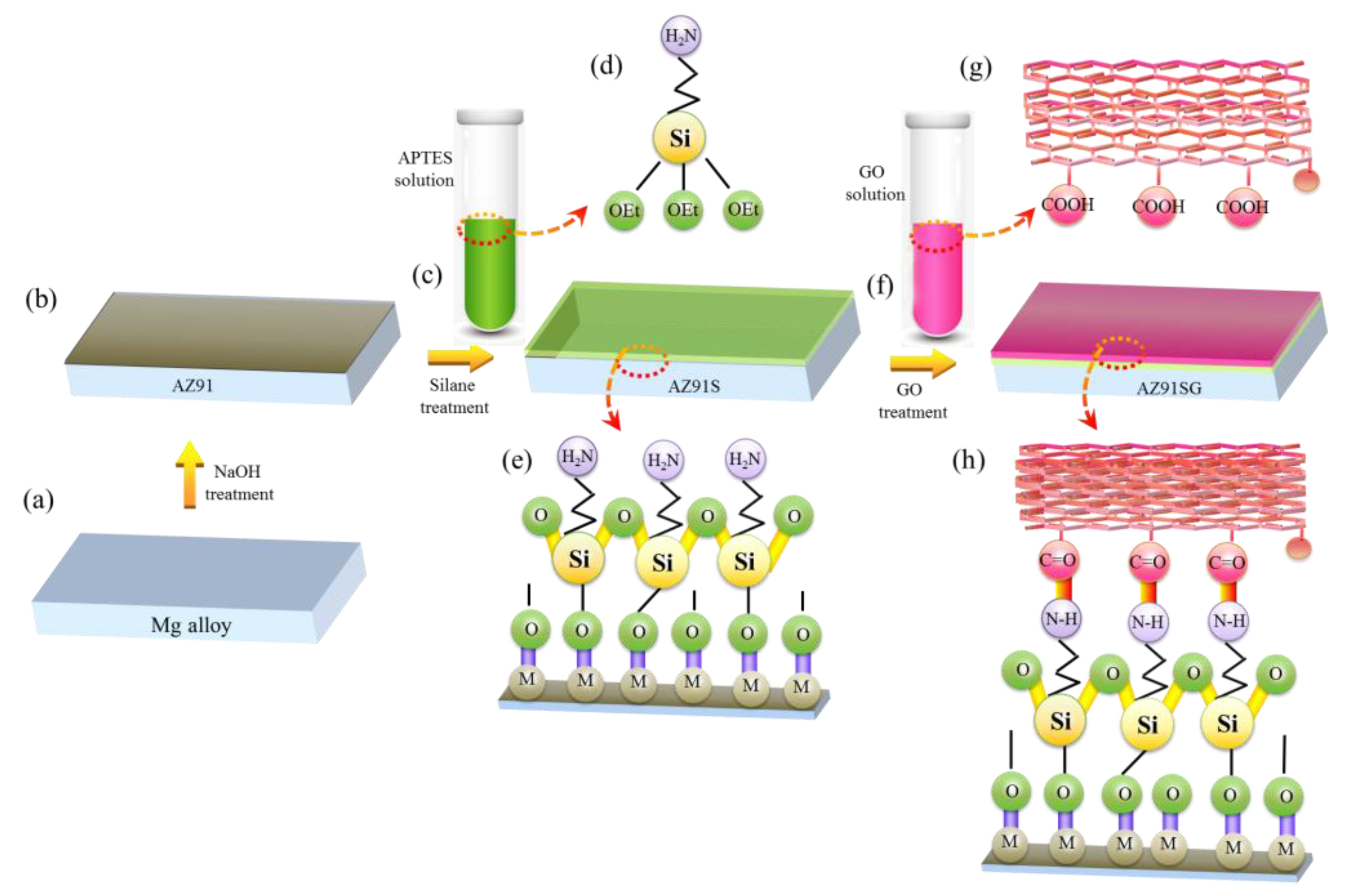
2.2. Substrate Treatment
2.2.1. Treatment with NaOH
2.2.2. Treatment with Silane
2.2.3. Treatment with GO
2.3. Characterization of Layers
2.4. Biodegradation Tests
2.4.1. Immersion Tests
2.4.2. H2 Release, pH Changes, and Mg Ion Measurements
2.5. Wear Tests
2.6. Cytocompatibility
2.7. Statistical Analysis
3. Results and Discussion
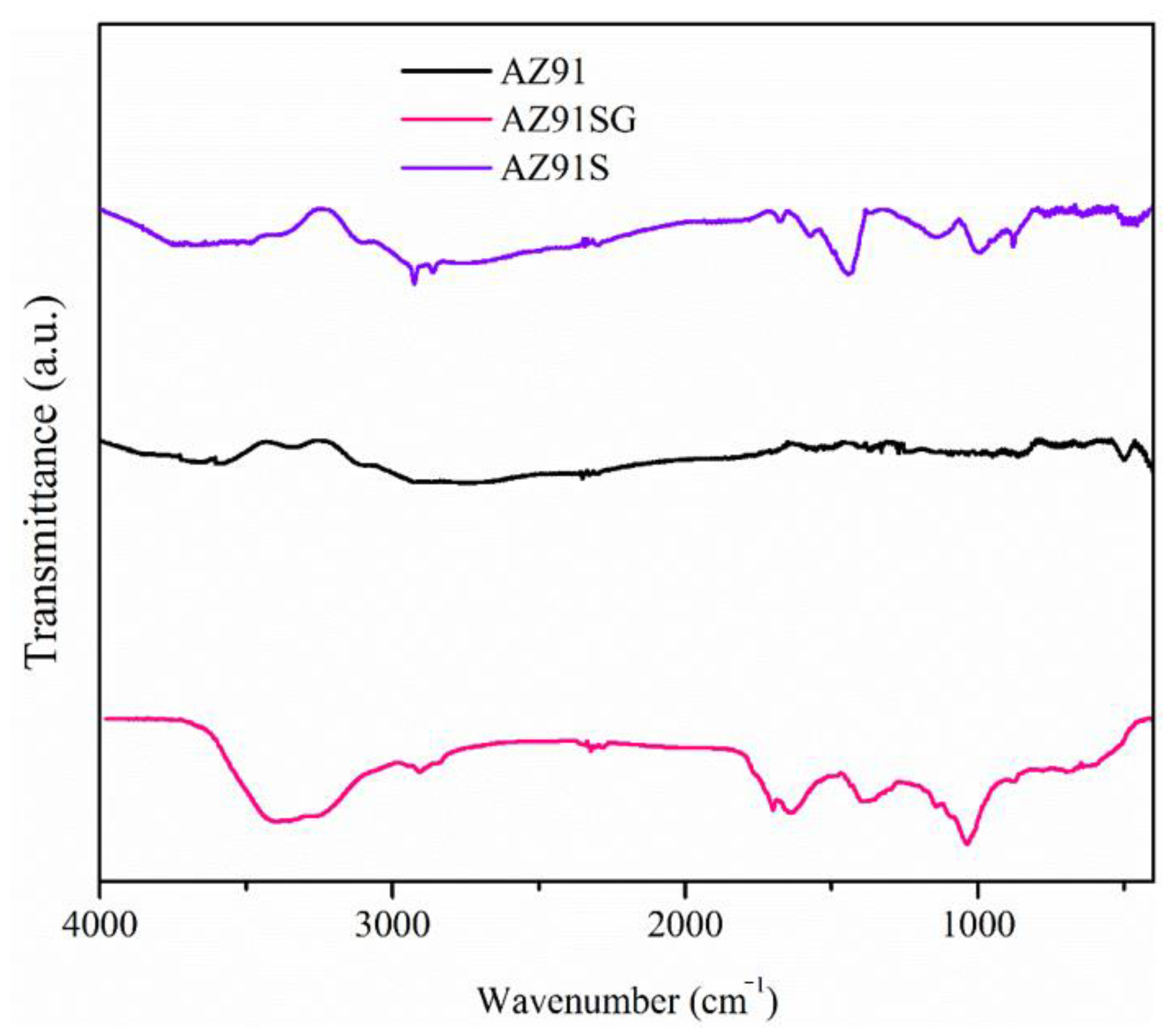
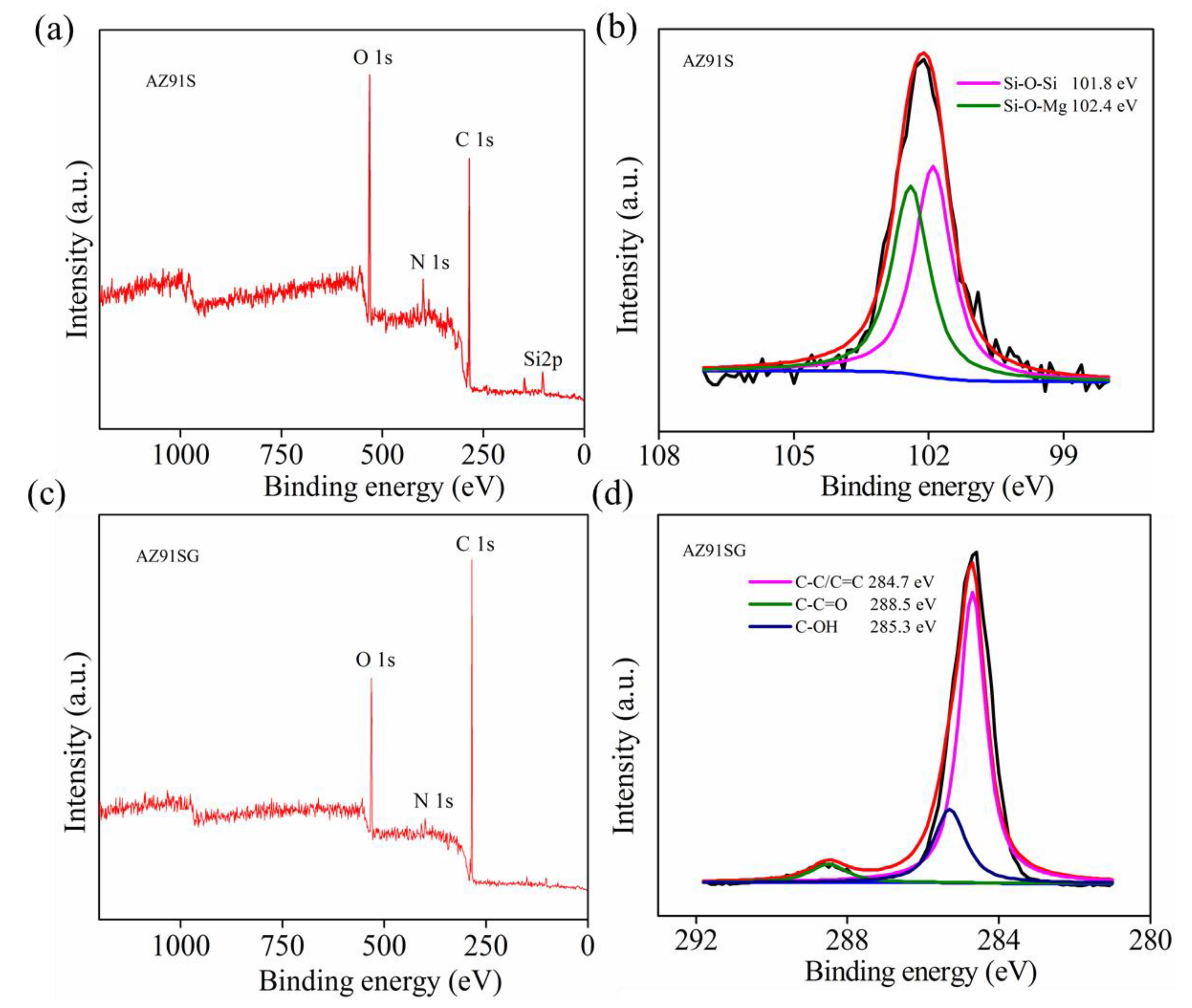
3.1. Surface Characteristics
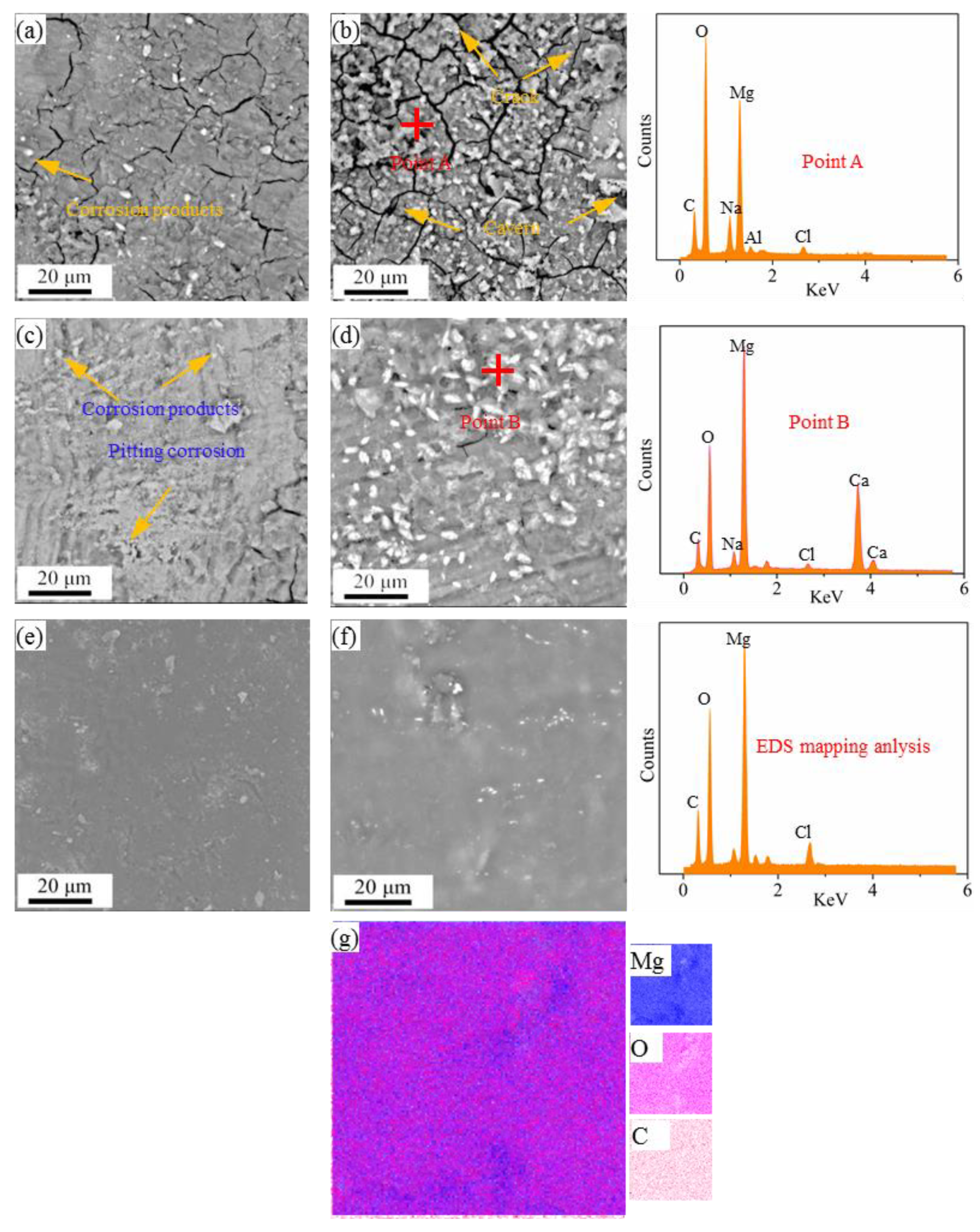
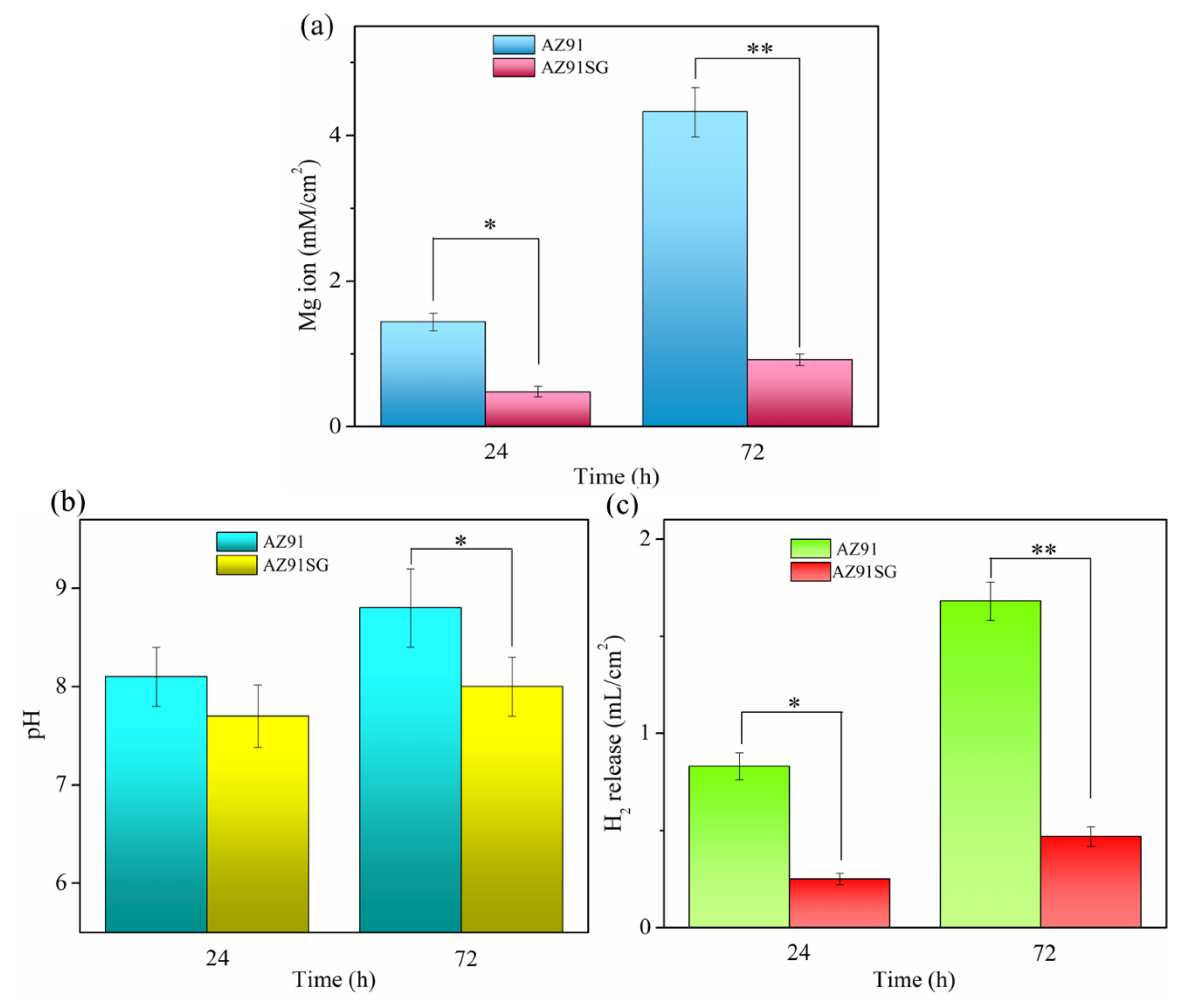
3.2. Corrosion Performance
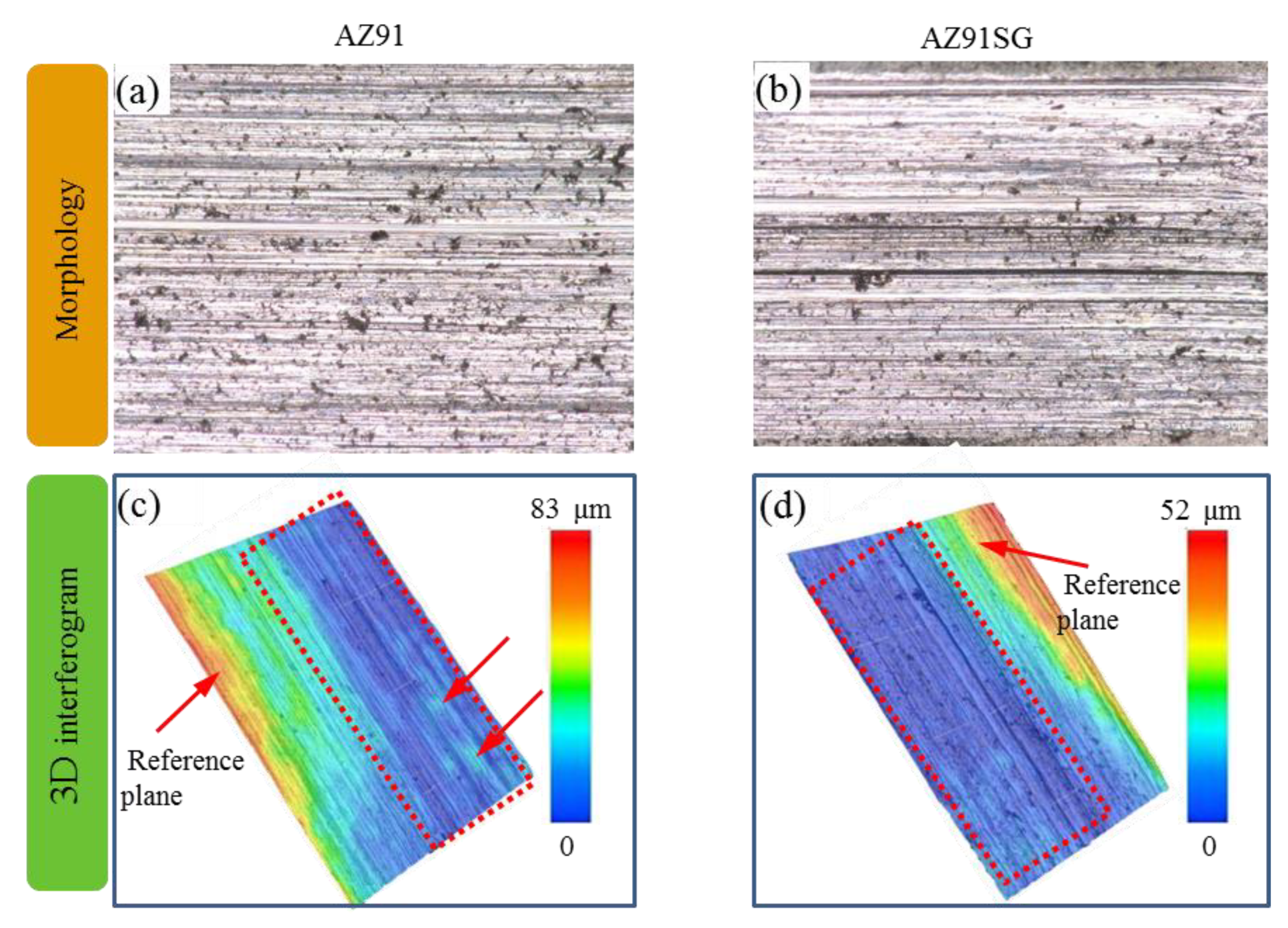
3.3. Wear Resistance
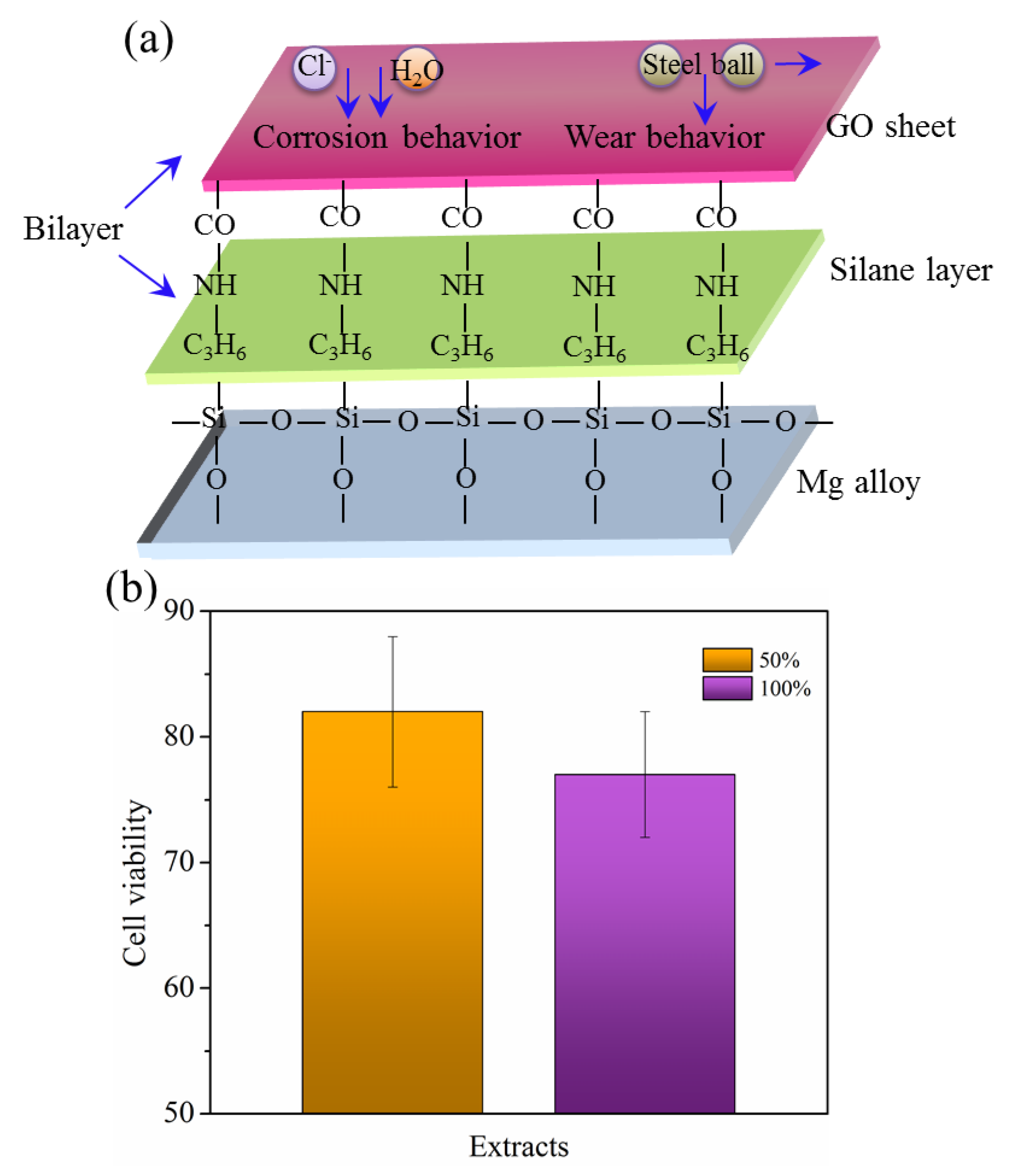
3.4. Protective Mechanism and Biomedical Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef]
- Xiang, D.; Li, S.; Yi, L.H.; Liu, T.F.; Liu, J.; Yang, C.M.; Zhou, L.H. In Situ Formation of Intermetallic Compound Bonding by Laser Cladding Al2O3 to Enhance the Corrosion Resistance of AZ31 Magnesium Alloy. Lasers Eng. 2020, 45, 309–323. [Google Scholar]
- Li, S.; Yi, L.; Liu, T.; Ji, B.; Yang, C.; Zhou, L. Al2O3 eliminating defects in the Al coating by laser cladding to improve corrosion and wear resistance of Mg alloy. Mater. Corros. 2019, 71, 980–991. [Google Scholar] [CrossRef]
- Özarslan, S.; Şevik, H.; Sorar, İ. Microstructure, mechanical and corrosion properties of novel Mg-Sn-Ce alloys produced by high pressure die casting. Mater. Sci. Eng. C 2019, 105, 1100641–1100648. [Google Scholar] [CrossRef]
- Liu, J.; Bian, D.; Zheng, Y.; Chu, X.; Lin, Y.; Wang, M.; Lin, Z.; Li, M.; Zhang, Y.; Guan, S. Comparative in v itro study on binary Mg-RE (Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu) alloy systems. Acta Biomater. 2020, 102, 508–528. [Google Scholar] [CrossRef]
- El-Kamel, R.S.; Ghoneim, A.A.; Fekry, A.M. Electrochemical, biodegradation and cytotoxicity of graphene oxide nanoparti-cles/polythreonine as a novel nano-coating on AZ91E Mg alloy staple in gastrectomy surgery. Mater. Sci. Eng. C 2019, 103, 109780–109788. [Google Scholar] [CrossRef] [PubMed]
- Elbashar, Y. Surface Treatment of Aluminium 6061-T6 Using Multi-shot Laser Plasma Shock Processing (LPSP) Without Confinement. Lasers Eng. 2019, 43, 13–20. [Google Scholar]
- Xia, D.; Liu, Y.; Wang, S.; Zeng, R.-C.; Liu, Y.; Zheng, Y.; Zhou, Y. In vitro and in vivo investigation on biodegradable Mg-Li-Ca alloys for bone implant application. Sci. China Mater. 2018, 62, 256–272. [Google Scholar] [CrossRef]
- Li, J.N.; Shi, C.W.; Wang, Z.; Zhang, B.-L. Surface Modification of a Titanium Alloy with a Fe3Al/Ti3Al Matrix Laser Clad Composite Coating. Lasers Eng. 2019, 43, 35–46. [Google Scholar]
- Singh, C.; Tiwari, S.; Singh, R. Exploring environment friendly nickel electrodeposition on AZ91 magnesium alloy: Effect of prior surface treatments and temperature of the bath on corrosion behaviour. Corros. Sci. 2019, 151, 1–19. [Google Scholar] [CrossRef]
- Wang, B.; Xu, D.; Sun, J.; Han, E.-H. Effect of grain structure on the stress corrosion cracking (SCC) behavior of an as-extruded Mg-Zn-Zr alloy. Corros. Sci. 2019, 157, 347–356. [Google Scholar] [CrossRef]
- Li, Y.; Yan, J.; Zhou, W.; Xiong, P.; Wang, P.; Yuan, W.; Zheng, Y.; Cheng, Y. In vitro degradation and biocompatibility evaluation of typical biodegradable metals (Mg/Zn/Fe) for the application of tracheobronchial stenosis. Bioact. Mater. 2019, 4, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Kunjukunju, S.; Roy, A.; Ramanathan, M.; Lee, B.; Candiello, J.E.; Kumta, P.N. A layer-by-layer approach to natural poly-mer-derived bioactive coatings on magnesium alloys. Acta Biomater. 2013, 9, 8690–8703. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, X.; Liu, X.; Xie, H.; Li, J. Effects of Al content on the corrosion properties of Mg–4Y–xAl alloys. Mater. Corros. 2020, 71, 1216–1225. [Google Scholar] [CrossRef]
- Soltan, A.; Dargusch, M.S.; Shi, Z.; Gerrard, D.; Al Shabibi, S.; Kuo, Y.; Atrens, A. Corrosion of Mg alloys EV31A, WE43B, and ZE41A in chloride- and sulfate-containing solutions saturated with magnesium hydroxide. Mater. Corros. 2020, 71, 956–979. [Google Scholar] [CrossRef]
- Li, G.; Su, J.; Zhao, X.; Chen, S.; Wu, R.; Wang, G. Effects of the addition of Na2SnO3 to NaCl electrolytes on Mg-14Li-3Al-1Gd electrode electrochemical behavior. Mater. Corros. 2019, 71, 564–570. [Google Scholar] [CrossRef]
- Kang, Y.; Du, B.; Li, Y.; Wang, B.; Sheng, L.; Shao, L.; Zheng, Y.; Xi, T. Optimizing mechanical property and cytocompatibility of the biodegradable Mg-Zn-Y-Nd alloy by hot extrusion and heat treatment. J. Mater. Sci. Technol. 2019, 35, 6–18. [Google Scholar] [CrossRef]
- Agarwal, S.; Morshed, M.; Labour, M.-N.; Hoey, D.; Duffy, B.; Curtin, J.; Jaiswal, S. Enhanced corrosion protection and biocompatibility of a PLGA–silane coating on AZ31 Mg alloy for orthopaedic applications. RSC Adv. 2016, 6, 113871–113883. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Zhao, H.; Tan, B.; Wang, L. Enhanced anticorrosion performance of copper by novel N-doped carbon dots. Corros. Sci. 2019, 161, 108193. [Google Scholar] [CrossRef]
- Qiang, Y.J.; Guo, L.; Lan, X.J. Fabrication of environmentally friendly Losartan potassium film for corrosion inhibition of mild steel in HCl medium. Chem. Eng. J. 2021, 406, 126863–126876. [Google Scholar] [CrossRef]
- Ma, L.; Qiang, Y.J.; Zhao, W.J. Designing novel organic inhibitor loaded MgAl-LDHs nanocontainer for enhanced corrosion resistance. Chem. Eng. J. 2021, 408, 127367–127379. [Google Scholar] [CrossRef]
- Thanigaivelan, R.; Arunachalam, R.; Nithish, A.; Venkatesh, S.; Naveenkumar, P.; Selvaganapathy, S.; Aravind, A.S. Optimi-zation of Laser and Electrochemical Process Parameters for Surface Modification of Hardness and Hydrophobicity on 316L Steel. Lasers Eng. 2020, 45, 69–84. [Google Scholar]
- Tian, Y.; Zhang, Z.; Li, J.N.; Huo, Y.S.; Su, M.L. Surface Modification of TA7 Titanium Alloy With Laser Clad La2O3 Amorphous Coatings. Lasers Eng. 2019, 44, 331–339. [Google Scholar]
- Shen, Z.; Zhao, M.; Bian, D.; Shen, D.; Zhou, X.; Liu, J.; Liu, Y.; Guo, H.; Zheng, Y. Predicting the degradation behavior of mag-nesium alloys with a diffusion-based theoretical model and in vitro corrosion testing. J. Mater. Sci. Technol. 2019, 035, 1393–1402. [Google Scholar] [CrossRef]
- Yilbas, B.; Ali, H.; Karatas, C.; Al-Sharafi, A.; Abu-Dheir, N.; Al-Qahtani, H.; Khaled, M. Laser Surface Treatment of Stainless Steel and Environmental Dust Effects. Lasers Eng. 2020, 45, 243–263. [Google Scholar]
- Sharma, P.; Pandey, P.M. Corrosion behaviour of the porous iron scaffold in simulated body fluid for biodegradable implant application. Mater. Sci. Eng. C 2019, 99, 838–852. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Q.; Huang, L.; Liu, X.; Liang, Y.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Zheng, Y.; et al. The effects of a phytic acid/calcium ion conversion coating on the corrosion behavior and osteoinductivity of a magnesium-strontium alloy. Appl. Surf. Sci. 2019, 484, 511–523. [Google Scholar] [CrossRef]
- Chang, J.-K.; Lin, C.-S.; Cheng, W.-J.; Lo, I.-H.; Wang, W.-R. Oxidation resistant silane coating for hot-dip galvanized hot stamping steel. Corros. Sci. 2020, 164, 108307. [Google Scholar] [CrossRef]
- Matsuda, T.; Kashi, K.B.; Fushimi, K.; Gelling, V.J. Corrosion protection of epoxy coating with pH sensitive microcapsules encapsulating cerium nitrate. Corros. Sci. 2019, 148, 188–197. [Google Scholar] [CrossRef]
- Montemor, M.; Pinto, R.; Ferreira, M. Chemical composition and corrosion protection of silane films modified with CeO2 nanoparticles. Electrochim. Acta 2009, 54, 5179–5189. [Google Scholar] [CrossRef]
- Frignani, A.; Zucchi, F.; Trabanelli, G.; Grassi, V. Protective action towards aluminium corrosion by silanes with a long al-iphatic chain. Corros. Sci. 2006, 48, 2258–2273. [Google Scholar] [CrossRef]
- Motte, C.; Poelman, M.; Roobroeck, A.; Fedel, M.; Deflorian, F.; Olivier, M.-G. Improvement of corrosion protection offered to galvanized steel by incorporation of lanthanide modified nanoclays in silane layer. Prog. Org. Coat. 2012, 74, 326–333. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Z.; Cao, Z.; Wang, D. Wear behavior of hollow glass beads (HGB) reinforced nitrile butadiene rubber: Effects of silane coupling agent and filler content. Mater. Today Commun. 2019, 19, 366–373. [Google Scholar] [CrossRef]
- Yuan, R.; Ju, P.; Wu, Y.; Ji, L.; Li, H.; Chen, L.; Zhou, H.; Chen, J. Silane-grafted graphene oxide improves wear and corrosion resistance of polyimide matrix: Molecular dynamics simulation and experimental analysis. J. Mater. Sci. 2019, 54, 11069–11083. [Google Scholar] [CrossRef]
- Kinoshita, H.; Nishina, Y.; Alias, A.A.; Fujii, M. Tribological properties of monolayer graphene oxide sheets as water-based lubricant additives. Carbon 2014, 66, 720–723. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Wang, T.; Pan, G. Preparation and tribological properties of graphene oxide/nitrile rubber nanocomposites. J. Mater. Sci. 2011, 47, 730–738. [Google Scholar] [CrossRef]
- Song, H.-J.; Jia, X.-H.; Li, N.; Yang, X.-F.; Tang, H. Synthesis of α-Fe2O3 nanorod/graphene oxide composites and their tribological properties. J. Mater. Chem. 2011, 22, 895–902. [Google Scholar] [CrossRef]
- Guo, B.; Chen, W.; Yan, L. Preparation of Flexible, Highly Transparent, Cross-Linked Cellulose Thin Film with High Mechanical Strength and Low Coefficient of Thermal Expansion. ACS Sustain. Chem. Eng. 2013, 1, 1474–1479. [Google Scholar] [CrossRef]
- Saud, S.N.; Bakhsheshi-Rad, H.; Yaghoubidoust, F.; Iqbal, N.; Hamzah, E.; Ooi, C.R. Corrosion and bioactivity performance of graphene oxide coating on TiNb shape memory alloys in simulated body fluid. Mater. Sci. Eng. C 2016, 68, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, Y.; Kamboj, A.; Rekha, M.; Rao, N.N.; Srivastava, C. Copper-graphene oxide composite coatings for corrosion protection of mild steel in 3.5% NaCl. Thin Solid Film 2017, 636, 107–115. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. Graphene oxide/hydroxyapatite composite coatings fabricated by electrophoretic nanotechnology for biological applications. Carbon 2014, 67, 185–197. [Google Scholar] [CrossRef]
- Raza, M.A.; Rehman, Z.U.; Ghauri, F.A. Corrosion study of silane-functionalized graphene oxide coatings on copper. Thin Solid Film 2018, 663, 93–99. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.; Liu, J.; Jiang, J.; Yuan, N.; Pu, J.; Ding, J. An organic/inorganic composite multi-layer coating to improve the corrosion resistance of AZ31B Mg alloy. Surf. Coatings Technol. 2019, 360, 276–284. [Google Scholar] [CrossRef]
- Li, P.F.; Zhou, H.; Cheng, X. Investigation of a hydrothermal reduced graphene oxide nano coating on Ti substrate and its nano-tribological behavior. Surf. Coatings Technol. 2014, 254, 298–304. [Google Scholar] [CrossRef]
- Qi, S.; Li, X.; Dong, H. Improving the macro-scale tribology of monolayer graphene oxide coating on stainless steel by a silane bonding layer. Mater. Lett. 2017, 209, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.H.; Tong, L.B.; Jiang, Z.H. Effect of Graphene Oxide/Silane Self-assemble Coating on Corrosion and Wear Resistance of Mg Alloy. Surf. Technol. 2019, 48, 62–68. [Google Scholar]
- Schmidt, J.C.; Astasov-Frauenhoffer, M.; Waltimo, T.; Walter, C.; Weiger, R. Efficacy of various side-to-side toothbrushes and impact of brushing parameters on noncontact biofilm removal in an interdental space model. Clin. Oral Investig. 2016, 21, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, N.; Birbilis, N.; Staiger, M. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; He, X.; Li, X.; Qiu, J.; Xu, H.; Zhang, N.; Liu, S. Limitations of MTT and CCK-8 assay for evaluation of graphene cyto-toxicity. Rsc Adv. 2015, 5, 53240–53244. [Google Scholar] [CrossRef]
- Ciofani, G.; Danti, S.; D’Alessandro, D.; Moscato, S.; Menciassi, A. Assessing cytotoxicity of boron nitride nanotubes: Inter-ference with the MTT assay. Biochem. Biophys. Res. Commun. 2010, 394, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.D.M.; Alves, A.M.H.; Ribeiro, D.M.; Santos, L.G.P.; Simões, C.M.O.; Felippe, W.T.; Felippe, M.C.S. Effect of milk renewal on cell viability in vitro at different time frames. Braz. Dent. J. 2017, 28, 435–439. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yue, Z.; Romeo, T.; Weber, J.K.R.; Scheuermann, T.; Moulton, S.E.; Wallace, G.G. Biofunctionalized anti-corrosive silane coatings for magnesium alloys. Acta Biomater. 2013, 9, 8671–8677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.R.; Wang, X.; Zhao, X.Y. Preparation and characterization of graphene oxide modified by silane coupling agent. Appl. Chem. Ind. 2019, 48, 97–100. [Google Scholar]
- Wojciechowski, J.; Szubert, K.; Peipmann, R.; Fritz, M.; Schmidt, U.; Bund, A.; Lota, G. Anti-corrosive properties of silane coatings deposited on anodised aluminium. Electrochimica Acta 2016, 220, 1–10. [Google Scholar] [CrossRef]
- Tong, L.; Zhang, J.; Xu, C.; Wang, X.; Song, S.; Jiang, Z.; Kamado, S.; Cheng, L.; Zhang, H. Enhanced corrosion and wear resistances by graphene oxide coating on the surface of Mg-Zn-Ca alloy. Carbon 2016, 109, 340–351. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, Y.; Song, Q. ChemInform Abstract: Cu/Fe-Cocatalyzed Formation of β-Ketophosphonates by a Domino Knoevenagel-Decarboxylation-Oxyphosphorylation Sequence from Aromatic Aldehydes and H-Phosphonates. Chemin- 2015, 46. [Google Scholar] [CrossRef]
- Lei, L.; Shi, J.; Wang, X.; Liu, D.; Xu, H. Microstructure and electrochemical behavior of cerium conversion coating modified with silane agent on magnesium substrates. Appl. Surf. Sci. 2016, 376, 161–171. [Google Scholar] [CrossRef]
- Lu, X.; Zuo, Y.; Zhao, X.; Tang, Y. The improved performance of a Mg-rich epoxy coating on AZ91D magnesium alloy by silane pretreatment. Corros. Sci. 2012, 60, 165–172. [Google Scholar] [CrossRef]
- Xue, B.; Yu, M.; Liu, J.; Li, S.; Xiong, L.; Kong, X. Corrosion Protective Properties of Silane Functionalized Graphene Oxide Film on AA2024-T3 Aluminum Alloy. J. Electrochem. Soc. 2016, 163, C798–C806. [Google Scholar] [CrossRef]
- Zhang, D.F.; Fan, L.Z.; Guo, R.H.; Fan, Z.T. Preparation of FGO/PBMA Composites with Improved Thermal Stability. Chem. J. Chin. Univ. 2014, 35, 2466–2471. [Google Scholar]
- Liu, Y.-C.; Liu, D.-B.; Zhao, Y.; Chen, M.-F. Corrosion degradation behavior of Mg–Ca alloy with high Ca content in SBF. Trans. Nonferrous Met. Soc. China 2015, 25, 3339–3347. [Google Scholar] [CrossRef]
- Frutos, G. Local Surface Environment and Degradation Processes of Degradable Magnesium Biomaterials under Simulated Physiological Conditions. Ph.D. Thesis, University of Kiel, Kiel, Germany, 27 April 2020. [Google Scholar]
- Gu, X.; Zheng, W.; Cheng, Y.; Zheng, Y. A study on alkaline heat treated Mg—Ca alloy for the control of the biocorrosion rate. Acta Biomater. 2009, 5, 2790–2799. [Google Scholar] [CrossRef]
- Zhong, H.; Dai, L.; Yue, Y.; Zhang, B.; Feng, Z.; Zhang, X.; Ma, M.; Khosla, T.; Xiao, J.; Liu, R. Friction and wear behavior of an-nealed Ti-20Zr-6.5 Al-4V alloy sliding against 440 °C steel in vacuum. Tribol. Int. 2017, 109, 571–577. [Google Scholar] [CrossRef]
- Richard, S.; SelwinRajadurai, J.; Manikandan, V.; Thanu, M.C.; Arumugaprabu, V.; Johnson, R.D.J. Study of tribological prop-erties of nano-sized red mud particle-reinforced polyester composites. Trans. Indian Inst. Met. 2019, 72, 2417–2431. [Google Scholar] [CrossRef]
- Du, B.; Hu, Z.; Wang, J.; Sheng, L.; Zhao, H.; Zheng, Y.; Xi, T. Effect of extrusion process on the mechanical and in vitro degradation performance of a biomedical Mg-Zn-Y-Nd alloy. Bioact. Mater. 2020, 5, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kent, D.; Doan, N.; Dargusch, M.; Wang, G. Effects of deformation twinning on the mechanical properties of biode-gradable Zn-Mg alloys. Bioact. Mater. 2019, 4, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ranade, V.V.; Somberg, J.C. Bioavailability and Pharmacokinetics of Magnesium After Administration of Magnesium Salts to Humans. Am. J. Ther. 2001, 8, 345–357. [Google Scholar] [CrossRef]
- Sun, Z.L.; Wataha, J.C.; Hanks, C.T. Effects of metal ions on osteoblast-like cell metabolism and differentiation. J. Biomed. Mater. Res. 1997, 34, 29–37. [Google Scholar] [CrossRef]
- Danscher, G.; Stoltenberg, M. Silver enhancement of quantum dots resulting from (1) metabolism of toxic metals in animals and humans, (2) in vivo, in vitro and immersion created zinc–sulphur/zinc–selenium nanocrystals, (3) metal ions liberated from metal implants and particles. Prog. Histochem. Cytochem. 2006, 41, 57–139. [Google Scholar] [CrossRef]
- Krendel, D. Hypermagnesemia and Neuromuscular Transmission. Semin. Neurol. 1990, 10, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A. Diagnosis and management of pre-eclampsia: An update. Int. J. Women’s Health 2010, 2, 327–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Rui, S.; Li, S. Layer-by-Layer Self-Assembly Composite Coatings for Improved Corrosion and Wear Resistance of Mg Alloy for Biomedical Applications. Coatings 2021, 11, 515. https://doi.org/10.3390/coatings11050515
Liu T, Rui S, Li S. Layer-by-Layer Self-Assembly Composite Coatings for Improved Corrosion and Wear Resistance of Mg Alloy for Biomedical Applications. Coatings. 2021; 11(5):515. https://doi.org/10.3390/coatings11050515
Chicago/Turabian StyleLiu, Tongfang, Song Rui, and Sheng Li. 2021. "Layer-by-Layer Self-Assembly Composite Coatings for Improved Corrosion and Wear Resistance of Mg Alloy for Biomedical Applications" Coatings 11, no. 5: 515. https://doi.org/10.3390/coatings11050515




