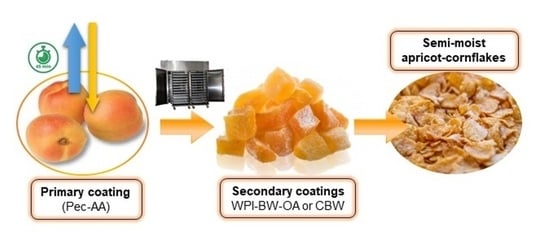
Quality Characteristics of Semi-Moist Apricot-Cornflakes: Effect of Different Composite Coating Application and Storage Time
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Preparation of Fruit Samples
2.2. Primary Coating Application before Hot-Air Drying (HAD)
2.3. Secondary Coating Application after HAD
2.3.1. Chitosan-Bees Wax (CBW) Coating
2.3.2. Whey Protein Isolate-Bees Wax Oleic Acid (WPI-BW-OA) Coating
2.4. Storage of the Dried Apricot-Cornflakes Product
2.5. Sample Analysis
2.5.1. Total Phenolic Compounds (TPC)
2.5.2. β-Carotene Content
2.5.3. Total Antioxidant Activity (TAA)
2.5.4. Color Analysis
2.5.5. Browning Value
2.5.6. Water Activity and Moisture Content
2.5.7. Texture Analysis of Breakfast Cereals and Dried Apricot Cubes
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Osmotic Dehydration and Active Pectin-Based Coating on Bioactive Compounds and Water Activity of Dried Apricot
3.2. Effect of Composite Coating Type and Storage Time on Bioactive Compounds of Semi-Moist Apricots in Breakfast Cereals
3.3. Effect of Secondary Coating Type and Storage Time on Color Properties of Semi-Moist Apricots Cubes
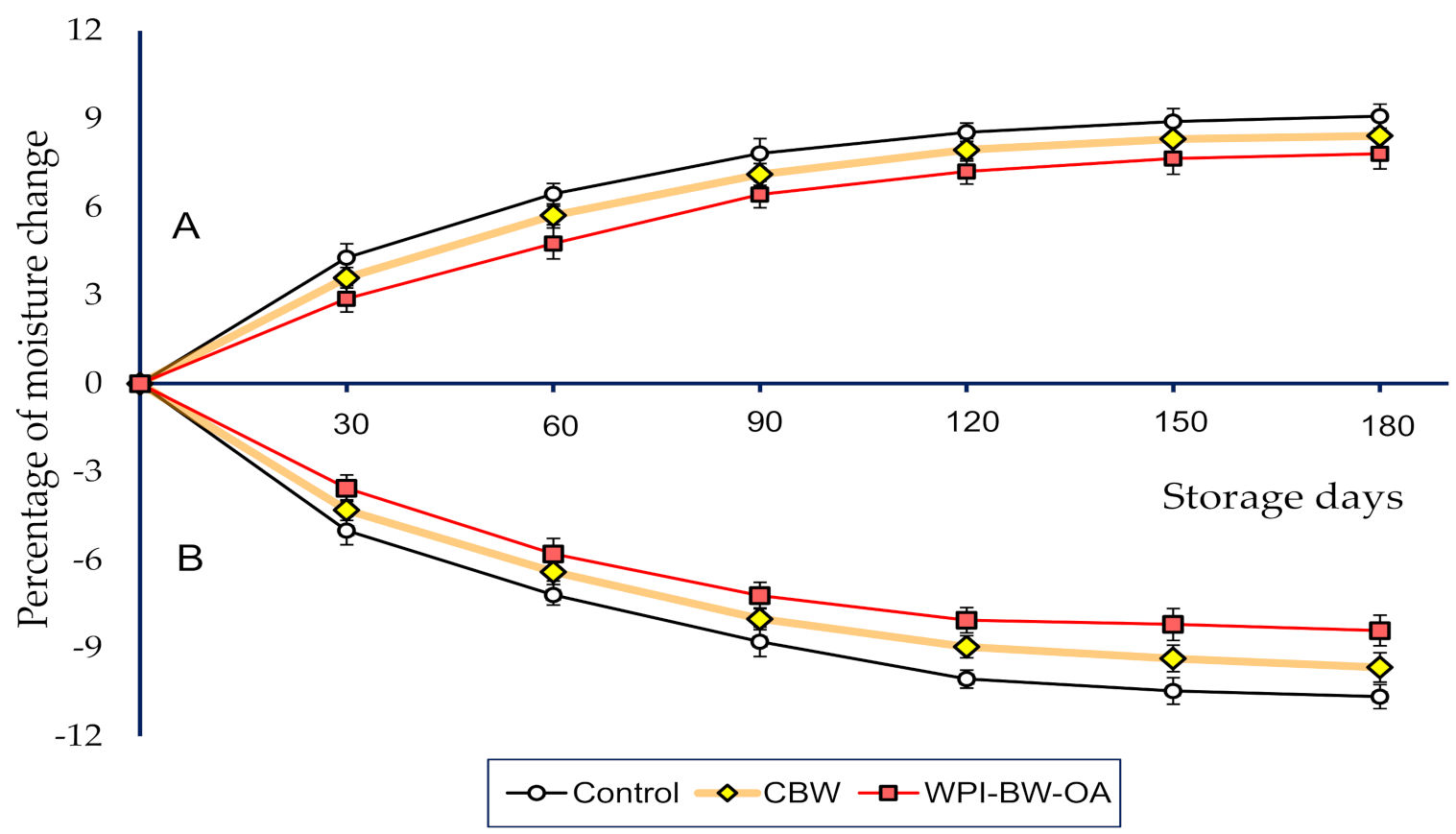
3.4. Effect of Composite Coating Type and Storage Time on Moisture Changes of Semi-Moist Apricot Cubes and Cornflakes in the Apricot-Cereal System
3.5. Effect of Composite Coating Type and Storage Time on Firmness of Semi-Moist Apricot Cubes and Cornflakes in the Apricot-Cereals System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saracoglu, S.; Tuzen, M.; Soylak, M. Evaluation of trace element contents of dried apricot samples from Turkey. J. Hazard. Mater. 2009, 167, 647–652. [Google Scholar] [CrossRef]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J. Nanocomposite films containing organoclay nanoparticles as an antimicrobial (active) packaging for potential food application. J. Food Process. Preserv. 2018, 42. [Google Scholar] [CrossRef]
- Golshan Tafti, A.; Peighambardoust, S.H.; Hesari, J.; Bahrami, A.; Shakuoie Bonab, E. Physico-chemical and functional properties of spray-dried sourdough in breadmaking. Food Sci. Technol. Int. 2013, 19, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A. Development of novel active polypropylene based packaging films containing different concentrations of sorbic acid. Food Packag. Shelf Life 2018, 18, 87–94. [Google Scholar] [CrossRef]
- Nottagh, S.; Hesari, J.; Peighambardoust, S.H.; Rezaei-Mokarram, R.; Jafarizadeh-Malmiri, H. Development of a biodegradable coating formulation based on the biological characteristics of the Iranian ultra-filtrated cheese. Biologia 2018, 73, 403–413. [Google Scholar] [CrossRef]
- Nottagh, S.; Hesari, J.; Peighambardoust, S.H.; Rezaei-Mokarram, R.; Jafarizadeh-Malmiri, H. Effectiveness of edible coating based on chitosan and Natamycin on biological, physico-chemical and organoleptic attributes of Iranian ultra-filtrated cheese. Biologia 2020, 75, 605–611. [Google Scholar] [CrossRef]
- Talens, P.; Pérez-Masía, R.; Fabra, M.J.; Vargas, M.; Chiralt, A. Application of edible coatings to partially dehydrated pineapple for use in fruit-cereal products. J. Food Eng. 2012. [Google Scholar] [CrossRef]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Peressini, D. Effects of osmotic dehydration (with and without sonication) and pectin-based coating pretreatments on functional properties and color of hot-air dried apricot cubes. Food Chem. 2020, 311, 125978. [Google Scholar] [CrossRef]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Soltanzadeh, M.; Peressini, D. Properties of dried apricots pretreated by ultrasound-assisted osmotic dehydration and application of active coatings. Food Technol. Biotechnol. 2020, 58. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; van der Goot, A.J.; Boom, R.M.; Hamer, R.J. Mixing behaviour of a zero-developed dough compared to a flour-water mixture. J. Cereal Sci. 2006, 44, 12–20. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Beigmohammadi, F.; Peighambardoust, S.J. Application of organoclay nanoparticle in low-density polyethylene films for packaging of UF cheese. Packag. Technol. Sci. 2016, 29, 355–363. [Google Scholar] [CrossRef]
- Barat, J.M.; Talens, P.; Barrera, C.; Chiralt, A.; Fito, P. Pineapple candying at mild temperature by applying vacuum impregnation. J. Food Sci. 2002. [Google Scholar] [CrossRef]
- Bourlieu, C.; Guillard, V.; Powell, H.; Vallès-Pàmies, B.; Guilbert, S.; Gontard, N. Modelling and control of moisture transfers in high, intermediate and low aw composite food. Food Chem. 2008. [Google Scholar] [CrossRef]
- Sapru, V.; Labuza, T. Moisture transfer simulation in packaged cereal-fruit systems. J. Food Eng. 1996. [Google Scholar] [CrossRef]
- Dehghan-Manshadi, A.; Peighambardoust, S.H.; Azadmard-Damirchi, S.; Niakousari, M.; Dehghan-Manshadi, A.; Peighambardoust, S.H.; Azadmard-Damirchi, S.; Niakousari, M. Effect of infrared-assisted spouted bed drying of flaxseed on the quality characteristics of its oil extracted by different methods. J. Sci. Food Agric. 2020, 100, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Pournasir, N.; Mohammadzadeh-Pakdel, P. Properties of active starch-based films incorporating a combination of Ag, ZnO and CuO nanoparticles for potential use in food packaging applications. Food Packag. Shelf Life 2019, 22, 100420. [Google Scholar] [CrossRef]
- Martínez-Navarrete, N.; Martínez-Monzó, J.; Pedro, R.; Chiralt, A. Water sorption and plasticization effect in breakfast cereals. Changes in texture. In Proceedings of the 3rd Karlsruhe Nutrition Symposium; Gaukel, V., Spiess, W.E.L., Eds.; Druckerei Grässer: Karlsruhe, Germany, 1998; pp. 485–492. [Google Scholar]
- Talens, P.; Fabra, M.J. Application of vacuum impregnation and edible films to improve the quality of raisin–cereal systems. In New Topics in Food Engineering; Comeau, M., Ed.; Nova Science Publisher, Inc.: New York, NY, USA, 2010; pp. 233–248. [Google Scholar]
- Vargas, M.; Albors, A.; Chiralt, A.; González-Martínez, C. Characterization of chitosan-oleic acid composite films. Food Hydrocoll. 2009. [Google Scholar] [CrossRef]
- Vargas, M.; Albors, A.; Chiralt, A.; González-Martínez, C. Quality of cold-stored strawberries as affected by chitosan-oleic acid edible coatings. Postharvest Biol. Technol. 2006. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Chiralt, A. Tensile properties and water vapor permeability of sodium caseinate films containing oleic acid-beeswax mixtures. J. Food Eng. 2008. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Chiralt, A. Microstructure and optical properties of sodium caseinate films containing oleic acid-beeswax mixtures. Food Hydrocoll. 2009. [Google Scholar] [CrossRef]
- Mendes de Souza, P.; Fernández, A.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Modified sodium caseinate films as releasing carriers of lysozyme. Food Hydrocoll. 2010. [Google Scholar] [CrossRef]
- Pereda, M.; Aranguren, M.I.; Marcovich, N.E. Caseinate films modified with tung oil. Food Hydrocoll. 2010. [Google Scholar] [CrossRef]
- Karami, Z.; Peighambardoust, S.H.; Hesari, J.; Akbari-Adergani, B.; Andreu, D. Antioxidant, anticancer and ACE-inhibitory activities of bioactive peptides from wheat germ protein hydrolysates. Food Biosci. 2019, 32, 100450. [Google Scholar] [CrossRef]
- Ekielski, A.; Mishra, P.K.; Żelaziński, T. Assessing the influence of roasting process parameters on mepiquat and chlormequat formation in dark barley malts. Food Bioprocess Technol. 2018, 11, 1177–1187. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, Y.; Peighambardoust, S.J.; Peighambardoust, S.H.; Karkaj, S.Z. Development of antibacterial carboxymethyl cellulose-based nanobiocomposite films containing various metallic nanoparticles for food packaging applications. J. Food Sci. 2019, 84, 2537–2548. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Effect of different drying methods and storage time on the retention of bioactive compounds and antibacterial activity of wine grape pomace (Pinot Noir and Merlot). J. Food Sci. 2012. [Google Scholar] [CrossRef]
- Moraga, G.; Igual, M.; García-Martínez, E.; Mosquera, L.H.; Martínez-Navarrete, N. Effect of relative humidity and storage time on the bioactive compounds and functional properties of grapefruit powder. J. Food Eng. 2012. [Google Scholar] [CrossRef]
- Miller, K.S.; Krochta, J.M. Oxygen and aroma barrier properties of edible films: A review. Trends Food Sci. Technol. 1997, 8, 228–237. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, L.; Luo, W. Effects of chitosan-beeswax composite coating on physiology and quality of frozen yellow-flesh peach. Trans. Chin. Soc. Agric. Eng. 2010, 26, 368–374. [Google Scholar]
- Dong, F.; Wang, X. Effects of carboxymethyl cellulose incorporated with garlic essential oil composite coatings for improving quality of strawberries. Int. J. Biol. Macromol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Murmu, S.B.; Mishra, H.N. The effect of edible coating based on Arabic gum, sodium caseinate and essential oil of cinnamon and lemon grass on guava. Food Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mahy, M.; Van Eycken, L.; Oosterlinck, A. Evaluation of uniform color spaces developed after the adoption of CIELAB and CIELUV. Color Res. Appl. 1994. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Cekmecelioglu, D. Optimization of hemicellulose coating as applied to apricot drying and comparison with chitosan coating and sulfite treatment. J. Food Process Eng. 2016. [Google Scholar] [CrossRef]
- Hamzaoğlu, F.; Türkyılmaz, M.; Özkan, M. Effect of SO2 on sugars, indicators of Maillard reaction, and browning in dried apricots during storage. J. Sci. Food Agric. 2018, 98, 4988–4999. [Google Scholar] [CrossRef]
- Acevedo, N.C.; Schebor, C.; Buera, P. Non-enzymatic browning kinetics analysed through water-solids interactions and water mobility in dehydrated potato. Food Chem. 2008, 108, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Peighambardoust, S.H.; Peighambardoust, S.J.; Hosseini, S.V.; Regenstein, J.M. Improved mechanical and antibacterial properties of active LDPE films prepared with combination of Ag, ZnO and CuO nanoparticles. Food Packag. Shelf Life 2019, 22. [Google Scholar] [CrossRef]
- Sarabandi, K.; Peighambardoust, S.H.; Mahoonak, A.S.; Samaei, S.P. Effect of carrier types and compositions on the production yield, microstructure and physical characteristics of spray dried sour cherry juice concentrate. J. Food Meas. Charact. 2017. [Google Scholar] [CrossRef]
- Sarabandi, K.; Peighambardoust, S.H.; Sadeghi Mahoonak, A.R.; Samaei, S.P. Effect of different carriers on microstructure and physical characteristics of spray dried apple juice concentrate. J. Food Sci. Technol. 2018, 55, 3098–3109. [Google Scholar] [CrossRef]
- Hajizadeh, H.; Peighambardoust, S.J.; Peighambardoust, S.H.; Peressini, D. Physical, mechanical, and antibacterial characteristics of bio-nanocomposite films loaded with Ag-modified SiO2 and TiO2 nanoparticles. J. Food Sci. 2020, 85, 1193–1202. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocoll. 2007. [Google Scholar] [CrossRef]
- Katz, E.E.; Labuza, T.P. Effect of water activity on the sensory crispness and mechanical deformation of snack food products. J. Food Sci. 1981. [Google Scholar] [CrossRef]
- Roudaut, G.; Dacremont, C.; Le Meste, M. Influence of water on the crispness of cereal-based foods: Acoustic, mechanical, and sensory studies. J. Texture Stud. 1998. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; van Brenk, S.; van der Goot, A.J.; Hamer, R.J.; Boom, R.M. Dough processing in a Couette-type device with varying eccentricity: Effect on glutenin macro-polymer properties and dough micro-structure. J. Cereal Sci. 2007, 45, 34–48. [Google Scholar] [CrossRef]
- Golshan Tafti, A.; Peighambardoust, S.H.; Behnam, F.; Bahrami, A.; Aghagholizadeh, R.; Ghamari, M.; Rafat, S.A. Effects of spray-dried sourdough on flour characteristics and rheological properties of dough. Czech J. Food Sci. 2013, 31, 361–367. [Google Scholar] [CrossRef] [Green Version]




| Sample | TPC (mg GA/100 g d.m.) | β-Carotene (mg/100 g d.m.) | TAA (%) | aW |
|---|---|---|---|---|
| Fresh apricot | 210 * (3.1) a | 25.1 (0.4) a | 11.7 (0.02) b | 0.93 (0.003) a |
| OD-Pec_AA coated- HAD | 109 (2.0) b | 13.6 (0.3) b | 23.3 (2.13) a | 0.55 (0.003) c |
| Non-pretreated- HAD | 37 (1.7) c | 12.2 (0.2) c | 10.7 (3.50) c | 0.63 (0.004) b |
| Storage (Days) | Samples | TPC (mg GA/100 g d.m.) | β-Carotene (mg/100 g d.m.) | TAA (%) |
|---|---|---|---|---|
| 0 | Control | 109.3 (2.1) aA | 13.5 (0.05) aA | 23.2 (0.25) aA |
| WPI-BW-OA coating | 104.5 (0.5) bA | 13.5 (0.02) aA | 21.9 (0.41) bA | |
| CBW coating | 103.9 (0.6) bA | 13.5 (0.01) aA | 21.7 (0.60) bA | |
| 30 | Control | 98.1 * (0.6) aB | 13.4 (0.02) aB | 21.4 (0.14) aB |
| WPI-BW-OA coating | 86.1 (2.3) bB | 12.7 (0.04) bB | 19.4 (0.13) bB | |
| CBW coating | 82.1 (0.1) cB | 12.1 (0.04) cB | 18.2 (0.24) cB | |
| 60 | Control | 85.2 (0.3) aC | 11.4 (0.04) aC | 19.8 (0.13) aC |
| WPI-BW-OA coating | 81.6 (2.1) bC | 12.4 (0.04) bC | 18.4 (0.42) bC | |
| CBW coating | 77.5 (1.9) cC | 11.7 (0.08) cC | 17.9 (0.06) bC | |
| 90 | Control | 71.6 (2.2) bD | 10.9 (0.02) cD | 16.3 (0.15) cD |
| WPI-BW-OA coating | 77.6 (3.2) aD | 12.0 (0.06) aD | 17.5 (0.27) aD | |
| CBW coating | 70.5 (2.1) bD | 11.2 (0.05) bD | 17.0 (0.35) bD | |
| 120 | Control | 60.5 (0.7) bE | 10.3 (0.02) cE | 14.8 (0.26) cE |
| WPI-BW-OA coating | 71.5 (2.4) aE | 11.8 (0.03) aE | 15.9 (0.30) aE | |
| CBW coating | 60.5 (1.9) bE | 10.8 (0.06) bE | 15.4 (0.36) bE | |
| 150 | Control | 52.1 (0.3) cF | 9.8 (0.01) cF | 12.3 (0.36) cF |
| WPI-BW-OA coating | 68.0 (2.6) aF | 11.4 (0.04) aF | 14.3 (0.18) aF | |
| CBW coating | 56.8 (2.1) bF | 10.4 (0.04) bF | 12.9 (0.30) bF | |
| 180 | Control | 40.8 (3.5) cG | 9.2 (0.02) cG | 11.0 (0.43) cG |
| WPI-BW-OA coating | 59.5 (0.8) aG | 11.1 (0.03) aG | 13.2 (0.26) aG | |
| CBW coating | 49.1 (2.2) bG | 10.1 (0.04) bG | 12.2 (0.41) bG |
| Storage (Day) | Samples | L* | a* | b* | C* (Chroma) | Browning Value (Abs/g d.m.) |
|---|---|---|---|---|---|---|
| - | Reference: OD45 + Pec-AA + HAD ** | 38.3 (1.8) | 4.3 (0.2) | 45.4 (1.2) | 45.55 (0.4) | - |
| 30 | Control | 27.5 (0.9) bA | 12.6 (0.2) aF | 37.2 (1.5) aA | 39.3 (0.2) cA | 0.09 (0.00) aF |
| CBW coating | 33.8 (0.8) aA | 11.8 (0.4) bE | 39.9 (0.9) bA | 41.6 (0.3) bA | 0.08 (0.00) bF | |
| WPI-BW-OA coating | 34.9 (0.9) aA | 9.4 (0.5) cE | 43.7 (0.6) cA | 43.7 (0.4) aA | 0.06 (0.00) cF | |
| 60 | Control | 21.2 (0.1) cB | 16.6 (0.3) aE | 30.9 (0.1) aB | 35.1 (0.2) bB | 0.12 (0.01) aE |
| CBW coating | 25.3 (0.3) bB | 16.4 (0.3) aD | 33.2 (0.3) bB | 35.7 (0.4) bB | 0.09 (0.00) bE | |
| WPI-BW-OA coating | 29.3 (0.2) aB | 13.6 (1.0) bE | 37.6 (0.9) bB | 40.0 (0.6) aB | 0.07 (0.00) cE | |
| 90 | Control | 20.6 (0.1) cC | 18.4 (0.3) aD | 28.4 (0.4) cC | 33.8 (0.1) cC | 0.21 (0.02) aD |
| CBW coating | 22.0 (0.5) bC | 18.9 (0.5) aC | 30.4 (0.4) aC | 35.8 (0.3) bB | 0.17 (0.01) bD | |
| WPI-BW-OA coating | 28.7 (0.6) aC | 13.3 (0.7) bD | 37.4 (0.4) bC | 39.7 (0.7) aB | 0.08 (0.00) cD | |
| 120 | Control | 11.0 (0.3) cD | 20.0 (0.5) aC | 12.4 (0.4) cD | 23.5 (0.5) cE | 0.32 (0.03) aC |
| CBW coating | 16.9 (0.4) bD | 18.4 (0.3) bC | 23.1 (0.4) aD | 29.5 (0.6) bC | 0.19 (0.01) bC | |
| WPI-BW-OA coating | 19.8 (0.4) aD | 18.6 (0.4) bC | 28.1 (0.4) bD | 33.7 (0.2) aC | 0.09 (0.00) cC | |
| 150 | Control | 6.2 (0.4) cE | 24.3 (0.2) aB | 3.1 (0.4) cE | 24.5 (0.4) bE | 0.34 (0.04) aB |
| CBW coating | 8.2 (0.2) bE | 21.2(0.9) bB | 6.5 (0.1) aE | 22.2 (0.1) cE | 0.22 (0.02) bB | |
| WPI-BW-OA coating | 21.9 (0.1) aE | 15.6 (0.1) cB | 24.7 (0.1) bE | 29.2 (0.3) aE | 0.09 (0.00) cB | |
| 180 | Control | 4.5 (0.4) cF | 25.9 (0.1) aA | 1.3 (0.4) cE | 26.0 (0.4) bD | 0.41 (0.06) aA |
| CBW coating | 6.5 (0.3) bF | 25.1 (0.2) bA | 3.4 (0.2) aF | 25.3 (0.1) cD | 0.28 (0.03) bA | |
| WPI-BW-OA coating | 16.8 (0.2) aF | 18.2 (0.4) cA | 24.0 (0.1) bF | 30.1 (0.3) aD | 0.12 (0.01) cA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakooei-Vayghan, R.; Peighambardoust, S.H.; Domínguez, R.; Pateiro, M.; Lorenzo, J.M. Quality Characteristics of Semi-Moist Apricot-Cornflakes: Effect of Different Composite Coating Application and Storage Time. Coatings 2021, 11, 516. https://doi.org/10.3390/coatings11050516
Sakooei-Vayghan R, Peighambardoust SH, Domínguez R, Pateiro M, Lorenzo JM. Quality Characteristics of Semi-Moist Apricot-Cornflakes: Effect of Different Composite Coating Application and Storage Time. Coatings. 2021; 11(5):516. https://doi.org/10.3390/coatings11050516
Chicago/Turabian StyleSakooei-Vayghan, Roghieh, Seyed Hadi Peighambardoust, Rubén Domínguez, Mirian Pateiro, and José M. Lorenzo. 2021. "Quality Characteristics of Semi-Moist Apricot-Cornflakes: Effect of Different Composite Coating Application and Storage Time" Coatings 11, no. 5: 516. https://doi.org/10.3390/coatings11050516