Carbon Aerogel Based Waterborne Ultra-Black Coatings with High Light Absorption
Abstract
1. Introduction
2. Experimental
2.1. Materials
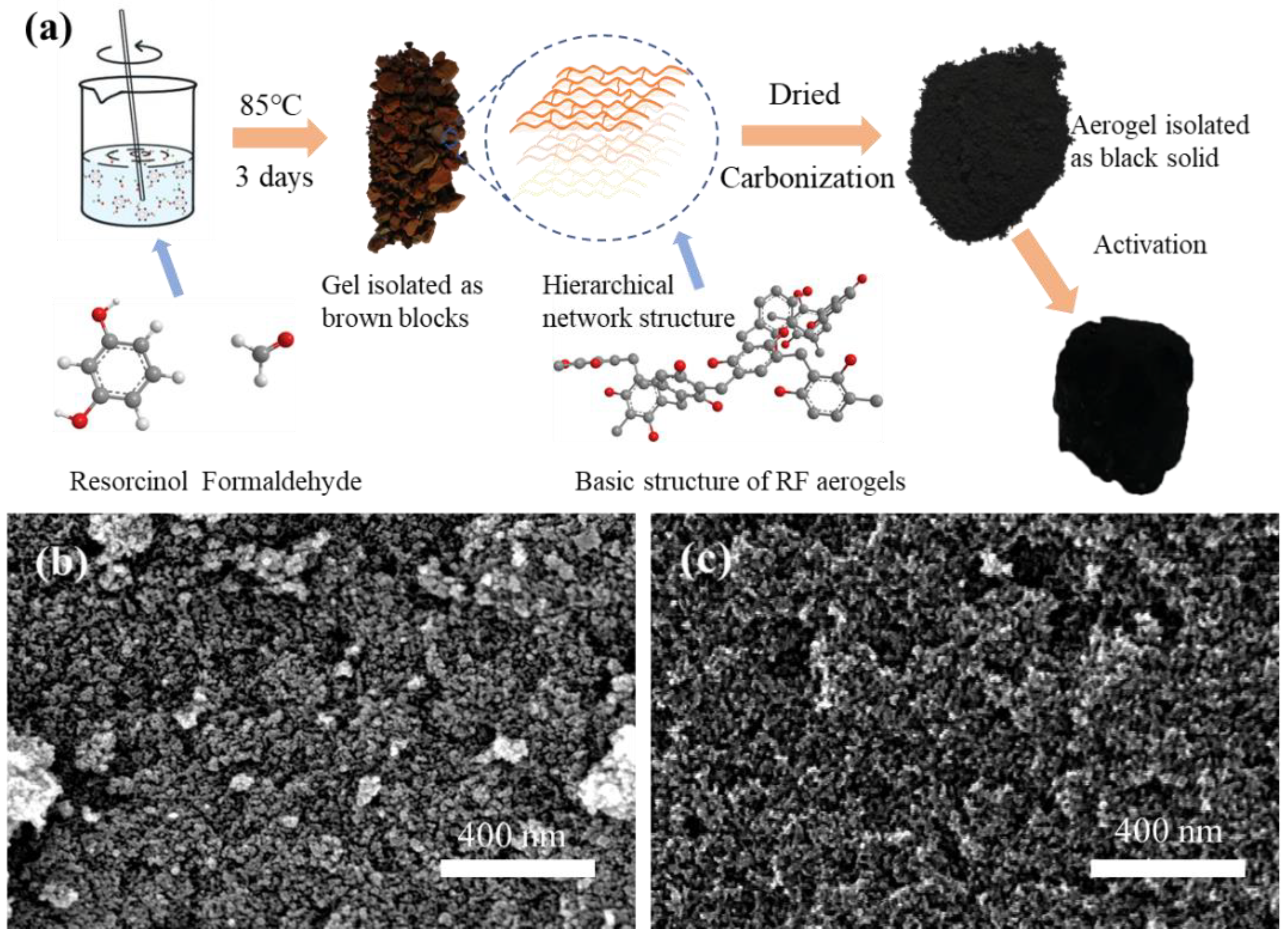
2.2. Synthesis of CAs Through the Sol-Gel Process
2.3. Activation of CAs with CO2
2.4. Preparation of Ultra-Black Coating
2.5. Characterization
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korb, A.R.; Salisbury, J.W.; D’Aria, D.M. Thermal-infrared remote sensing and Kirchhoff’s law: 2. Field measurements. J. Geophys. Res. Solid Earth 1999, 104, 15339–15350. [Google Scholar] [CrossRef]
- Revzen, M.; Opher, R.; Opher, M.; Mann, A. Kirchhoff’s theorem and the casimir effect. Europhys. Lett. 1997, 38, 245–248. [Google Scholar] [CrossRef]
- Watts, C.M.; Liu, X.; Padilla, W.J. Metamaterial electromagnetic wave absorbers. Adv. Mater. 2012, 24, OP98–OP120. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; O’Callahan, B.T.; Yang, H.U.; Raschke, M.B. The thermal near-field: Coherence, spectroscopy, heat-transfer, and optical forces. Prog. Surf. Sci. 2013, 88, 349–392. [Google Scholar] [CrossRef]
- Song, J.; Hao, X.P.; Yuan, Z.D.; Liu, Z.L.; Ding, L. Research of ultra-black coating emissivity based on a controlling the surrounding radiation method. Int. J. Thermophys. 2018, 39. [Google Scholar] [CrossRef]
- Salimian, S.; Zadhoush, A.; Naeimirad, M.; Kotek, R.; Ramakrishna, S. A review on aerogel 3D nanoporous structured fillers in polymer-based nanocomposites. Polym. Compos. 2017, 39, 3383–3408. [Google Scholar] [CrossRef]
- Jackson, J.J.; Puretzky, A.A.; More, K.L.; Rouleau, C.M.; Geohegan, D.B. Pulsed growth of vertically aligned nanotube arrays with variable density. ACS Nano 2010, 4, 7573–7581. [Google Scholar] [CrossRef]
- Zhai, M.; Liu, Y.; Huang, J.; Wang, Y.; Chen, K.; Fu, Y.; Li, H. Efficient suspension plasma spray fabrication of black titanium dioxide coatings with visible light absorption performances. Ceram. Int. 2019, 45, 930–935. [Google Scholar] [CrossRef]
- Xing, F.; Zhao, B.; Shi, W. Study on tunable fabrication of the ultra-black Ni–P film and its blacking mechanism. Electrochim. Acta. 2012, 100, 157–163. [Google Scholar] [CrossRef]
- Hadobás, K.; Kirsch, S.; Carl, A.; Acet, M.; Wassermann, E.F. Reflection properties of nanostructure-arrayed silicon surfaces. Nanotechnology 2000, 11, 161–164. [Google Scholar] [CrossRef]
- Steglich, M.; Lehr, D.; Ratzsch, S.; Käsebier, T.; Schrempel, F.; Kley, E.-B.; Tünnermann, A. An ultra-black silicon absorber. Laser Photonics Rev. 2014, 8, L13–L17. [Google Scholar] [CrossRef]
- Wang, Y.F.; Fu, W.G.; Feng, M.; Cao, X.W. Investigation of the structure and the physical properties of nickel-phosphorus ultra-black surfaces. Appl. Phys. A 2007, 90, 549–553. [Google Scholar] [CrossRef]
- Panagiotopoulos, N.T.; Diamanti, E.K.; Koutsokeras, L.E.; Baikousi, M.; Kordatos, E.; Matikas, T.E. Nanocomposite catalysts producing durable, super-black carbon nanotube systems: Applications in solar thermal harvesting. ACS Nano 2012, 6, 10475–10485. [Google Scholar] [CrossRef]
- Zha, Z.; Yue, X.; Ren, Q.; Dai, Z. Uniform polypyrrole nanoparticles with high photothermal conversion efficiency for photothermal ablation of cancer cells. Adv. Mater. 2013, 25, 777–782. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Cheminform abstract: Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2007, 41, 1578–1586. [Google Scholar] [CrossRef]
- Kaul, A.B.; Coles, J.B.; Eastwood, M.; Green, R.O.; Bandaru, P.R. Ultra-high optical absorption efficiency from the ultraviolet to the infrared using multi-walled carbon nanotube ensembles. Small 2013, 9, 1058–1065. [Google Scholar] [CrossRef]
- De Rosa, I.M.; Sarasini, F.; Sarto, M.S.; Tamburrano, A. EMC impact of advanced carbon fiber/carbon nanotube reinforced composites for next-generation aerospace applications. IEEE Trans. Electromagn. Compat. 2008, 50, 556–563. [Google Scholar] [CrossRef]
- Saxena, V.; Rani, R.U.; Sharma, A.K. Studies on ultra high solar absorber black electroless nickel coatings on aluminum alloys for space application. Surf. Coat. Technol. 2006, 201, 855–862. [Google Scholar] [CrossRef]
- Arunnellaippan, T.; Rama Krishna, L.; Anoop, S.; Uma Rani, R.; Ramesbabu, N. Fabrication of multifunctional black PEO coatings on AA7075 for spacecraft applications. Surf. Coat. Technol. 2016, 307, 735–746. [Google Scholar]
- Mousinho, A.P.; Mansano, R.D. Micropatterning of single-walled carbon nanotube forest. Prog. Org. Coat. 2011, 70, 326–329. [Google Scholar] [CrossRef]
- Sun, Y.; Evans, J.; Ding, F.; Liu, N.; Liu, W.; Zhang, Y.; He, S. Bendable, ultra-black absorber based on a graphite nanocone nanowire composite structure. Opt. Express 2015, 23, 20115–20123. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.J.; Hutley, M.C. The Optical Properties of ‘Moth Eye’ Antireflection Surfaces. Opt. Acta: Int. J. Opt. 2010, 29, 993–1009. [Google Scholar] [CrossRef]
- Yu, K.; Fan, T.; Lou, S.; Zhang, D. Biomimetic optical materials: Integration of nature’s design for manipulation of light. Prog. Mater. Sci. 2013, 58, 825–873. [Google Scholar] [CrossRef]
- Zhao, Q.; Fan, T.; Ding, J.; Zhang, D.; Guo, Q.; Kamada, M. Super black and ultrathin amorphous carbon film inspired by anti-reflection architecture in butterfly wing. Carbon 2011, 49, 877–883. [Google Scholar] [CrossRef]
- Yang, Z.P.; Hsieh, M.L.; Wilthan, B.; Bur, J.A.; Ajayan, P.M.; Ci, L.; Lin, S.Y.; Hanssen, L.M. Experimental observation of extremely weak optical scattering from an interlocking carbon nanotube array. Appl. Opt. 2011, 50, 1850–1855. [Google Scholar] [CrossRef]
- Azoubel, S.; Cohen, R.; Magdassi, S. Wet deposition of carbon nanotube black coatings for stray light reduction in optical systems. Surf. Coat. Technol. 2015, 262, 21–25. [Google Scholar] [CrossRef]
- Sun, X.; Wei, Y.; Li, J.; Zhao, J.; Zhao, L.; Li, Q. Ultralight conducting PEDOT:PSS/carbon nanotube aerogels doped with silver for thermoelectric materials. Sci. China Mater. 2017, 60, 159–166. [Google Scholar] [CrossRef]
- Wang, B.; Li, D.; Tang, M.; Ma, H.; Gui, Y.; Tian, X.; Quan, F.; Song, X.; Xia, Y. Alginate-based hierarchical porous carbon aerogel for high-performance supercapacitors. J. Alloys Compd. 2018, 749, 517–522. [Google Scholar] [CrossRef]
- Zeng, F.Y.; Sui, Z.Y.; Liu, S.; Liang, H.P.; Zhan, H.H.; Han, B.H. Nitrogen-doped carbon aerogels with high surface area for supercapacitors and gas adsorption. Mater. Today Commun. 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Farhan, S.; Wang, R.; Jiang, H.; Li, K.; Wang, C. A novel combination of simple foaming and freeze-drying processes for making carbon foam containing multiwalled carbon nanotubes. Ceram. Int. 2016, 42, 8980–8989. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Wang, P.; Xu, B.; Ma, Y.; Wen, W.; Yang, Y.; Fang, D. Porous carbon-bonded carbon fiber composites impregnated with SiO2-Al2O3 aerogel with enhanced thermal insulation and mechanical properties. Ceram. Int. 2018, 44, 3484–3487. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed. Engl. 2004, 43, 597–601. [Google Scholar] [CrossRef]
- Pilch-Pitera, B.; Czachor, D.; Kowalczyk, K.; Pavlova, E.; Wojturski, J.; Florczak, Ł.; Byczyński, Ł. Conductive polyurethane-based powder clear coatings modified with carbon nanotubes. Prog. Org. Coat. 2019, 137, 105367. [Google Scholar] [CrossRef]
- Sun, W.; Du, A.; Gao, G.; Shen, J.; Wu, G. Graphene-templated carbon aerogels combining with ultra-high electrical conductivity and ultra-low thermal conductivity. Microporous Mesoporous Mater. 2017, 253, 71–79. [Google Scholar] [CrossRef]
- Guo, J.; Li, D.; Zhao, H.; Zou, W.; Yang, Z.; Qian, Z.; Yang, S.; Yang, M.; Zhao, N.; Xu, J. Cast-and-use super black coating based on polymer-derived hierarchical porous carbon spheres. ACS Appl. Mater. Interfaces 2019, 11, 15945–15951. [Google Scholar] [CrossRef]
- Xie, P.; Sun, W.; Liu, Y.; Du, A.; Zhang, Z.; Wu, G.; Fan, R. Carbon aerogels towards new candidates for double negative metamaterials of low density. Carbon 2018, 129, 598–606. [Google Scholar] [CrossRef]
- Sun, W.; Du, A.; Feng, Y.; Shen, J.; Huang, S.; Tang, J.; Zhou, B. Super black material from low-density carbon aerogels with subwavelength structures. ACS Nano 2016, 10, 9123–9128. [Google Scholar] [CrossRef]
- Yu, Z.L.; Wu, Z.Y.; Xin, S.; Qiao, C.; Yu, Z.Y.; Cong, H.P.; Yu, S.H. General and straightforward synthetic route to phenolic resin gels templated by chitosan networks. Chem. Mater. 2014, 26, 6915–6918. [Google Scholar] [CrossRef]
- Li, F.; Xie, L.; Sun, G.; Kong, Q.; Su, F.; Cao, Y.; Wei, J.; Ahmad, A.; Guo, X.; Chen, C.M. Resorcinol-formaldehyde based carbon aerogel: Preparation, structure and applications in energy storage devices. Microporous Mesoporous Mater. 2019, 279, 293–315. [Google Scholar] [CrossRef]
- Pekala, R.W.; Alviso, C.T.; Kong, F.M.; Hulsey, S.S. Aerogels derived from multifuctional organic monomers. J. Non Cryst. Solids 1991, 145, 90–98. [Google Scholar] [CrossRef]
- Illera, D.; Mesa, J.; Gomez, H.; Maury, H. Cellulose aerogels for thermal insulation in buildings: Trends and challenges. Coatings 2018, 8, 345. [Google Scholar] [CrossRef]
- ISO 2409, Paints and Varnishes—Cross-cut Test. 2013.
- ASTM Stardard D 3359, Standard Test Methods for Measuring Adhesion by Tape Test, Method A; ASTM: Philadelphia, PA, USA, 2017.
- Xu, Y.; Wang, S.; Yan, M.; Zhang, L.; Liu, Z. Synthesis, characterization and electrochemical properties of S-doped carbon aerogels. Solid State Ionics 2018, 321, 91–97. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, B.; Wang, S.; Zhang, L.; Liu, Z. Carbon aerogel-based supercapacitors modified by hummers oxidation method. J. Colloid Interface Sci. 2018, 527, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, G.; Liu, S.; Ma, K.; Meng, L. The relationship between volatile fatty acids accumulation and microbial community succession triggered by excess sludge alkaline fermentation. J. Environ. Manag. 2018, 223, 85–91. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Xu, S.J.; Qiao, G.J.; Wang, H.J.; Li, D.C.; Lu, T.J. Preparation of mesoporous carbon derived from mixtures of phenol-formaldehyde resin and ethylene glycol. Mater. Lett. 2008, 62, 3716–3718. [Google Scholar] [CrossRef]
- Bartosik, L.G.; Babel, H.W. Space environmental effects on spacecraft thermal control coatings. In Proceedings of the Space Simulation Conference: Confirming Spaceworthiness Into the Next Millennium, Albuquerque, NM, USA, 5–8 November 1990. [Google Scholar]
- Li, M.; Qiang, X.; Xu, W.; Zhang, H. Synthesis, characterization and application of AFC-based waterborne polyurethane. Prog. Org. Coat. 2015, 84, 35–41. [Google Scholar] [CrossRef]







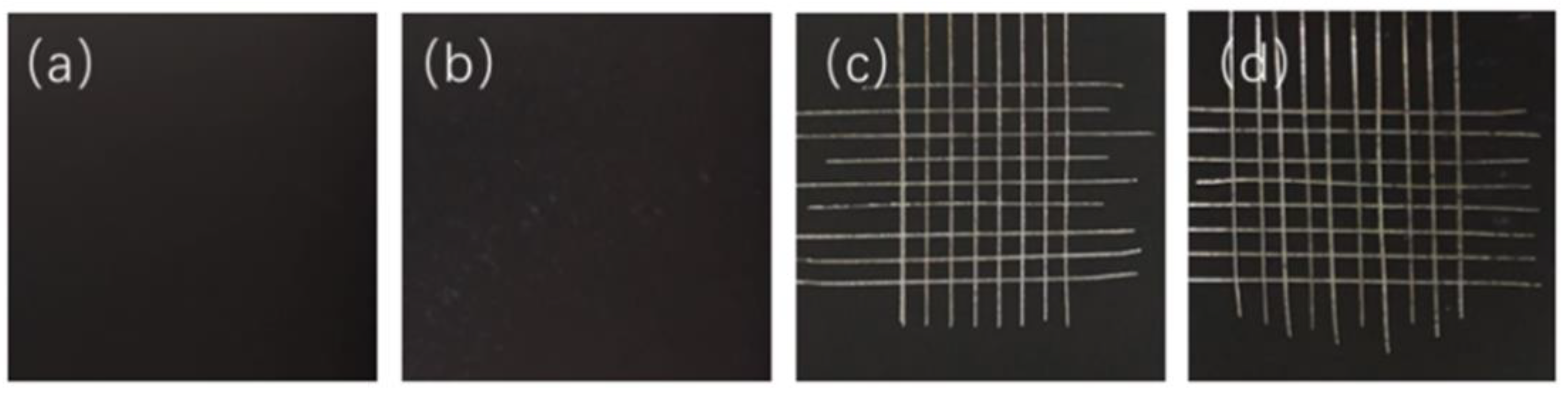
| PB Ratio | Coatings as Prepared | After Thermal Cycling Test | ||
|---|---|---|---|---|
| ISO 2409 | ASTM D3359 | ISO 2409 | ASTM D3359 | |
| 1.15 | Class 1 | Class 4B | Class 2 | Class 3B |
| 1.06 | Class 1 | Class 4B | Class 1 | Class 4B |
| 0.96 | Class 0 | Class 5B | Class 0 | Class 5B |
| 0.87 | Class 0 | Class 5B | Class 0 | Class 5B |
| 0.77 | Class 0 | Class 5B | Class 0 | Class 5B |
| 0.67 | Class 0 | Class 5B | Class 0 | Class 5B |
| 0.58 | Class 0 | Class 5B | Class 0 | Class 5B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Shi, Y.; Li, J.; Cui, G.; Gu, G. Carbon Aerogel Based Waterborne Ultra-Black Coatings with High Light Absorption. Coatings 2021, 11, 563. https://doi.org/10.3390/coatings11050563
Xu J, Shi Y, Li J, Cui G, Gu G. Carbon Aerogel Based Waterborne Ultra-Black Coatings with High Light Absorption. Coatings. 2021; 11(5):563. https://doi.org/10.3390/coatings11050563
Chicago/Turabian StyleXu, Jie, Yifan Shi, Jiangling Li, Guangzhen Cui, and Guangxin Gu. 2021. "Carbon Aerogel Based Waterborne Ultra-Black Coatings with High Light Absorption" Coatings 11, no. 5: 563. https://doi.org/10.3390/coatings11050563
APA StyleXu, J., Shi, Y., Li, J., Cui, G., & Gu, G. (2021). Carbon Aerogel Based Waterborne Ultra-Black Coatings with High Light Absorption. Coatings, 11(5), 563. https://doi.org/10.3390/coatings11050563






