Heterogeneous Bonding of PMMA and Double-Sided Polished Silicon Wafers through H2O Plasma Treatment for Microfluidic Devices
Abstract
1. Introduction
2. Experimental Procedure
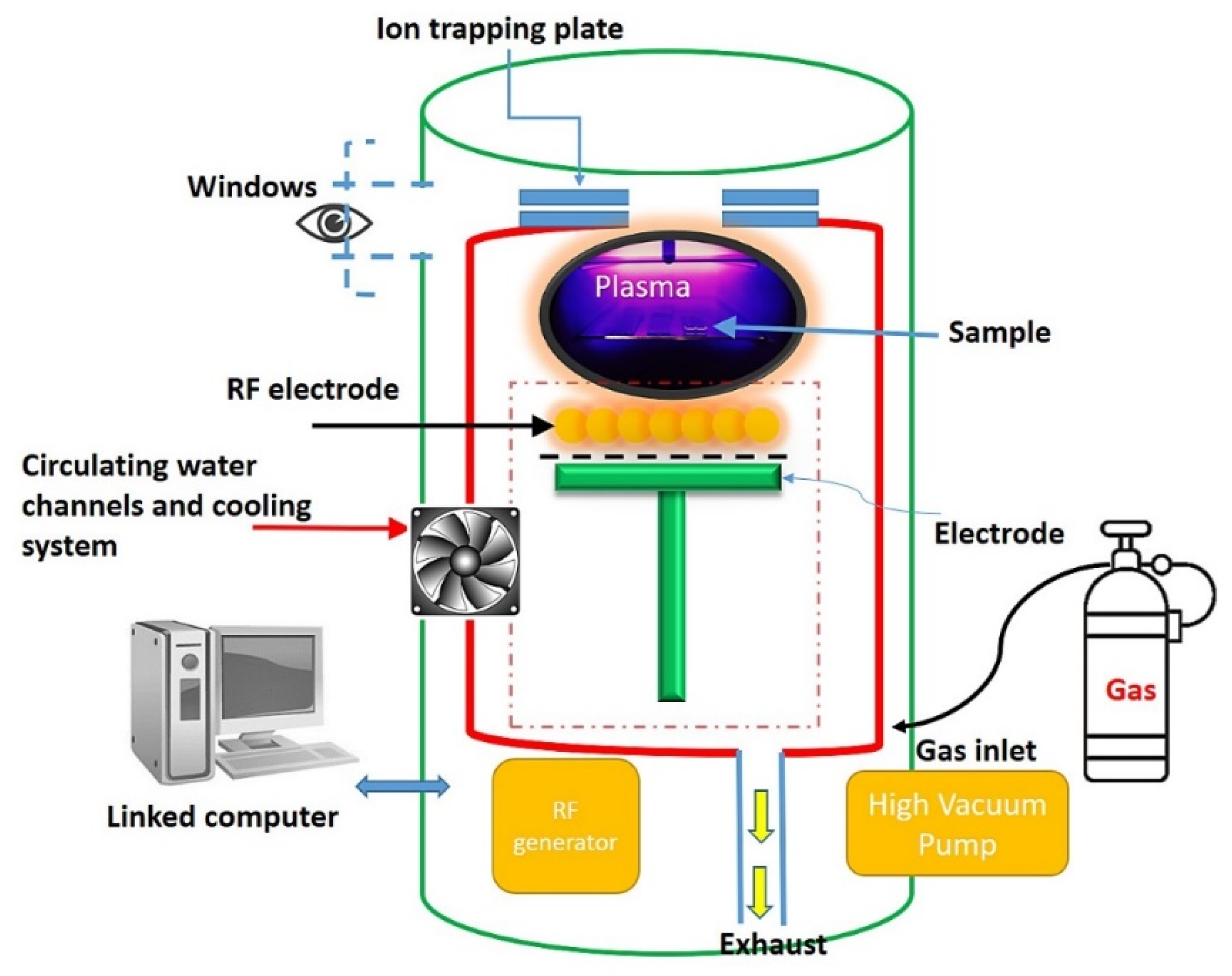
2.1. Fabrication of PMMA Microchannels and Bonding with Silicon Substrates through H2O Plasma
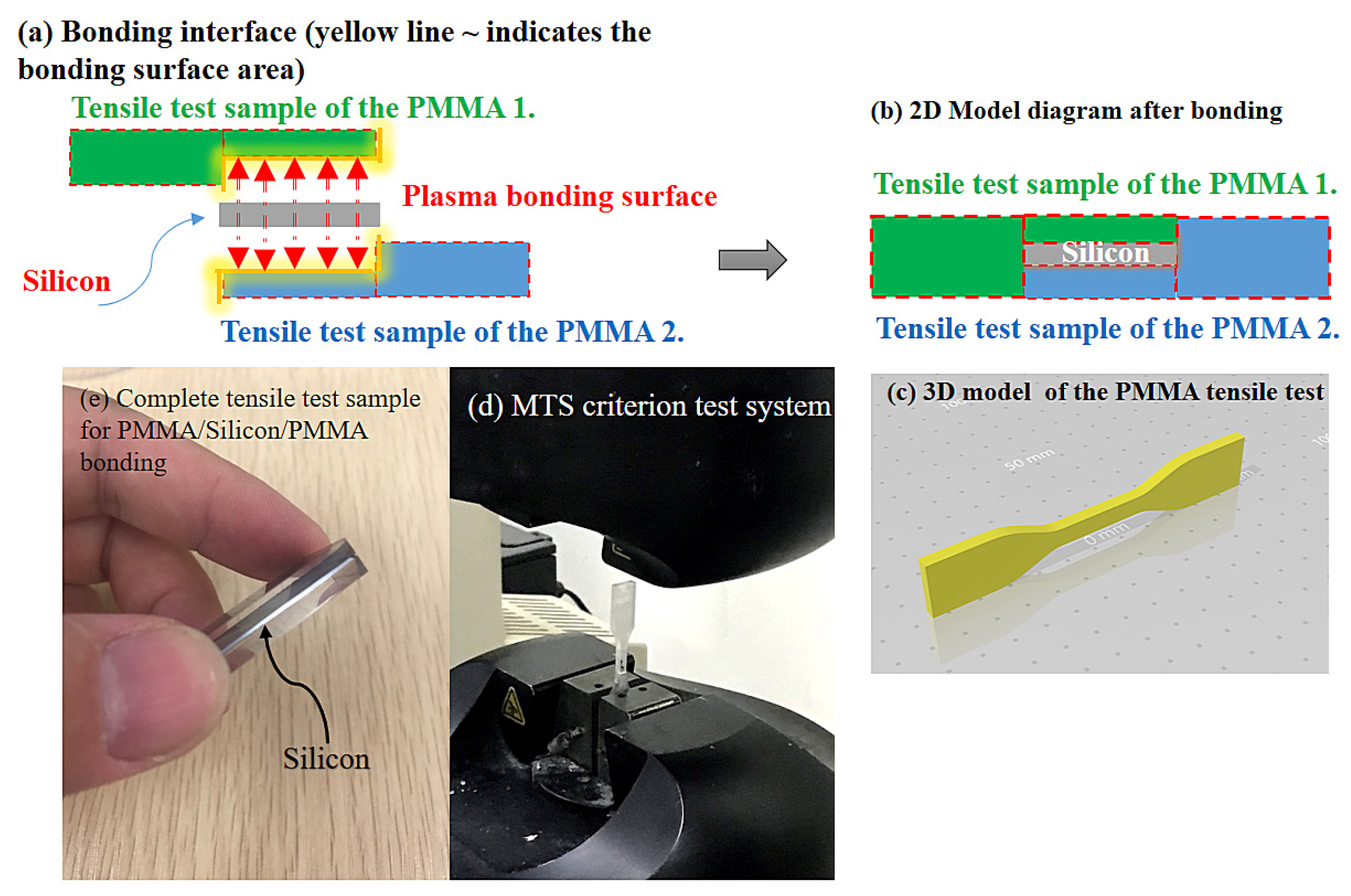
2.2. Tensile Testing of Bond Strength
2.3. Optical Emission Spectroscopy
2.4. UV–Visible Transmission Spectrum
2.5. Interface Surface Analysis
3. Results and Discussion
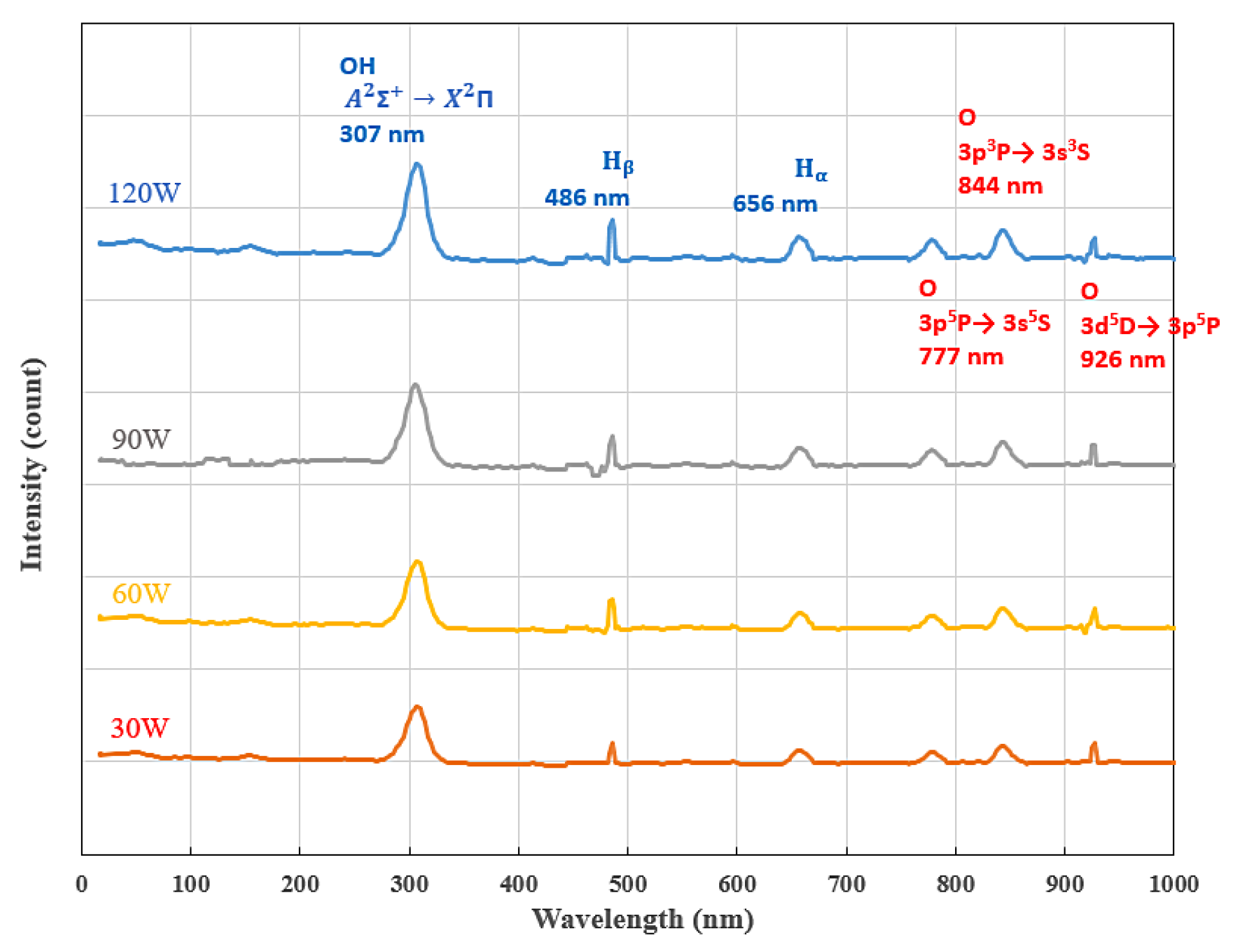
3.1. Chemical Energy Analysis by Optical Emission Spectroscopy (OES)
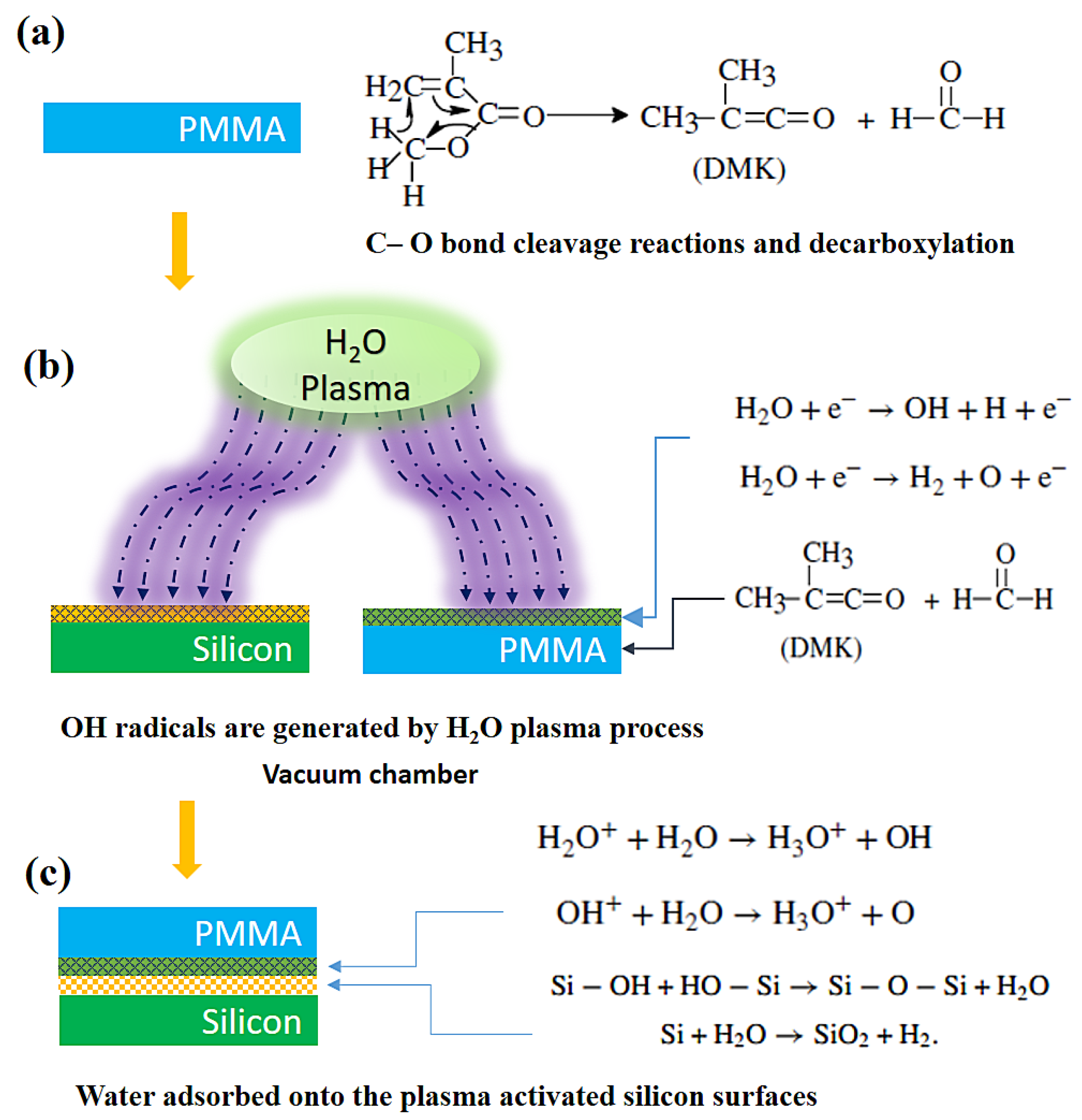
3.2. Bond Strength and Bonding Mechanism
3.3. Surface Morphology and Characteristics
3.4. Optical Properties of the PMMA Layer
3.5. UV Fluorescence Inspection after Heterogeneous Bonding to Determine the Integrity, Morphology and Leakage of the Microchannels
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, I.-H.; Park, T. PMMA solution assisted room temperature bonding for PMMA–PC hybrid devices. Micromachines 2017, 8, 284. [Google Scholar] [CrossRef]
- Tran, H.H.; Wu, W.; Lee, N.Y. Ethanol and UV-assisted instantaneous bonding of PMMA assemblies and tuning in bonding reversibility. Sens. Actuators B Chem. 2013, 181, 955–962. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, Y.; Lin, J.; Su, R.; Xie, Y. Vacuum-assisted thermal bonding of plastic capillary electrophoresis microchip imprinted with stainless steel template. J. Chromatogr. A 2004, 1038, 239–245. [Google Scholar] [CrossRef]
- Koval, Y. Mechanism of etching and surface relief development of PMMA under low-energy ion bombardment. J. Vac. Science Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2004, 22, 843–851. [Google Scholar] [CrossRef]
- Funano, S.-I.; Ota, N.; Sato, A.; Tanaka, Y. A method of packaging molecule/cell-patterns in an open space into a glass microfluidic channel by combining pressure-based low/room temperature bonding and fluorosilane patterning. Chem. Commun. 2017, 53, 11193–11196. [Google Scholar] [CrossRef] [PubMed]
- Amirfeiz, P.; Bengtsson, S.; Bergh, M.; Zanghellini, E.; Börjesson, L. Formation of silicon structures by plasma-activated wafer bonding. J. Electrochem. Soc. 2000, 147, 2693. [Google Scholar] [CrossRef]
- Dong, P.; Chen, Y.-K.; Duan, G.-H.; Neilson, D.T. Silicon photonic devices and integrated circuits. Nanophotonics 2014, 3, 215–228. [Google Scholar] [CrossRef]
- Yang, H.; Huang, M.; Wu, J.; Lan, Z.; Hao, S.; Lin, J. The polymer gel electrolyte based on poly (methyl methacrylate) and its application in quasi-solid-state dye-sensitized solar cells. Mater. Chem. Phys. 2008, 110, 38–42. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, H.; Zhang, L.; Chen, G. Fabrication of PMMA CE microchips by infrared-assisted polymerization. Electrophoresis 2008, 29, 4922–4927. [Google Scholar] [CrossRef]
- Fung, C.K.; Zhang, M.Q.; Chan, R.H.; Li, W.J. In A PMMA-based micro pressure sensor chip using carbon nanotubes as sensing elements. In Proceedings of the 18th IEEE International Conference on Micro Electro Mechanical Systems, Miami Beach, FL, USA, 30 January–3 February 2005; pp. 251–254. [Google Scholar]
- Hong, J.; Lee, S.; Seo, J.; Pyo, S.; Kim, J.; Lee, T. A highly sensitive hydrogen sensor with gas selectivity using a PMMA membrane-coated Pd nanoparticle/single-layer graphene hybrid. ACS Appl. Mater. Interfaces 2015, 7, 3554–3561. [Google Scholar] [CrossRef]
- Mathur, A.; Roy, S.; Tweedie, M.; Mukhopadhyay, S.; Mitra, S.; McLaughlin, J. Characterisation of PMMA microfluidic channels and devices fabricated by hot embossing and sealed by direct bonding. Curr. Appl. Phys. 2009, 9, 1199–1202. [Google Scholar] [CrossRef]
- Romoli, L.; Tantussi, G.; Dini, G. Experimental approach to the laser machining of PMMA substrates for the fabrication of microfluidic devices. Opt. Lasers Eng. 2011, 49, 419–427. [Google Scholar] [CrossRef]
- Xu, J.; Locascio, L.; Gaitan, M.; Lee, C.S. Room-temperature imprinting method for plastic microchannel fabrication. Anal. Chem. 2000, 72, 1930–1933. [Google Scholar] [CrossRef] [PubMed]
- Mair, D.A.; Rolandi, M.; Snauko, M.; Noroski, R.; Svec, F.; Fréchet, J.M. Room-temperature bonding for plastic high-pressure microfluidic chips. Anal. Chem. 2007, 79, 5097–5102. [Google Scholar] [CrossRef]
- Takagi, H.; Kikuchi, K.; Maeda, R.; Chung, T.; Suga, T. Surface activated bonding of silicon wafers at room temperature. Appl. Phys. Lett. 1996, 68, 2222–2224. [Google Scholar] [CrossRef]
- Howlader, M.; Zhang, F.; Kibria, M. Void nucleation at a sequentially plasma-activated silicon/silicon bonded interface. J. Micromechanics Microeng. 2010, 20, 065012. [Google Scholar] [CrossRef]
- Matsumae, T.; Fengwen, M.; Fukumoto, S.; Hayase, M.; Kurashima, Y.; Higurashi, E.; Takagi, H.; Suga, T. Heterogeneous GaN-Si integration via plasma activation direct bonding. J. Alloys Compd. 2021, 852, 156933. [Google Scholar] [CrossRef]
- Howlader, M.; Suehara, S.; Takagi, H.; Kim, T.; Maeda, R.; Suga, T. Room-temperature microfluidics packaging using sequential plasma activation process. IEEE Trans. Adv. Packag. 2006, 29, 448–456. [Google Scholar] [CrossRef]
- Budraa, N.K.; Jackson, H.; Barmatz, M.; Pike, W.T.; Mai, J.D. In Low pressure and low temperature hermetic wafer bonding using microwave heating, Technical Digest. In Proceedings of the IEEE International MEMS 99 Conference. Twelfth IEEE International Conference on Micro Electro Mechanical Systems (Cat. No. 99CH36291), Orlando, FL, USA, 21 January 1999; pp. 490–492. [Google Scholar]
- Ashraf, M.W.; Tayyaba, S.; Afzulpurkar, N. Micro electromechanical systems (MEMS) based microfluidic devices for biomedical applications. Int. J. Mol. Sci. 2011, 12, 3648–3704. [Google Scholar] [CrossRef]
- Tsao, C.; Hromada, L.; Liu, J.; Kumar, P.; DeVoe, D. Low temperature bonding of PMMA and COC microfluidic substrates using UV/ozone surface treatment. Lab Chip 2007, 7, 499–505. [Google Scholar] [CrossRef]
- Frazer, R.Q.; Byron, R.T.; Osborne, P.B.; West, K.P. PMMA: An essential material in medicine and dentistry. J. Long-Term Eff. Med Implant. 2005, 15, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, R.; Jin, Z.; Fan, Y.; Zhou, X.; Zhang, Y. Injection molding and characterization of PMMA-based microfluidic devices. Microsyst. Technol. 2020, 26, 1317–1324. [Google Scholar] [CrossRef]
- Roman, G.T.; Hlaus, T.; Bass, K.J.; Seelhammer, T.G.; Culbertson, C.T. Sol−gel modified poly (dimethylsiloxane) microfluidic devices with high electroosmotic mobilities and hydrophilic channel wall characteristics. Anal. Chem. 2005, 77, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Burns, M.A. Surface-modified polyolefin microfluidic devices for liquid handling. J. Micromech. Microeng. 2005, 15, 2156. [Google Scholar] [CrossRef]
- Chen, C.-S.; Breslauer, D.N.; Luna, J.I.; Grimes, A.; Chin, W.-c.; Lee, L.P.; Khine, M. Shrinky-Dink microfluidics: 3D polystyrene chips. Lab A Chip 2008, 8, 622–624. [Google Scholar] [CrossRef]
- Fan, Y.; Li, H.; Yi, Y.; Foulds, I.G. PMMA to Polystyrene bonding for polymer based microfluidic systems. Microsyst. Technol. 2014, 20, 59–64. [Google Scholar] [CrossRef]
- Yu, H.; Chong, Z.; Tor, S.; Liu, E.; Loh, N. Low temperature and deformation-free bonding of PMMA microfluidic devices with stable hydrophilicity via oxygen plasma treatment and PVA coating. RSC Adv. 2015, 5, 8377–8388. [Google Scholar] [CrossRef]
- Ashok, P.C.; Dholakia, K. Microfluidic Raman spectroscopy for bio-chemical sensing and analysis. In Optical Nano-and Microsystems for Bioanalytics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 247–268. [Google Scholar]
- Vollrath, A.; Pretzel, D.; Pietsch, C.; Perevyazko, I.; Schubert, S.; Pavlov, G.M.; Schubert, U.S. Preparation, cellular internalization, and biocompatibility of highly fluorescent PMMA nanoparticles. Macromol. Rapid Commun. 2012, 33, 1791–1797. [Google Scholar] [CrossRef]
- Roy, S.; Yue, C.; Wang, Z.; Anand, L. Thermal bonding of microfluidic devices: Factors that affect interfacial strength of similar and dissimilar cyclic olefin copolymers. Sens. Actuators B Chem. 2012, 161, 1067–1073. [Google Scholar] [CrossRef]
- Brown, L.; Koerner, T.; Horton, J.H.; Oleschuk, R.D. Fabrication and characterization of poly (methylmethacrylate) microfluidic devices bonded using surface modifications and solvents. Lab Chip 2006, 6, 66–73. [Google Scholar] [CrossRef]
- Bagiatis, V.; Critchlow, G.; Price, D.; Wang, S. The effect of atmospheric pressure plasma treatment (APPT) on the adhesive bonding of poly (methyl methacrylate)(PMMA)-to-glass using a polydimethylsiloxane (PDMS)-based adhesive. Int. J. Adhes. Adhes. 2019, 95, 102405. [Google Scholar] [CrossRef]
- Pfleging, W.; Baldus, O. Laser Patterning and Welding of Transparent Polymers for Microfluidic Device Fabrication, Laser-Based Micropackaging; International Society for Optics and Photonics: Bellingham, WA, USA, 2006; p. 610705. [Google Scholar]
- Chen, X.; Shen, J.; Zhou, M. Rapid fabrication of a four-layer PMMA-based microfluidic chip using CO2-laser micromachining and thermal bonding. J. Micromech. Microeng. 2016, 26, 107001. [Google Scholar] [CrossRef]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A review of the properties and applications of poly (methyl methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Lynh, H.D.; Pin-Chuan, C. Novel solvent bonding method for creation of a three-dimensional, non-planar, hybrid PLA/PMMA microfluidic chip. Sens. Actuators A Phys. 2018, 280, 350–358. [Google Scholar] [CrossRef]
- Terai, H.; Funahashi, R.; Hashimoto, T.; Kakuta, M. Heterogeneous bonding between cyclo-olefin polymer (COP) and glass-like substrate by newly developed water vapor-assisted plasma, Aqua Plasma Cleaner. Electr. Eng. Jpn. 2018, 205, 48–56. [Google Scholar] [CrossRef]
- Immanuel, P.N.; Chao-Ching, C.; Yang, C.-R.; Subramani, M.; Lee, T.-H.; Huang, S.-J. Surface activation of poly (methyl methacrylate) for microfluidic device bonding through a H2O plasma treatment linked with a low-temperature annealing. J. Micromech. Microeng. 2021, 31, 055004. [Google Scholar]
- Tang, L.; Lee, N.Y. A facile route for irreversible bonding of plastic-PDMS hybrid microdevices at room temperature. Lab Chip 2010, 10, 1274–1280. [Google Scholar] [CrossRef]
- Aran, K.; Sasso, L.A.; Kamdar, N.; Zahn, J.D. Irreversible, direct bonding of nanoporous polymer membranes to PDMS or glass microdevices. Lab Chip 2010, 10, 548–552. [Google Scholar] [CrossRef]
- Liston, E.; Martinu, L.; Wertheimer, M. Plasma surface modification of polymers for improved adhesion: A critical review. J. Adhes. Sci. Technol. 1993, 7, 1091–1127. [Google Scholar] [CrossRef]
- Zeng, W.; Li, S.; Chow, W.K. Review on chemical reactions of burning poly (methyl methacrylate) PMMA. J. Fire Sci. 2002, 20, 401–433. [Google Scholar] [CrossRef]
- Matsutani, A.; Ohtsuki, H.; Koyama, F. Characterization of H2O-inductively coupled plasma for dry etching. J. Phys. Conf. Ser. 2008, 100, 062022. [Google Scholar] [CrossRef]
- Annušová, A.; Krištof, J.; Veis, P.; Foissac, C.; Supiot, P. Spectroscopic study of an Ar-H2O discharge excited by a RF helical cavity. WDS’12 Proc. Contrib. Pap. 2012, 2, 99–104. [Google Scholar]
- Tennico, Y.H.; Koesdjojo, M.T.; Kondo, S.; Mandrell, D.T.; Remcho, V.T. Surface modification-assisted bonding of polymer-based microfluidic devices. Sens. Actuators B Chem. 2010, 143, 799–804. [Google Scholar] [CrossRef]
- Wang, R.; Shen, Y.; Zhang, C.; Yan, P.; Shao, T. Comparison between helium and argon plasma jets on improving the hydrophilic property of PMMA surface. Appl. Surf. Sci. 2016, 367, 401–406. [Google Scholar] [CrossRef]
- Lee, W.-J.; Chang, J.-G.; Ju, S.-P. Hydrogen-bond structure at the interfaces between water/poly (methyl methacrylate), water/poly (methacrylic acid), and water/poly (2-aminoethylmethacrylamide). Langmuir 2010, 26, 12640–12647. [Google Scholar] [CrossRef]
- ASTM D638-14:2014. Standard Test Method for Tensile Properties of Plastics; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Ravarian, R.; Wei, H.; Rawal, A.; Hook, J.; Chrzanowski, W.; Dehghani, F. Molecular interactions in coupled PMMA–bioglass hybrid networks. J. Mater. Chem. B 2013, 1, 1835–1845. [Google Scholar] [CrossRef]
- Joonlapak, K.; Wiwattanakul, P.; Jirathampradhab, T.; Akhadejdamrong, T.; Wongariyakawee, A.; Srikhirin, T. The Preparation of Highly Transparent Layered Double Hydroxides (LDHs)-Poly (Methyl Methacrylate) (PMMA) Nanocomposites Sheets by Batch Cell Polymerization Method. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; p. 012009. [Google Scholar]
- Abdel–Fattah, E. Surface activation of poly (methyl methacrylate) with atmospheric pressure ar+ h2o plasma. Coatings 2019, 9, 228. [Google Scholar] [CrossRef]
- Pan, Y.V.; Denton, D.D. Plasma dissociation reaction kinetics. I. Methyl Methacrylate J. Appl. Polym. Sci. 1999, 73, 1–16. [Google Scholar]
- Bamshad, A.; Nikfarjam, A.; Khaleghi, H. A new simple and fast thermally-solvent assisted method to bond PMMA–PMMA in micro-fluidics devices. J. Micromech. Microeng. 2016, 26, 065017. [Google Scholar] [CrossRef]
- Gilliam, M.; Farhat, S.; Zand, A.; Stubbs, B.; Magyar, M.; Garner, G. Atmospheric plasma surface modification of PMMA and PP micro-particles. Plasma Process. Polym. 2014, 11, 1037–1043. [Google Scholar] [CrossRef]
- Shimizu, M. Ion chemistry in the cometary atmosphere. Astrophys. Space Sci. 1975, 36, 353–361. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Tian, Y.; Wang, C.; Suga, T. Room-temperature direct bonding of silicon and quartz glass wafers. Appl. Phys. Lett. 2017, 110, 221602. [Google Scholar] [CrossRef]
- Shobhana, E. X-Ray diffraction and UV-visible studies of PMMA thin films. Int. J. Mod. Eng. Res. 2012, 2, 1092–1095. [Google Scholar]
- Saggiomo, V.; Velders, A.H. Simple 3D printed scaffold-removal method for the fabrication of intricate microfluidic devices. Adv. Sci. 2015, 2, 1500125. [Google Scholar] [CrossRef]
- Shah, J.J.; Geist, J.; Locascio, L.E.; Gaitan, M.; Rao, M.V.; Vreeland, W.N. Capillarity induced solvent-actuated bonding of polymeric microfluidic devices. Anal. Chem. 2006, 78, 3348–3353. [Google Scholar] [CrossRef]
- Kotz, F.; Mader, M.; Dellen, N.; Risch, P.; Kick, A.; Helmer, D.; Rapp, B.E. Fused deposition modeling of microfluidic chips in polymethylmethacrylate. Micromachines 2020, 11, 873. [Google Scholar] [CrossRef]
- Dorranian, D.; Abedini, Z.; Hojabri, A.; Ghoranneviss, M. Structural and optical characterization of PMMA surface treated in low power nitrogen and oxygen RF plasmas. J. Non-Oxide Glasses 2009, 1, 217–229. [Google Scholar]
- Jafari, M.; Dorranian, D. Surface modification of PMMA polymer in the interaction with oxygen-argon RF plasma. J. Theor. Appl. Phys. 2011, 5, 59–66. [Google Scholar]
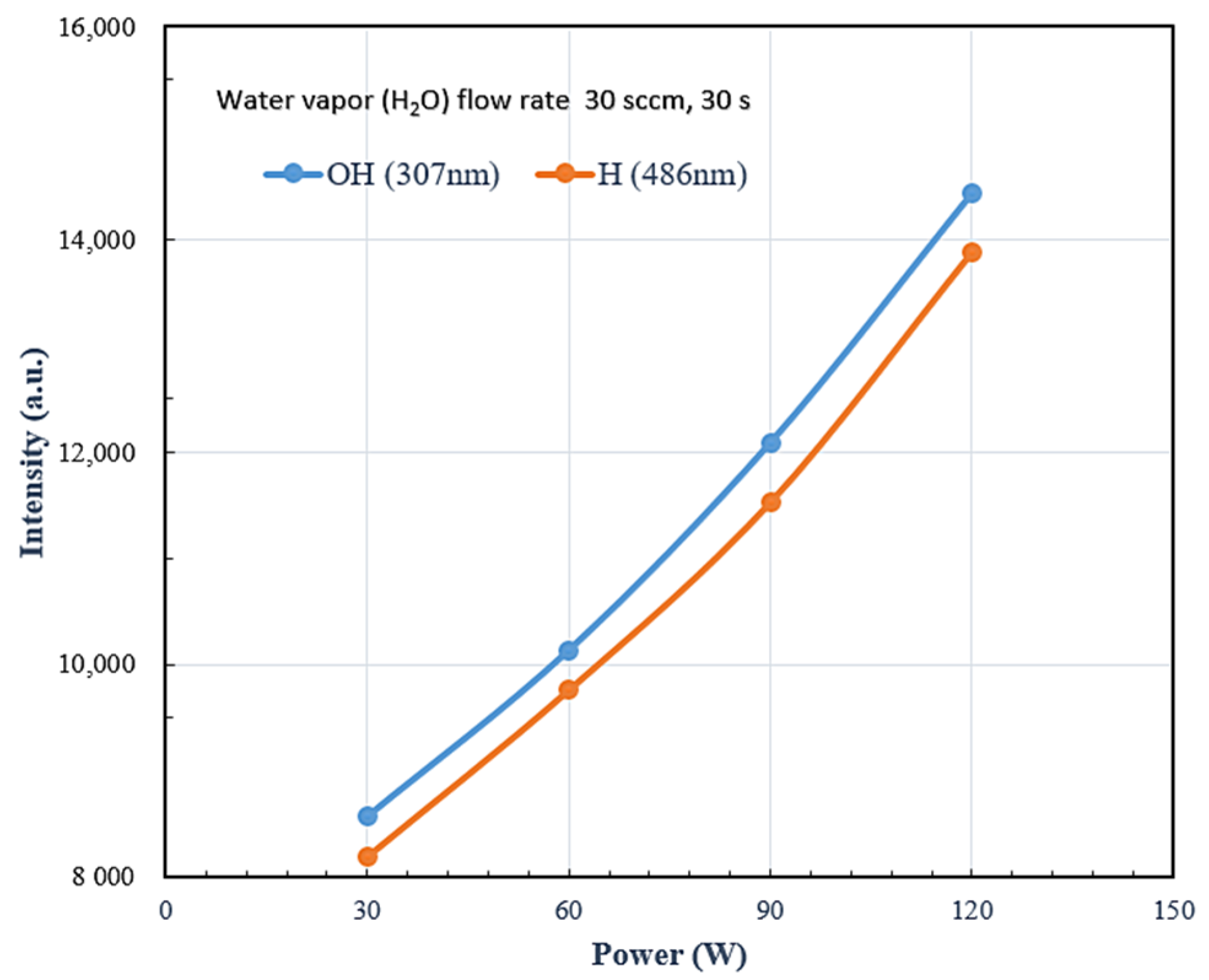

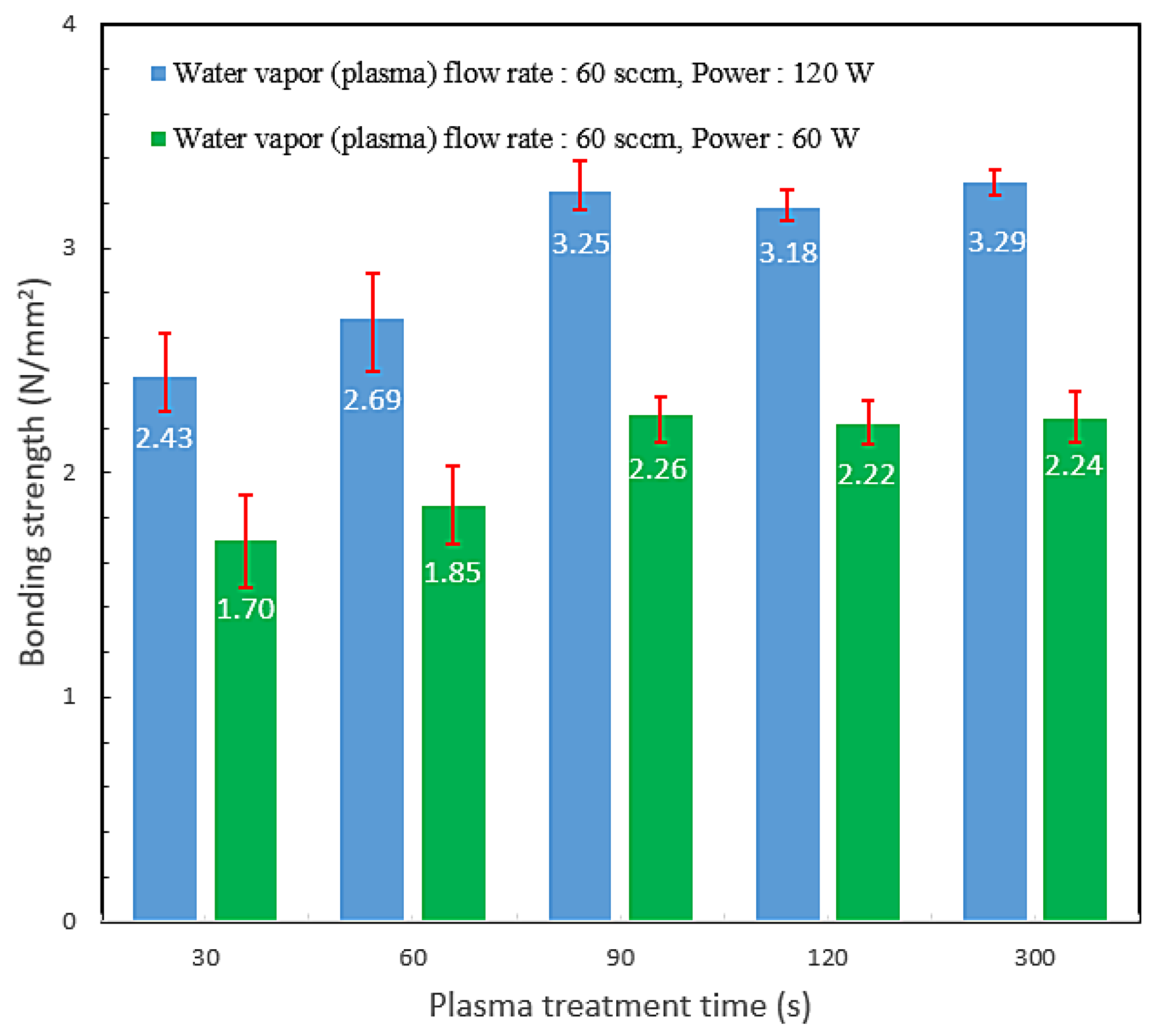

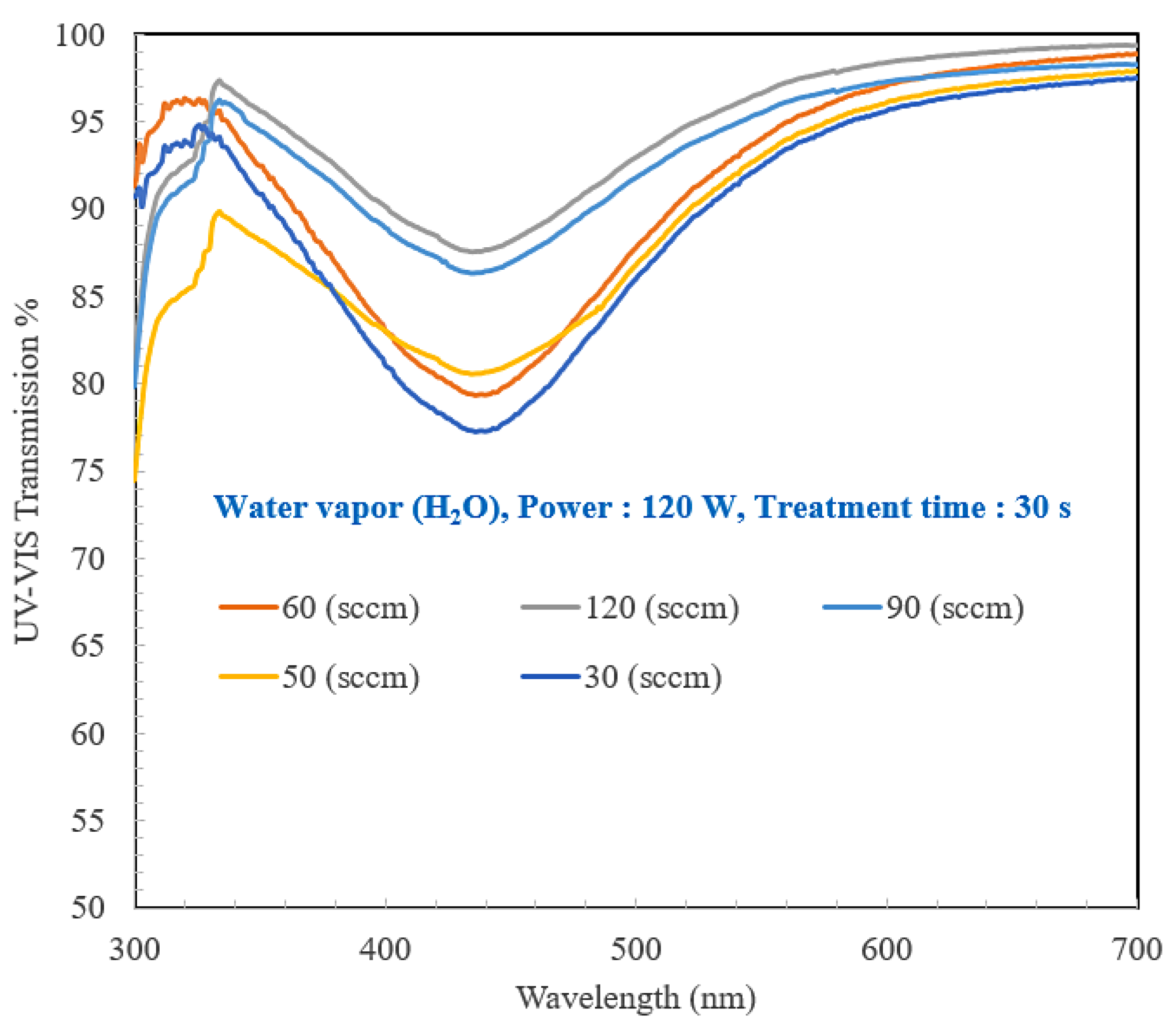


| H2O Flow Rate (sccm) | 30 | 50 | 60 | 90 | 120 |
| Power 120 W, Bond strength (N/mm2) | 1.56 | 2.18 | 2.43 | 1.68 | 1.32 |
| Power 60 W, Bond strength (N/mm2) | 1.08 | 1.52 | 1.7 | 1.1 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, C.-C.; Immanuel, P.N.; Chiu, Y.-H.; Huang, S.-J. Heterogeneous Bonding of PMMA and Double-Sided Polished Silicon Wafers through H2O Plasma Treatment for Microfluidic Devices. Coatings 2021, 11, 580. https://doi.org/10.3390/coatings11050580
Chiang C-C, Immanuel PN, Chiu Y-H, Huang S-J. Heterogeneous Bonding of PMMA and Double-Sided Polished Silicon Wafers through H2O Plasma Treatment for Microfluidic Devices. Coatings. 2021; 11(5):580. https://doi.org/10.3390/coatings11050580
Chicago/Turabian StyleChiang, Chao-Ching, Philip Nathaniel Immanuel, Yi-Hsiung Chiu, and Song-Jeng Huang. 2021. "Heterogeneous Bonding of PMMA and Double-Sided Polished Silicon Wafers through H2O Plasma Treatment for Microfluidic Devices" Coatings 11, no. 5: 580. https://doi.org/10.3390/coatings11050580
APA StyleChiang, C.-C., Immanuel, P. N., Chiu, Y.-H., & Huang, S.-J. (2021). Heterogeneous Bonding of PMMA and Double-Sided Polished Silicon Wafers through H2O Plasma Treatment for Microfluidic Devices. Coatings, 11(5), 580. https://doi.org/10.3390/coatings11050580








