1. Introduction
Microreactors with micro-sized channels are being applied in various fields, and, in particular, studies of various reactions (e.g., steam reforming [
1] and methane combustion [
2]) have been conducted in chemical engineering. Microreactors have a large surface area per unit volume (10,000 to 50,000 m
2/m
3) compared to conventional reactors (<1000 m
2/m
3, but generally ~100 m
2/m
3), resulting in improved heat and mass transfer rates [
3,
4].
Zhang et al. [
5] fabricated a plate that had 32 channels with a width and depth of 0.5 mm on stainless steel 316 L and cleaned it using 5% NaOH, 30% HNO
3 and acetone sequentially. In order to generate a uniform alumina whisker, substrates are filled with a mixture of Al, Al
2O
3, and NH
4Cl powder and heated at 850 ℃ for 10 h to form an aluminum layer, and then they are heated at 900 ℃ for 10 h with a rate of 3 ℃/min. For comparison, the surface pretreatment was performed on the same substrate with a mixed solution of HCl and HNO
3. These were denominated with aluminizing pretreatment and acid pretreatment, respectively. Polyvinyl alcohol (PVA), γ-Al
2O
3 (3 µm), etc. were used to prepare an alumina sol and a suspension of pH 3.5, and the acid pretreatment substrate was additionally coated with alumina sol, dried, and then calcined at 600 ℃ for 5 h. Thereafter, the two pretreated substrates were calcined at 600 ℃ for 2 h. The surfaces before and after coating were compared through two pretreatment methods, and it was confirmed that there was no crack on the aluminizing pretreated substrate, and the weight loss was 1.24%, which was relatively lower than that of the acid pretreatment. It was suggested that the aluminizing pretreatment could enhance the adhesion when applying γ-Al
2O
3 coating on a metallic substrate lacking an aluminum component.
Peela et al. [
6] used aluminum-free stainless steel 304 as a substrate to investigate the effects of slurry pH, viscosity, and binders and primers of various compositions on coating properties, such as morphology, uniformity, and loading. Microchannels were fabricated with a depth, width, and length of 0.4, 0.5 and 20 mm, respectively (25 channels per plate). The channels were chemically pretreated with a mixture of HCl and HNO
3, followed by a soap solution, and finally acetone, whereas no thermal pretreatment was performed. In this study, it was confirmed that 2% DISPERAL
® P2 and 4% PVA aqueous solution was the optimum primer composition. On this primer, a catalyst slurry of 20% γ-alumina and 4% PVA was washcoated and after 1 h of sonication, and 2.9% of weight loss, was measured. In addition, it was reported that a uniform and well-adhered surface was achieved when the catalyst slurry had pH 3.5 and viscosity in the range of 90–120 mPa at a shear rate of 200 s
−1; moreover, decreased cracks and improved adherence were achieved when colloidal alumina was added as a binder.
Katheria et al. [
1] investigated the effect of the thermal surface pretreatment method, pH, and binder composition on the coating. They used FeCrAlloy foils as substrate with a width, length, and depth of 30, 30, and 0.1 mm, respectively. The substrate was cleaned with acetone and then chemically pretreated with 10 wt.% NaOH and 10 wt.% HNO
3. Thermal surface pretreatment was performed at 850, 900, 950 ℃ for 10 h and 900 ℃ for 15 h with a rate of 2 ℃/min. After both pretreatments, they analyzed the surface using scanning electron microscopy (SEM) and confirmed the presence of well-grown whiskers on the calcined surface at 900 ℃ for 15 h. The stable Ni/MgAl
2O
4 slurry was obtained at pH 4, and a uniform coating was formed on the surface of FeCrAlloy at this pH. The authors also noted a 5.5% weight loss, which is the smallest value when using a binder of 2 wt.% PVA and 4 wt.% colloidal alumina among the various compositions.
Wu et al. [
7] investigated the effect of adhesion of alumina sol on chemical and thermal pretreatment using FeCrAlloy foil. Substrates with a width, length, and thickness of 20, 50, and 0.1 mm, respectively, were used, and after polishing the surface with sandpaper, ultrasonic cleaning was performed for 30 min using acetone. For chemical pretreatment, H
2SO
4, HCl, HNO
3, and NaOH were individually used at 1 mol/L each, and thermal pretreatment was performed by heating at 600, 800, and 1000 ℃ for 5, 10 or 15 h. It was confirmed that the combination of chemical and thermal pretreatment on the rough metal surface contributes to the formation of α-Al
2O
3 whiskers, which leads to an improvement in the adhesion of the washcoated surface.
The substrate made of stainless steel or FeCrAlloy was used, and thermal surface pretreatment and/or chemical surface pretreatment were performed in previous studies. While a number of studies on thermal surface pretreatment with various heating temperatures and times have been investigated, studies on various chemical surface pretreatments have been difficult to investigate.
Meanwhile, in the case of FeCrAlloy, which has a relatively high thermal resistance and oxidation resistance, Al atoms preferentially migrate to the surface and form the alumina layer when exposed to high temperatures and a high-oxidation environment [
8]. This alumina layer provides uniform slurry distribution and improves the adhesion. However, stainless steel has no or very little aluminum components compared with FeCrAlloy, so another pretreatment and/or coating is required to form the alumina layer [
5,
9].
In order to increase the adhesion of the catalyst to operate the microchannel reactor for a long time, the formation of alumina on the surface of the channel is required. For this surface treatment, various chemical surface pretreatment methods were performed on FeCrAlloy to investigate the surface condition of the substrate, and based on the results, qualitative and quantitative analyses of the surface condition of the substrate were conducted in this study.
2. Materials and Methods
FeCrAlloy
® (Goodfellow, Huntingdon, UK) plates were fabricated with dimensions of 20 mm × 20 mm × 1 mm and channels of a rectangular shape with a width and depth of 1 and 0.5 mm, respectively. The FeCrAlloy plate and its composition are shown in
Figure 1 and
Table 1, respectively. To remove impurities and organic substances on the substrates, all substrates were first pre-washed with acetone. Moreover, three chemical pretreatments were additionally performed using sodium hydroxide (NaOH), hydrochloric acid (HCl), and nitric acid (HNO
3). The solutions and compositions used in various treatment methods are shown in
Table 2. Pre-washing and each treatment were performed in an ultrasonic bath at room temperature for 30 min, and residual solutions on the substrates were removed using distilled water and an air gun.
All chemically pretreated substrates were thermally treated at 900 ℃ for 15 h with a rate of 2 ℃/min [
1]. Treated substrates were named according to the treatment method and were as follows: AH (treated by treatment method 1), AcH (treated by treatment method 2), BH (treated by treatment method 3), and BAcH (treated by treatment method 4) [
10]. A, Ac, B, and H stand for acetone, acidic solution, basic (alkaline) solution, and heating, respectively.
In order to operate the microchannel reactor for a long time and to improve the adhesion of the catalyst, the surface area of the substrate must be increased so that it is well fixed inside the channels. For this, the formation of alumina is required, as it increases the surface area to which the catalyst is to be bonded and fixes it well. SEM, EDS and XRD analyses were performed to identify on which substrate among the various chemical pretreatments the alumina was well formed.
3. Results
Scanning electron microscopy (SEM, SU8230, Hitachi, Tokyo, Japan), energy dispersive X-ray spectroscopy (EDS, SU8230, Hitachi, Tokyo, Japan) and X-ray diffraction (XRD, SmartLab, Rigaku, Tokyo, Japan) analyses were performed to analyze the surface of the substrate thermally treated with different chemical pretreatments.
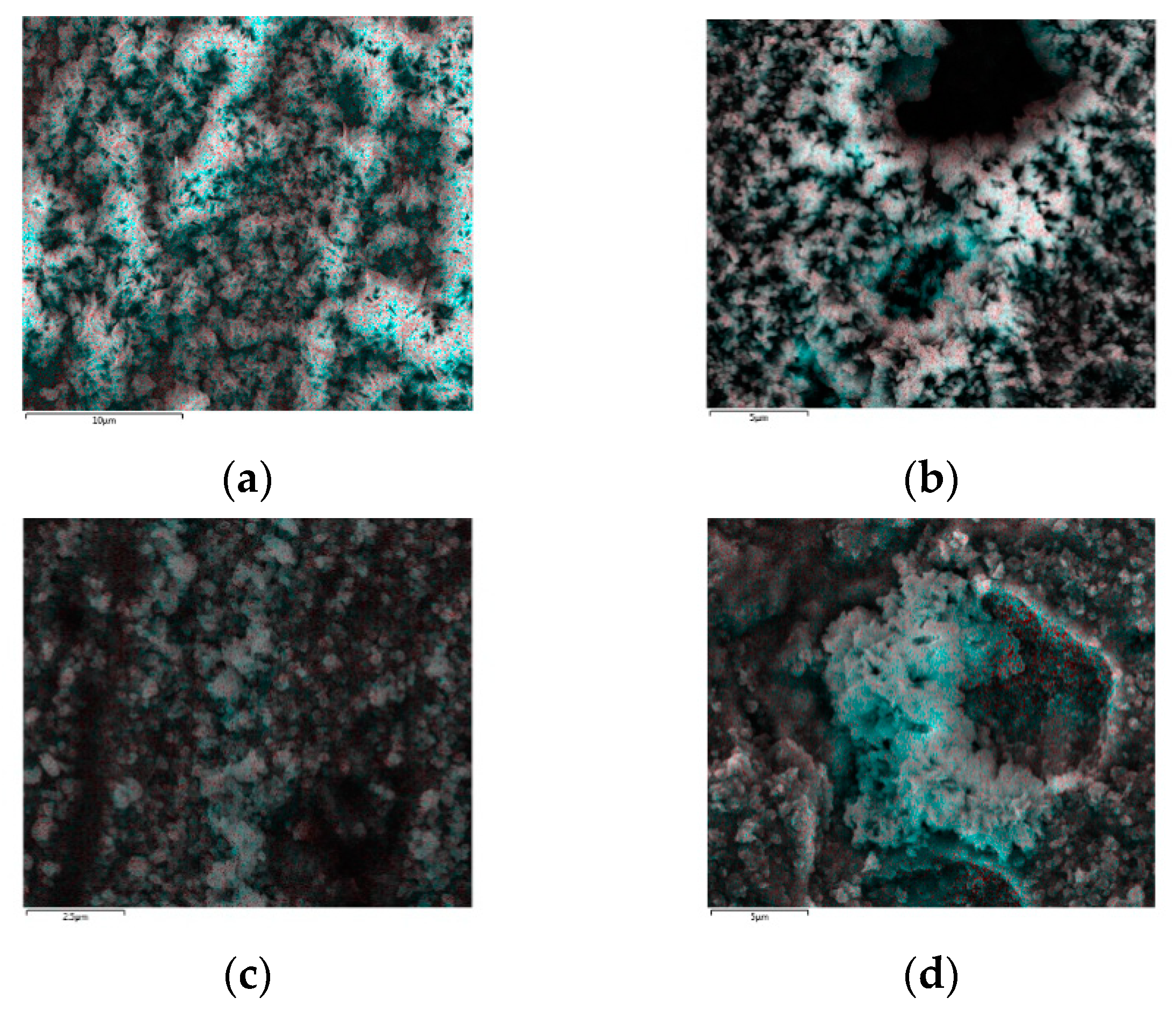
Figure 2 depicts the SEM images of the surface treated with different chemical treatment methods. Although alumina whiskers were observed on the substrate treated with treatment methods 2, 3, and 4, the corroded surfaces were also found in several places, and it was confirmed that the whiskers were not properly generated on these surfaces (
Figure 2b–d). On the other hand, it was confirmed that there was rarely corrosion on the surface treated with treatment method 1, and the needle-like α-alumina whisker [
2] was also relatively evenly generated compared to that of the other three surfaces (
Figure 2a).
Figure 3 depicts EDS mapping images of the surface treated with different chemical treatment methods. The shape of the alumina whisker on all the substrates was qualitatively analyzed. Unlike the other three substrates (
Figure 3b–d), the alumina whisker shape was observed on the surface of the substrate treated with only acetone (AH,
Figure 3a), and it was confirmed that it was formed evenly over the entire surface. In the AcH substrate (
Figure 3b), alumina whiskers were generated relatively well in most places, but the surface of the alumina layer was corroded in several places, and the exposed part of metal substrate was observed. In the BH substrate (
Figure 3c), the alumina whisker could be observed, but its shape was considerably blunt in most places when compared to that of the AH substrate (
Figure 3a), and the metal surfaces were exposed in some places. While the surfaces of the AcH and BH (
Figure 2b,c) substrates were mixed, in the BAcH substrate (
Figure 3d), well-formed alumina whiskers and whiskers that were not so well formed were observed. Many of them were blunt, and the corrosion of the alumina layer was also observed in several places.
Figure 4 depicts EDS mapping images of only the aluminum and oxygen of the surface treated with different chemical treatment methods. Compared to the remaining three substrates, it was found that aluminum and oxygen were evenly distributed in the AH substrate (
Figure 4a). It was noted that the BH substrate (
Figure 4c) also showed good distribution when compared to that of the AcH and BAcH substrates (
Figure 4b,d), but its degree was relatively weak compared to that of the AH substrate.
Table 3 shows compositions of only the aluminum and oxygen of the surface treated with different chemical treatment methods. The weight and atomic composition were also quantitatively analyzed in EDS analysis, and since the focus was on alumina whiskers, compositions of iron, chromium, and other trace elements were not indicated. As shown in the weight percent in
Table 3, it was found that the proportion of aluminum and oxygen in the AH and AcH substrates was relatively higher than that of the other two substrates.
Figure 5 depicts the XRD patterns of the pretreated surface of each substrate. All of the substrates show the patterns of α-Al
2O
3 as the major phase formed during thermal treatment. For the AH substrate (line 1 in
Figure 5), the peaks at 2θ values of α-Al
2O
3 (ICDD No. 04-014-1368) were observed at 25.42°, 35.01°, 39.78°, 42.33°, and 57.25°. For the AcH substrate (line 2 in
Figure 5), the peaks at 2θ values of α-Al
2O
3 (ICDD No. 01-088-0826) were observed at 25.49°, 35.04°, 37.69°, 43.25°, 52.49°, 57.46°, and 66.25°. For the BH substrate (line 3 in
Figure 5), the peaks at 2θ values of α-Al
2O
3 (ICDD No. 01-088-0826) were observed at 25.46°, 35.01°, 37.78°, 43.17°, 52.35°, and 57.27°. For the BAcH substrate (line 4 in
Figure 5), the peaks at 2θ values of α-Al
2O
3 (ICDD No. 00-046-1212) were observed at 25.46°, 35.03°, 43.2°, and 57.39°. The diffraction patterns of each substrate appeared to be similar. The presence of α-Al
2O
3 was confirmed on the surface of the test samples.
Each substrate was coated with primer slurry to test the adhesion according to the chemical treatment. Primer slurry was prepared by mixing PVA (polyvinyl alcohol) aqueous solution (74.3%), γ-Al
2O
3 (22.3%), boehmite (2.5%), and acetic acid (1%). A total of 100 mg of slurry was coated on each substrate by the fill-and-dry method. For the adhesion test, the substrate was immersed in acetone, and then the vibration test was performed in an ultrasonic bath (Elmasonic P300H, Elma, Singen, Germany) at room temperature. There was no significant difference in weight loss in each substrate. The results of the vibration test are shown in
Table 4.
4. Conclusions
In this study, the surface pretreatments of the FeCrAlloy substrate were performed through various chemical treatments and a thermal treatment. Pretreated surfaces were qualitatively and quantitatively analyzed by scanning electron microscopy (SEM), energy dispersive spectrometry (EDS) and X-ray diffraction (XRD).
Qualitative analysis through SEM showed the alumina whisker on the AH and AcH substrates, and EDS mapping showed that the distribution of aluminum and oxygen, the main components of the alumina whisker, was evenly spread compared to the other substrates. Moreover, in the quantitative analysis through EDS, it was confirmed that the AH and AcH substrates achieved a relatively high weight ratio of aluminum and oxygen compared to the other two substrates. It was also confirmed through XRD that α-Al2O3 was the main oxide phase that formed during the thermal pretreatment process. In addition, each substrate was coated with primer slurry to perform adhesion tests on the substrates according to different chemical pretreatments. There was no significant difference in weight loss according to different chemical pretreatments. Therefore, it is considered that the use of relatively harmful acid and/or basic solutions in the pretreatment process can be reduced, and the time required to complete the pretreatment process can also be reduced.