Electrodeposition Based Preparation of Zn–Ni Alloy and Zn–Ni–WC Nano-Composite Coatings for Corrosion-Resistant Applications
Abstract
1. Introduction
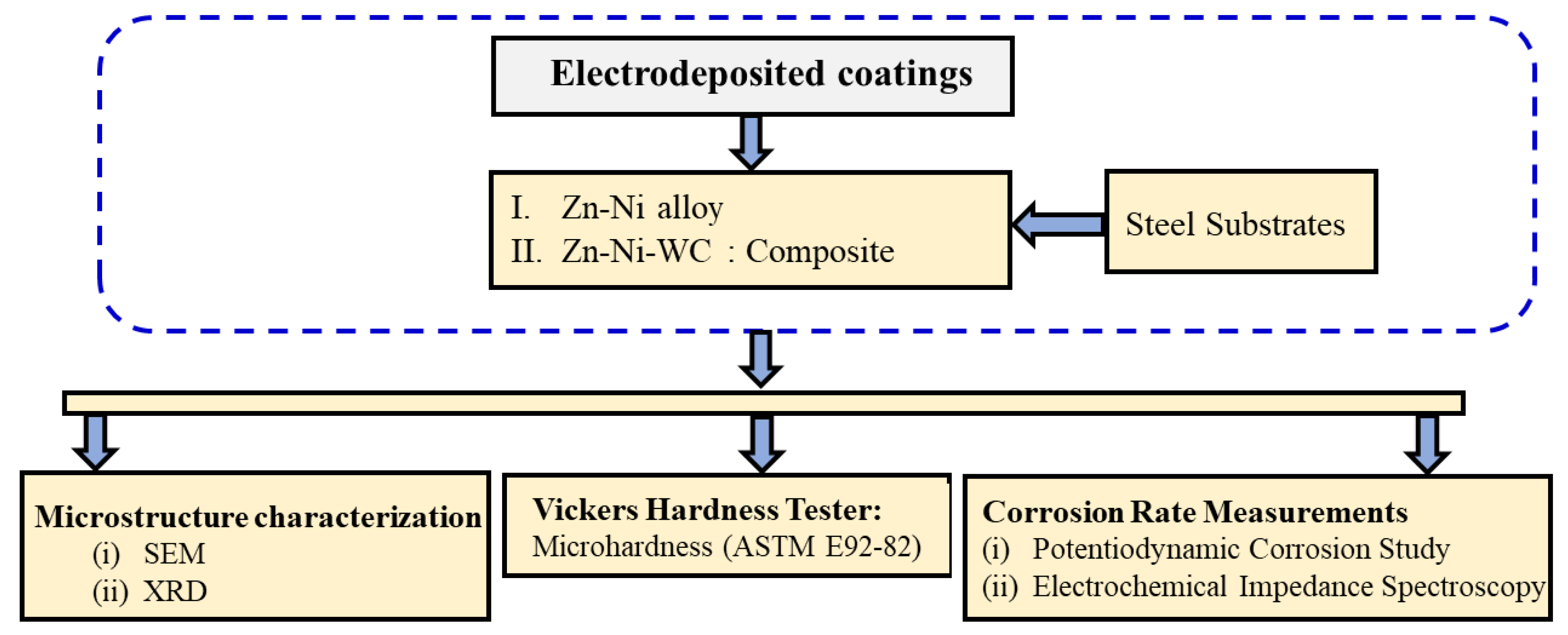
2. Materials and Methods
2.1. Materials and Electrodeposition Method
2.2. Microstructural Characterization
2.3. Microhardness Measurement
2.4. Corrosion Rate Measurements
3. Results
3.1. Tungsten Carbide (WC) Particle Characterization
3.2. Electrodeposition
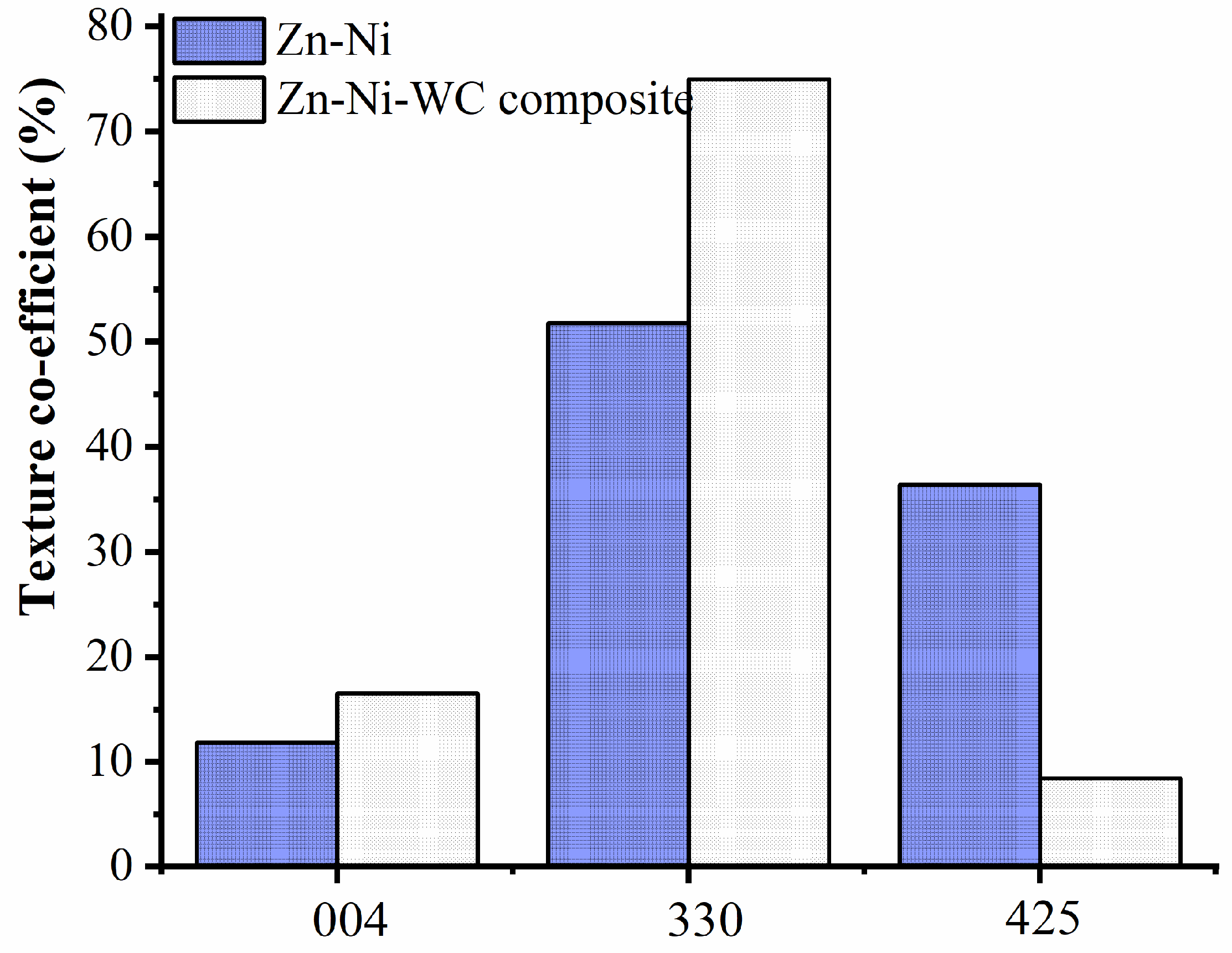
3.3. Characterization of the Deposits
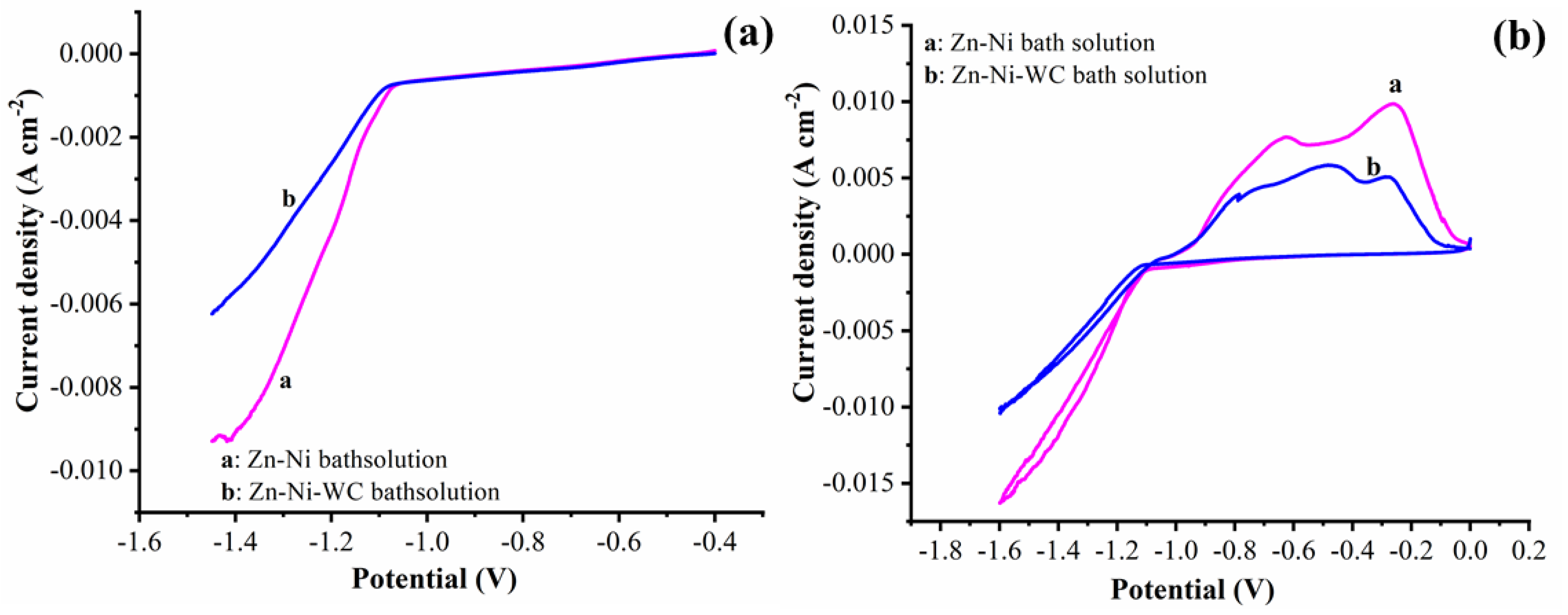
3.4. Corrosion Behavior of the Electrodeposited Coatings
4. Conclusions
- On mild steel specimens, Zn–Ni alloy and Zn–Ni–WC coatings were successfully developed, which can be used in industrial applications to protect parts from chemical/electrochemical deterioration.
- The incorporation of WC particles into the Zn–Ni matrix was confirmed from the SEM and EDAX spectroscopy.
- The composite coating’s SEM images show a smoother grained deposit than the Zn–Ni alloy coating.
- The XRD results shows that the composite coating has smaller crystallites than the Zn–Ni coating.
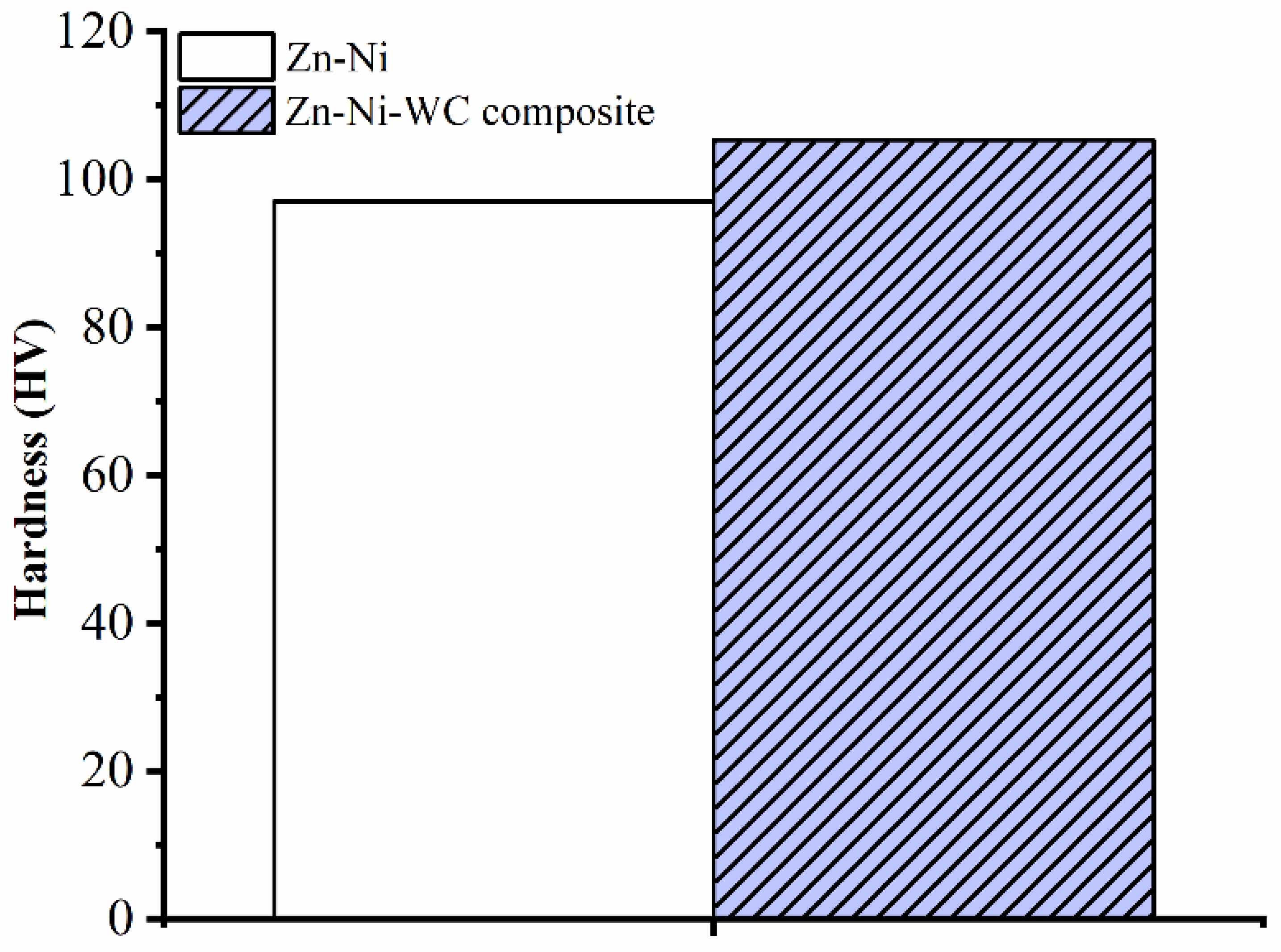
- The hardness test revealed that the Zn–Ni–WC composite coating has a higher hardness compared to the Zn–Ni coating. The addition of Ni metallic particles to pure Zn, i.e., Zn–Ni alloy, enhanced microhardness by 103.78% compared to pure Zn. The addition of WC particles to the Zn–Ni alloy i.e., Zn–Ni–WC composites, resulted in 8.56% increased microhardness in the Zn–Ni alloy.
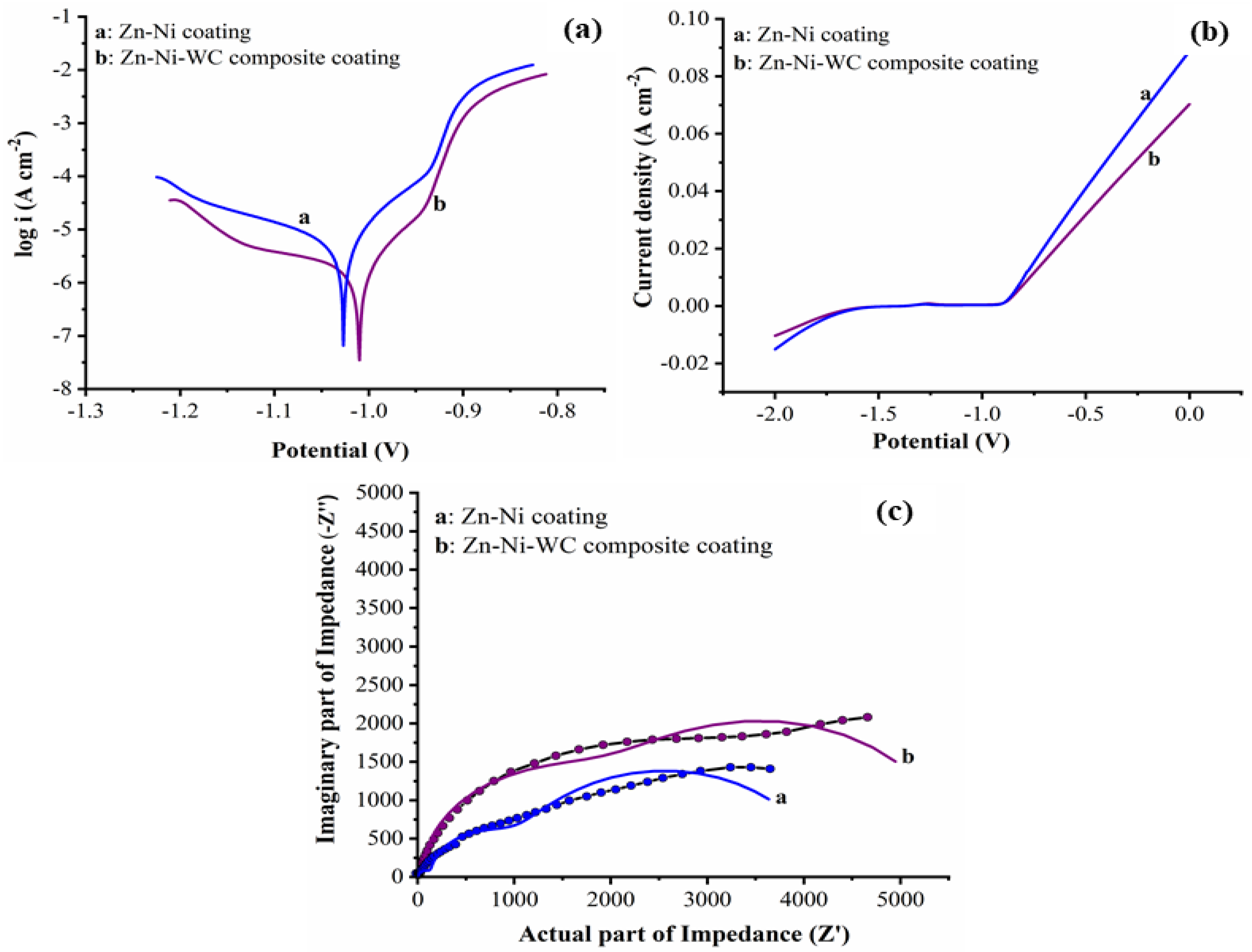
- Polarization, potentiodynamic curves, and impedance spectra techniques were used to investigate the improved corrosion rate of the Zn–Ni–WC composite coating. Ni metallic ions alloyed with the Zn matrix exhibited a 57.47% decreased corrosion rate in coatings compared to WO3 particles. The addition of WC nanoparticles to Zn–Ni alloy, i.e., Zn–Ni–WC composites, resulted in a 41.71% decreased corrosion rate than the Zn–Ni alloy.
- Zn–Ni–WC composite nanocoatings could protect the steel surface effectively from electrochemical deterioration subjected to corrosive media.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bajaj, P.; Hariharan, A.; Kini, A.; Kürnsteiner, P.; Raabe, D.; Jägle, E.A. Steels in additive manufacturing: A review of their microstructure and properties. Mater. Sci. Eng. A 2020, 772, 138633. [Google Scholar] [CrossRef]
- Qudeiri, J.E.A.; Zaiout, A.; Mourad, A.H.I.; Abidi, M.H.; Elkaseer, A. Principles and characteristics of different EDM processes in machining tool and die steels. Appl. Sci. 2020, 10, 2082. [Google Scholar] [CrossRef]
- Wei, H.; Chen, Y.L.; Yu, W.; Su, L.; Wang, X.; Tang, D. Study on corrosion resistance of high-strength medium-carbon spring steel and its hydrogen-induced delayed fracture. Constr. Build. Mater. 2020, 239, 117815. [Google Scholar] [CrossRef]
- Sumita, M.; Hanawa, T.; Teoh, S.H. Development of nitrogen-containing nickel-free austenitic stainless steels for metallic biomaterials. Mater. Sci. Eng. C 2004, 24, 753–760. [Google Scholar] [CrossRef]
- Keerthana, A.K.; Ashraf, P.M. Carbon nanodots synthesized from chitosan and its application as a corrosion inhibitor in boat-building carbon steel BIS2062. Appl. Nanosci. 2020, 10, 1061–1071. [Google Scholar] [CrossRef]
- Di Schino, A. Manufacturing and Applications of Stainless Steels. Metals 2020, 10, 327. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, B.; Zhou, Y.; Wu, Y.; Zhu, H. Evaluation of pitting corrosion in duplex stainless steel Fe20Cr9Ni for nuclear power application. Acta Mater. 2020, 197, 172–183. [Google Scholar] [CrossRef]
- Schmitt, G. Global Needs for Knowledge Dissemination, Research, and Development in Materials Deterioration and Corrosion Control; World Corrosion Organization: New York, NY, USA, 2009. [Google Scholar]
- The World Corrosion Organization. Available online: https://corrosion.org/ (accessed on 2 June 2021).
- Zotov, S.V. Analysis of galvanized coatings applied on general purpose wire. In Solid State Phenomena; Trans Tech Publications Ltd.: Bäch, Switzerland, 2020; Volume 299, pp. 827–832. [Google Scholar] [CrossRef]
- Hegyi, A.; Dico, C.; Constantinescu, H.; Baera, C. Influence of hot-dip galvanizing of reinforcement on the kinetics and thermodynamics of corrosion process in concrete. Procedia Eng. 2017, 181, 226–233. [Google Scholar] [CrossRef]
- Song, P.; Liu, M.; Jiang, X.; Feng, Y.; Wu, J.; Zhang, G.; Wang, D.; Dong, J.; Chen, X.; Lou, L. Influence of alloying elements on hot corrosion resistance of nickel-based single crystal superalloys coated with Na2SO4 salt at 900 °C. Mater. Des. 2021, 197, 109197. [Google Scholar] [CrossRef]
- Nady, H.; El-Rabiei, M.M.; Samy, M.; Badawy, W.A. The Influence of Alloying El-ements (Al, Ni and Zn) on the Corrosion Resistance of Some Cu-Ternary Alloys in Na2SO4 Solutions. J. Biol. Tribocorros. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Han, J.; Wu, H.C.; Yang, B.; Wang, X.T. Effect of sigma phase precipitation on the mechanical and wear properties of Z3CN20. 09M cast duplex stainless steel. Nucl. Eng. Des. 2013, 259, 1–7. [Google Scholar] [CrossRef]
- Baruwa, A.D.; Akinlabi, E.T.; Oladijo, O.P. Surface coating processes: From conventional to the advanced methods: A short review. In Advances in Manufacturing Engineering, Lecture Notes in Mechanical Engineering; Emamian, S.S., Awang, M., Yusof, F., Eds.; Spring: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Koga, G.Y.; Wolf, W.; Schulz, R.; Savoie, S.; Bolfarini, C.; Kiminami, C.S.; Botta, W.J. Corrosion and wear properties of FeCrMnCoSi HVOF coatings. Surf. Coat. Technol. 2019, 357, 993–1003. [Google Scholar] [CrossRef]
- Gu, Y.; Xia, K.; Wu, D.; Mou, J.; Zheng, S. Technical characteristics and wear-resistant mechanism of nano coatings: A review. Coatings 2020, 10, 233. [Google Scholar] [CrossRef]
- Chalker, P.R.; Bull, S.J.; Rickerby, D.S. A review of the methods for the evaluation of coating-substrate adhesion. Mater. Sci. Eng. A 1991, 140, 583–592. [Google Scholar] [CrossRef]
- Schrooten, J.; Helsen, J.A. Adhesion of bioactive glass coating to Ti6Al4V oral implant. Biomaterials 2000, 21, 1461–1469. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Arango, S.; Peláez-Vargas, A.; García, C. Coating and surface treatments on orthodontic metallic materials. Coatings 2013, 3, 1–15. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and engineering of superhydrophobic surfaces: Review of corrosion resistance, chemical and mechanical stability. Arab. J. Chem. 2020, 13, 1763–1802. [Google Scholar] [CrossRef]
- Chen, Z.; Hao, L.; Chen, A.; Song, Q.; Chen, C. A rapid one-step process for fabrication of superhydrophobic surface by electrodeposition method. Electrochim. Acta 2012, 59, 168–171. [Google Scholar] [CrossRef]
- She, X.; Wu, J.; Xu, H.; Mo, Z.; Lian, J.; Song, Y.; Liu, L.; Du, D.; Li, H. Enhancing charge density and steering charge unidirectional flow in 2D non-metallic semiconductor-CNTs-metal coupled photocatalyst for solar energy conversion. Appl. Catal. B Environ. 2017, 202, 112–117. [Google Scholar] [CrossRef]
- Walsh, F.C.; Wang, S.; Zhou, N. The electrodeposition of composite coatings: Diversity, applications and challenges. Curr. Opin. Electrochem. 2020, 20, 8–19. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Q.; Liu, T.; Zhang, B. The preservation damage of hydrophobic polymer coating materials in conservation of stone relics. Prog. Org. Coat. 2013, 76, 1127–1134. [Google Scholar] [CrossRef]
- Chanana, M.; Liz-Marzán, L.M. Coating matters: The influence of coating materials on the optical properties of gold nanoparticles. Nanophotonics 2012, 1, 199–220. [Google Scholar] [CrossRef]
- DeMasi-Marcin, J.T.; Gupta, D.K. Protective coatings in the gas turbine engine. Surf. Coat. Technol. 1994, 68, 522. [Google Scholar] [CrossRef]
- Vignesh, S.; Shanmugam, K.; Balasubramanian, V.; Sridhar, K. Identifying the optimal HVOF spray parameters to attain minimum porosity and maximum hardness in iron based amorphous metallic coatings. Def. Technol. 2017, 13, 101–110. [Google Scholar] [CrossRef]
- Rajahram, S.S.; Harvey, T.J.; Wood, R.J.K. Erosion–corrosion resistance of engineering materials in various test conditions. Wear 2009, 267, 244–254. [Google Scholar] [CrossRef]
- Chavan, N.M.; Kiran, B.; Jyothirmayi, A.; Phani, P.S.; Sundararajan, G. The corrosion behavior of cold sprayed zinc coatings on mild steel substrate. J. Therm. Spray Technol. 2013, 22, 463–470. [Google Scholar] [CrossRef]
- Proskurkin, E.V.; Sukhomlin, D.A. A Study of the corrosion resistance of pump–compressor pipes with a diffusion zinc coating under the complicated conditions of gas-producing wells. Prot. Met. Phys. Chem. Surf. 2017, 53, 1288–1294. [Google Scholar] [CrossRef]
- Roghanian, N.; Banthia, N. Development of a sustainable coating and repair material to prevent bio-corrosion in concrete sewer and waste-water pipes. Cem. Concr. Compos. 2019, 100, 99–107. [Google Scholar] [CrossRef]
- Lee, L.; Behera, P.; Sriraman, K.R.; Chromik, R.R. The effect of contact stress on the sliding wear behaviour of Zn-Ni electrodeposited coatings. Wear 2018, 400, 82–92. [Google Scholar] [CrossRef]
- Conde, A.; Arenas, M.A.; De Damborenea, J.J. Electrodeposition of Zn–Ni coatings as Cd replacement for corrosion protection of high strength steel. Corros. Sci. 2011, 53, 1489–1497. [Google Scholar] [CrossRef]
- Sriraman, K.R.; Brahimi, S.; Szpunar, J.A.; Osborne, J.H.; Yue, S. Tribocorrosion behavior of Zn, Zn–Ni, Cd and Cd–Ti electrodeposited on low carbon steel substrates. Surf. Coat. Technol. 2013, 224, 126–137. [Google Scholar] [CrossRef]
- El Hajjami, A.; Gigandet, M.P.; De Petris-Wery, M.; Catonne, J.C.; Duprat, J.J.; Thiery, L.; Raulin, F.; Pommier, N.; Starck, B.; Remy, P. Characterization of thin Zn–Ni alloy coatings electrodeposited on low carbon steel. Appl. Surf. Sci. 2007, 254, 480–489. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Li, G.; Li, C. Effects of zinc coating on interfacial microstructures and mechanical properties of aluminum/steel bimetallic composites. J. Alloys Compd. 2016, 678, 249–257. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, B.; Zhang, X.; Sun, T.; Li, G.; Cui, J. Mechanical properties and interfacial microstructures of magnetic pulse welding joints with aluminum to zinc-coated steel. Mater. Sci. Eng. A. 2020, 788, 139425. [Google Scholar] [CrossRef]
- Klekotka, M.; Zielińska, K.; Stankiewicz, A.; Kuciej, M. Tribological and anticorrosion performance of electroplated zinc based nanocomposite coatings. Coatings 2020, 10, 594. [Google Scholar] [CrossRef]
- Malatji, N.; Popoola, A.P.I.; Fayomi, O.S.I. The effect of nanoparticulate loading on the fabrication and characterization of multi-doped Zn-Al2O3-Cr2O3 hybrid coatings on mild steel. Int. J. Adv. Manuf. Tech. 2017, 90, 2443–2452. [Google Scholar] [CrossRef]
- Popoola, A.P.I.; Daniyan, A.A.; Umoru, L.E.; Fayomi, O.S.I. Effect of WO3 nanoparticle loading on the microstructural, mechanical and corrosion resistance of Zn matrix/TiO2-WO3 nanocomposite coatings for marine application. J. Mar. Sci. Technol. 2017, 16, 102–109. [Google Scholar] [CrossRef]
- Utu, I.D.; Muntean, R.; Mitelea, I. Corrosion and Wear Properties of Zn-Based Composite Coatings. J. Mater. Eng. Perform. 2020, 29, 5360–5365. [Google Scholar] [CrossRef]
- PraveenKumar, C.M.; Venkatesha, T.V.; Vathsala, K.; Nayana, K.O. Electrodeposition and corrosion behavior of Zn–Ni and Zn–Ni–Fe2O3 coatings. J. Coat. Technol. Res. 2012, 9, 71–77. [Google Scholar] [CrossRef]
- Lotfi, N.; Aliofkhazraei, M.; Rahmani, H.; Darband, G.B. Zinc–nickel alloy electrodeposition: Characterization, properties, multilayers and composites. Prot. Met. Phys. Chem. S 2018, 54, 1102–1140. [Google Scholar] [CrossRef]
- Bhat, R.S.; Shet, V.B. Development and characterization of Zn–Ni, Zn–Co and Zn–Ni–Co coatings. Surf. Eng. 2020, 36, 429–437. [Google Scholar] [CrossRef]
- Arrighi, C.; Savall, C.; Cohendoz, S.; Grosseau-Poussard, J.L.; Baissac, L.; Olivier, M.G.; Creus, J. Optimization of the morphology, structure and properties of high iron content Zn–Fe coatings by pulse electrodeposition. Mater. Chem. Phys. 2021, 263, 124366. [Google Scholar] [CrossRef]
- Dini, J.W.; Johnson, H.R. Electrodeposition of Zinc—Nickel Alloys Coatings; (No. SAND-77–8511; CONF-771094–1); Sandia National Lab. (SNL-NM): Albuquerque, NM, USA, 1977. [Google Scholar]
- Park, J.H.; Kosugi, D.; Hagio, T.; Kamimoto, Y.; Ichino, R.; Lee, M.H. Improvement in corrosion resistance of ternary Zn-Fe-Mo plating by additional Mo-oxide coating. Surf. Coat. Technol. 2020, 389, 125567. [Google Scholar] [CrossRef]
- Toghan, A.; Abou-krisha, M.M.; Assaf, F.H.; El-Sheref, F. Effect of deposition potential on the mechanism and corrosion behavior of Zn-Fe-Co thin coatings electrochemically deposited on a steel substrate. Int. J. Electrochem. Sci. 2021, 16, 151044. [Google Scholar] [CrossRef]
- Kumar, C.M.P.; Chandrashekarappa, M.P.G.; Kulkarni, R.M.; Pimenov, D.Y.; Giasin, K. The Effect of Zn and Zn–WO3 Composites Nano-Coatings Deposition on Hardness and Corrosion Resistance in Steel Substrate. Materials 2021, 14, 2253. [Google Scholar] [CrossRef]
- Miller, R.J.; Adeleye, A.S.; Page, H.M.; Kui, L.; Lenihan, H.S.; Keller, A.A. Nano and traditional copper and zinc antifouling coatings: Metal release and impact on marine sessile invertebrate communities. J. Nanopart. Res. 2020, 22, 547. [Google Scholar] [CrossRef]
- Roventi, G.; Bellezze, T.; Fratesi, R. Electrodeposition of Zn–SiC nanocomposite coatings. J. Appl. Electrochem. 2013, 43, 839–846. [Google Scholar] [CrossRef]
- Guan, Z.; Linsley, C.S.; Pan, S.; DeBenedetto, C.; Liu, J.; Wu, B.M.; Li, X. Highly Ductile Zn-2Fe-WC Nanocomposite as Biodegradable Material. Metall. Mater. Trans. A 2020, 51, 4406–4413. [Google Scholar] [CrossRef]
- Blagoveshchenskiy, Y.V.; Isaeva, N.V.; Sinaiskiy, M.A.; Ankudinov, A.B.; Zelensky, V.A. Tuning the properties of refractory carbide nanopowders. Org. Mater. Appl. Res. 2018, 9, 924–929. [Google Scholar] [CrossRef]
- Farahmand, P.; Liu, S.; Zhang, Z.; Kovacevic, R. Laser cladding assisted by induction heating of Ni–WC composite enhanced by nano-WC and La2O3. Ceram. Int. 2014, 40, 15421–15438. [Google Scholar] [CrossRef]
- Paul, C.P.; Mishra, S.K.; Tiwari, P.; Kukreja, L.M. Solid-particle erosion behaviour of WC/Ni composite clad layers with different contents of WC particles. Opt. Laser Technol. 2013, 50, 155–162. [Google Scholar] [CrossRef]
- Fernández, M.R.; García, A.; Cuetos, J.M.; González, R.; Noriega, A.; Cadenas, M. Effect of actual WC content on the reciprocating wear of a laser cladding NiCrBSi alloy reinforced with WC. Wear 2015, 324, 80–89. [Google Scholar] [CrossRef]
- Pan, S.; Yao, G.; Sokoluk, M.; Guan, Z.; Li, X. Enhanced thermal stability in Cu-40 wt% Zn/WC nanocomposite. Mater. Des. 2019, 180, 107964. [Google Scholar] [CrossRef]
- Guan, Z.; Linsley, C.S.; Hwang, I.; Yao, G.; Wu, B.M.; Li, X. Novel zinc/tungsten carbide nanocomposite as bioabsorbable implant. Mater. Lett. 2020, 263, 127282. [Google Scholar] [CrossRef]
- He, L.; Liu, H.; Chen, D.; Chen, Z.; Bai, X. Fabrication of HAp/Ni biomedical coatings using an electro-codeposition technique. Surf. Coat. Technol. 2002, 160, 109–113. [Google Scholar] [CrossRef]
- Ruthradevi, T.; Akbar, J.; Kumar, G.S.; Thamizhavel, A.; Kumar, G.A.; Vatsa, R.K.; Dannangoda, G.C.; Martirosyan, K.S.; Girija, E.K. Investigations on nickel ferrite embedded calcium phosphate nanoparticles for biomedical applications. J. Alloys Compd. 2017, 695, 3211–3219. [Google Scholar] [CrossRef]
- Kumar, C.P.; Venkatesha, T.V.; Shabadi, R. Preparation and corrosion behavior of Ni and Ni–graphene composite coatings. Mater. Res. Bull. 2013, 48, 1477–1483. [Google Scholar] [CrossRef]
- Mbugua, N.S.; Kang, M.; Zhu, J.; Liu, Y.; Zhang, Y.; Ndiithi, N.J. Synthesis and characterization of Ni–W/Cr2O3 nanocomposite coatings using electrochemical deposition technique. Coatings 2019, 9, 815. [Google Scholar] [CrossRef]
- Sugiyama, S.; Ogawa, T.; He, L.; Wang, Z.; Adachi, Y. Quantitative analysis of the recovery process in pure iron using x-ray diffraction line profile analysis. Materials 2021, 14, 895. [Google Scholar] [CrossRef] [PubMed]
- Lagos, K.J.; Marinkovic, B.A.; Debut, A.; Vizuete, K.; Guerrero, V.H.; Pardo, E.; Pontón, P.I. Towards iron-titanium oxide nanostructures from ecuadorian black mineral sands. Minerals 2021, 11, 122. [Google Scholar] [CrossRef]
- Pardhasaradhy, N.V. Practical Electroplating Hand Book; Prentice Hall Inc Publications: Hoboken, NJ, USA, 1987. [Google Scholar]
- Praveen, B.M.; Venkatesha, T.V. Electrodeposition and properties of Zn–Ni–CNT composite coatings. J. Alloys Compd. 2009, 482, 53–57. [Google Scholar] [CrossRef]
- Chen, X.H.; Chen, C.S.; Xiao, H.N.; Cheng, F.Q.; Zhang, G.; Yi, G.J. Corrosion behavior of carbon nanotubes–Ni composite coating. Surf. Coat. Technol. 2005, 191, 351–356. [Google Scholar] [CrossRef]
- Lee, H.K.; Lee, H.Y.; Jeon, J.M. Codeposition of micro- and nano-sized SiC Particles in the nickel matrix composite coatings obtained by electroplating. Surf. Coat. Technol. 2007, 201, 4711–4717. [Google Scholar] [CrossRef]
- Fustes, J.; Gomes, A.; da Silva Pereira, M.I. Electrodeposition of Zn–TiO2 nanocomposite films—effect of bath composition. J. Solid State Electr. 2008, 12, 1435–1443. [Google Scholar] [CrossRef]
- Niu, Z.X.; Cao, F.H.; Wei, W.A.N.G.; Zhang, Z.; Zhang, J.Q.; Cao, C.N. Electrodeposition of Ni-SiC nanocomposite film. Trans. Nonferr. Metals Soc. China 2007, 17, 9–15. [Google Scholar] [CrossRef]
- Deepa, K.; Venkatesha, T.; Nagaraja, C.; Vinutha, M. Electrochemical corrosion studies of Zn-CuO and Zn-NiO-CuO composite coatings on mild steel. Anal. Bioanal. Electrochem. 2017, 9, 374–389. Available online: https://www.sid.ir/en/journal/ViewPaper.aspx?id=660641 (accessed on 2 June 2021).
- Mohankumar, P.C.; Venkatesha, V.T. Surfactants Effect on Zn–Si3N4 coating, electrochemical properties, and their corrosion behaviors. Ind. Eng. Chem. Res. 2013, 52, 12827–12837. [Google Scholar] [CrossRef]
- Shi, L.; Sun, C.F.; Gao, P.; Zhou, F.; Liu, W.M. Electrodeposition and characterization of Ni–Co–carbon nanotubes composite coatings. Surf. Coat. Technol. 2006, 200, 4870–4875. [Google Scholar] [CrossRef]
- Li, S.; Han, Z.; Meng, Q.; Zhao, X.; Cao, X.; Liu, B. Effect of WC nanoparticles on the microstructure and properties of WC-bronze-Ni-Mn based diamond composites. Appl. Sci. 2018, 8, 1501. [Google Scholar] [CrossRef]
- Zhang, P.; Pang, Y.; Yu, M. Effects of WC particle types on the microstructures and properties of WC-reinforced Ni60 composite coatings produced by laser cladding. Metals 2019, 9, 583. [Google Scholar] [CrossRef]
- Mohajeri, S.; Dolati, A.; Rezagholibeiki, S. Electrodeposition of Ni/WC nano composite in sulfate solution. Mater. Chem. Phys. 2011, 129, 746–750. [Google Scholar] [CrossRef]
- Frankel, G.S. Electrochemical techniques in corrosion: Status, limitations, and needs. J. Test. Eval. 2014, 42, 517–538. [Google Scholar] [CrossRef]
- Cai, S.Y.; Wen, L.; Jin, Y. A comparative study on corrosion kinetic parameter estimation methods for the early stage corrosion of Q345B steel in 3.5 wt% NaCl solution. Int. J. Min. Met. Mater. 2017, 24, 1112–1124. [Google Scholar] [CrossRef]
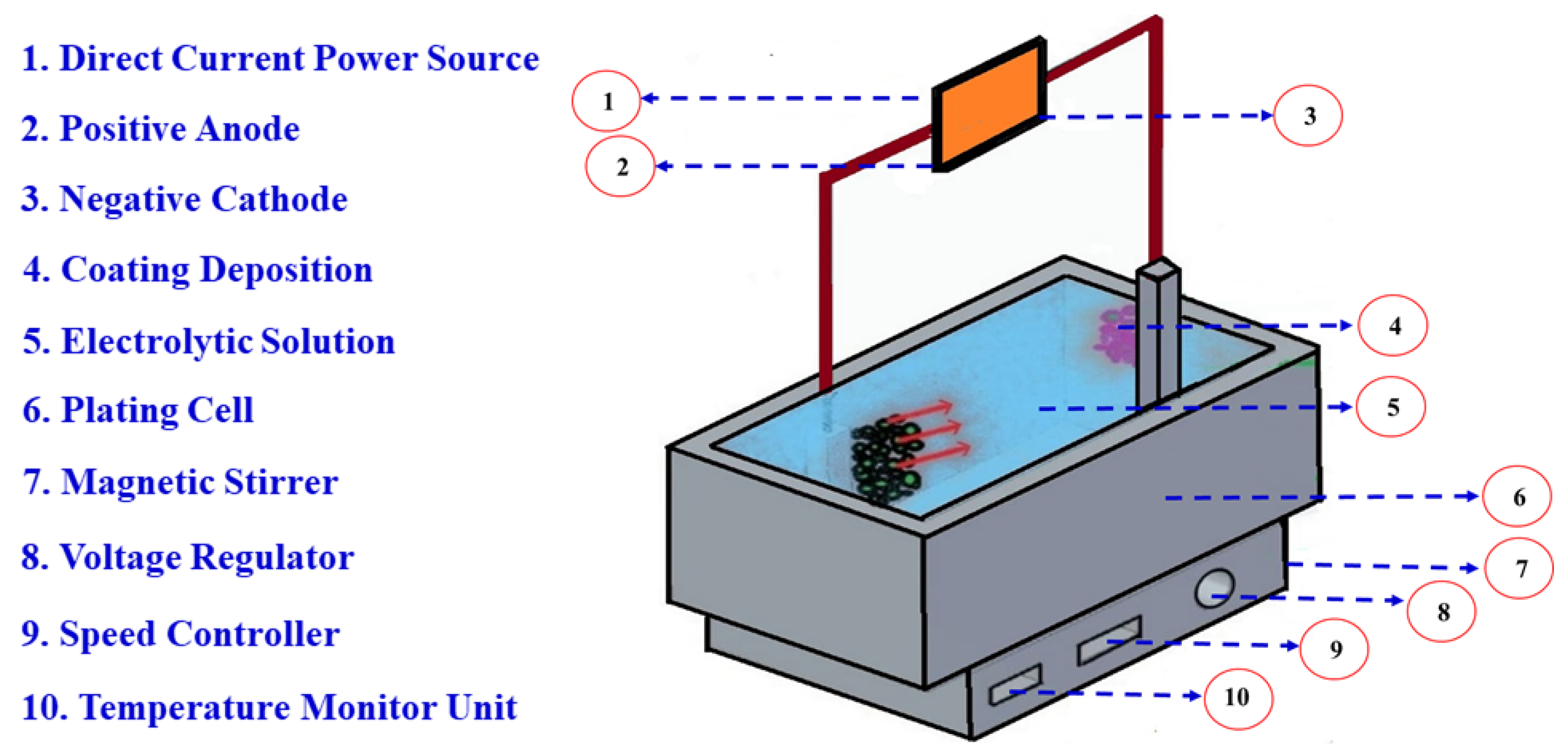




| Constituents of Zn–Ni Bath Solution in (g/L) | Experimental Parameters |
|---|---|
| NiSO4—60 | pH—3.0 |
| Na2SO4—40 | Anode—Zn metal |
| H3BO3—24 | Cathode—Mild steel |
| ZnSO4—240 | Current density—4 A/dm2 |
| SDS (Sodium Dodecyl Sulphate)—2 | Stirring rate—300 rpm |
| WC—1.0 | Cathode dimension—50 mm × 40 mm × 1 mm |
| - | Plating time—20 min |
| Samples | βa (V−1) | βc (V−1) | Error (V) | icorr (A) | Corrosion Rate (Å/min) |
|---|---|---|---|---|---|
| Zn–Ni | 27.531 | 4.914 | −1.027 | 4.180 × 10−6 | 1.192 |
| Zn–Ni–WC coating | 23.994 | 3.759 | −1.007 | 2.437 × 10−6 | 0.6948 |
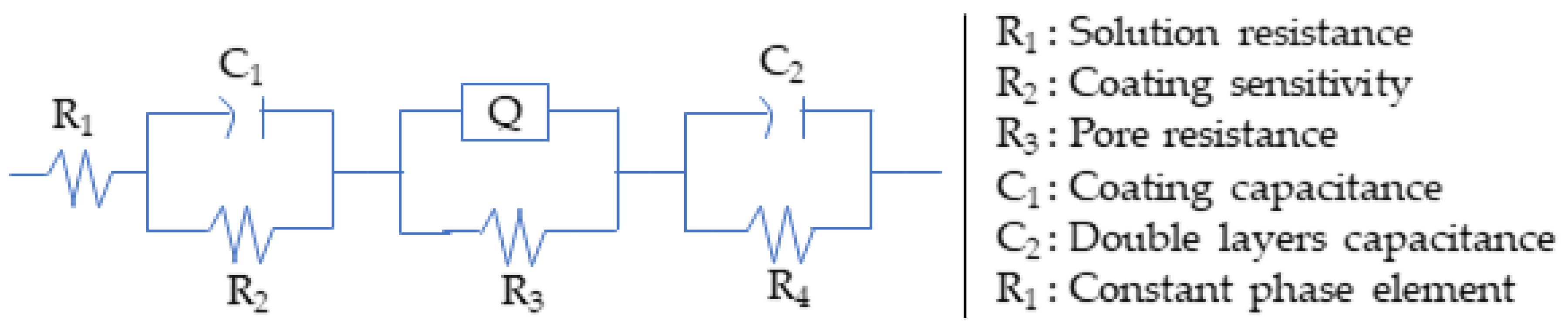
| Samples | R1 Ω·cm2 | R2 Ω·cm2 | C1 (F) | R3 Ω·cm2 | Q | R4 Ω·cm2 | C2 (F) | Rp Ω·cm2 |
|---|---|---|---|---|---|---|---|---|
| Zn–Ni | 3.88 × 10−8 | 128.1 | 2.08 × 10−7 | 3332 | 2.163 × 10−5 | 806.9 | 2.138 × 10−6 | 4267.0 |
| Zn–Ni–WC coating | 23.994 | 3.759 | −1.007 | 2.437 × 10−6 | 0.6948 | 47.45 | 9.546 × 10−7 | 5621.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, C.M.P.; Lakshmikanthan, A.; Chandrashekarappa, M.P.G.; Pimenov, D.Y.; Giasin, K. Electrodeposition Based Preparation of Zn–Ni Alloy and Zn–Ni–WC Nano-Composite Coatings for Corrosion-Resistant Applications. Coatings 2021, 11, 712. https://doi.org/10.3390/coatings11060712
Kumar CMP, Lakshmikanthan A, Chandrashekarappa MPG, Pimenov DY, Giasin K. Electrodeposition Based Preparation of Zn–Ni Alloy and Zn–Ni–WC Nano-Composite Coatings for Corrosion-Resistant Applications. Coatings. 2021; 11(6):712. https://doi.org/10.3390/coatings11060712
Chicago/Turabian StyleKumar, Channagiri Mohankumar Praveen, Avinash Lakshmikanthan, Manjunath Patel Gowdru Chandrashekarappa, Danil Yurievich Pimenov, and Khaled Giasin. 2021. "Electrodeposition Based Preparation of Zn–Ni Alloy and Zn–Ni–WC Nano-Composite Coatings for Corrosion-Resistant Applications" Coatings 11, no. 6: 712. https://doi.org/10.3390/coatings11060712
APA StyleKumar, C. M. P., Lakshmikanthan, A., Chandrashekarappa, M. P. G., Pimenov, D. Y., & Giasin, K. (2021). Electrodeposition Based Preparation of Zn–Ni Alloy and Zn–Ni–WC Nano-Composite Coatings for Corrosion-Resistant Applications. Coatings, 11(6), 712. https://doi.org/10.3390/coatings11060712









