Effects of Surface Morphology on Erosion–Corrosion and Corrosion Resistance of Highly Hydrophobic Nickel-Tungsten Electrodeposited Film
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
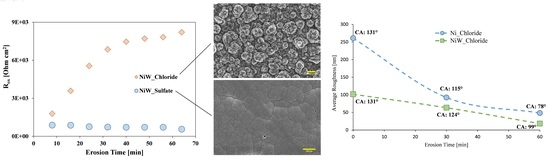
3.1. Morphology and Surface Considerations by SEM
3.2. Corrosion Resistance of the Coatings
3.2.1. Polarization Readings
3.2.2. EIS Studies of the Coatings
3.3. Erosion–Corrosion Behavior of the Electrodeposited Coatings
3.3.1. OCP vs. Time
3.3.2. EIS Characterization
3.3.3. EIS Modeling
3.4. Hydrophobicity and Roughness of the Coatings
4. Conclusions
- During static immersion in aggressive solution, due to the thickening of the corrosion products, barrier properties of both coatings increased, regardless of their different morphologies. During EC, the smooth NiW coating lost its barrier property whereas the barrier action of the rough NiW coating exceeded its value during static immersion.
- The hierarchical structure obtained from the chloride-based electrolyte improved durability of hydrophobicity behavior of NiW coatings during EC tests. In this regard, the NiWchloride resumed its hydrophobicity by storing in the air after EC tests. However, the NiWsulfate lost approximately 72% of its initial contact angle after EC and was not hydrophobic anymore.
- The capacitive behavior of the two NiW coatings was widely different. The Ceff values of the NiWchloride were much lower than those obtained for the NiWsulfate coating each time, indicating the higher barrier performance of hierarchical morphology.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| EC | erosion–corrosion |
| OCP | open circuit potential |
| EIS | electrochemical impedance spectroscopy |
| WCA | water contact angle |
| DC polarization | direct current polarization |
| Ecorr | corrosion potential |
| icorr | apparent corrosion current density |
| Rp | polarization resistance |
| CPEox | constant phase element of the passive oxide |
| CPEc | constant phase element of cracks and pores in coating |
| Rs | electrolyte resistance |
| Rox | passive oxide resistance |
| Rpore | pore resistance |
| ESA | exposed surface area |
| Ceff | effective capacitance |
| α | constant phase element power |
References
- Allahyarzadeh, M.H.; Aliofkhazraei, M.; Rezvanian, A.R.; Torabinejad, V.; Rouhaghdam, A.R.S. Ni-W electrodeposited coatings: Characterization, properties and applications. Surf. Coat. Tech. 2016, 307, 978–1010. [Google Scholar] [CrossRef]
- Karimzadeh, A.; Aliofkhazraei, M.; Walsh, F.C. A review of electrodeposited Ni-Co alloy and composite coatings: Microstructure, properties and applications. Surf. Coat. Tech. 2019, 372, 463–498. [Google Scholar] [CrossRef]
- Liu, T.; Yin, Y.S.; Dong, L.H. New application of the “underwater super-hydrophobic” surface in the corrosion protection. Adv. Mater. Res. 2009, 79–82, 1115–1118. [Google Scholar] [CrossRef]
- Ferrari, M.; Benedetti, A. Superhydrophobic surfaces for applications in seawater. Adv. Colloid Interface Sci. 2015, 222, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef] [Green Version]
- Khorsand, S.; Raeissi, K.; Ashrafizadeh, F.; Arenas, M.A. Relationship between the Structure and Water Repellency of Nickel–Cobalt Alloy Coatings Prepared by Electrodeposition Process. Surf. Coat. Technol. 2015, 276, 296–304. [Google Scholar] [CrossRef]
- Hashemzadeh, M.; Raeissi, K.; Ashrafizadeh, F.; Khorsand, S. Effect of ammonium chloride on microstructure, super-hydrophobicity and corrosion resistance of nickel coatings. Surf. Coat. Tech. 2015, 283, 318–328. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Hierarchical roughness optimization for biomimetic superhydrophobic surfaces. Ultramicroscopy 2007, 107, 969–979. [Google Scholar] [CrossRef]
- Hang, T.; Hu, A.; Ling, H.; Li, M.; Mao, D. Super-hydrophobic nickel films with micro-nano hierarchical structure prepared by electrodeposition. Appl. Surf. Sci. 2010, 256, 2400–2404. [Google Scholar] [CrossRef]
- Xu, X.H.; Zhang, Z.Z.; Yang, J.; Zhu, X.T. Study of the corrosion resistance and loading capacity of superhydrophobic meshes fabricated by spraying method. Colloid. Surf. A Physiochem. Eng. Asp. 2011, 377, 70–75. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Tech. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Toloei, A.S.; Stoilov, V.; Northwood, D.O. The Relationship between Surface Roughness and Corrosion; International Mechanical Engineering Congress and Exposition; ASME: New York, NY, USA, 2013; pp. 1–10. [Google Scholar]
- Herminghaus, S. Roughness-induced non-wetting. Europhys. Lett. 2000, 52, 165–170. [Google Scholar] [CrossRef]
- Quere, D. Wetting and roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.G.; Ke, W.; Sun, W.H.; Hou, W.L.; Chang, X.C. Slurry erosion–corrosion behaviour of high-velocity oxy-fuel (HVOF) sprayed Fe-based amorphous metallic coatings for marine pump in sand-containing NaCl solutions. Corros. Sci. 2011, 53, 3177–3185. [Google Scholar] [CrossRef]
- Bayer, I.S. On the durability and wear resistance of transparent superhydrophobic coatings. Coatings 2017, 7, 12. [Google Scholar] [CrossRef]
- Garcia-Giron, A.; Romano, J.M.; Batal, A.; Dashtbozorg, B.; Dong, H.; Solanas, E.M. Durability and wear resistance of laser-textured hardened stainless steel surfaces with hydrophobic properties. Langmuir 2019, 35, 5353–5363. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Hao, X.; Yang, Y.; Li, L.; He, N.; Li, H. Feasible fabrication of a wear-resistant hydrophobic surface. Appl. Surf. Sci. 2019, 463, 923–930. [Google Scholar] [CrossRef]
- Salehikahrizsangi, P.; Raeissi, K.; Karimzadeh, F.; Calabrese, L.; Patane, S.; Proverbio, E. Erosion-corrosion behavior of highly hydrophobic hierarchical Nickel coatings. Colloid. Surf. A Physiochem. Eng. Asp. 2018, 558, 446–454. [Google Scholar] [CrossRef]
- Calderón, J.A.; Henao, J.E.; Gómez, M.A. Erosion–corrosion resistance of Ni composite coatings with embedded SiC nanoparticles. Electrochim. Acta 2014, 124, 190–198. [Google Scholar] [CrossRef]
- Burstein, G.T.; Sasaki, K. Effect of impact angle on the slurry erosion–corrosion of 304L stainless steel. Wear 2000, 240, 80–94. [Google Scholar] [CrossRef]
- Kumar, U.P.; Shanmugan, S.; Kennady, C.J.; Shibli, S.M.A. Anti-corrosion and microstructural properties of Ni–W alloy coatings: Effect of 3,4-Dihydroxybenzaldehyde. Heliyon 2019, 5, e01288. [Google Scholar] [CrossRef] [Green Version]
- Khorsand, S.; Raeissi, K.; Ashrafizadeh, F. Corrosion resistance and long-term durability of superhydrophobic nickel film prepared by electrodeposition process. Appl. Surf. Sci. 2014, 305, 498–505. [Google Scholar] [CrossRef]
- She, Z.; Li, Q.; Wang, Z.; Tan, C.; Zhou, J.; Li, L. Highly anticorrosion, self-cleaning superhydrophobic Ni–Co surface fabricated on AZ91D magnesium alloy. Surf. Coat. Technol. 2014, 251, 7–14. [Google Scholar] [CrossRef]
- Liu, T.; Yin, Y.; Chen, S.; Chang, X.; Cheng, S. Super-hydrophobic surfaces improve corrosion resistance of copper in seawater. Electrochim. Acta 2007, 52, 3709–3713. [Google Scholar] [CrossRef]
- Esmailzadeh, S.; Khorsand, S.; Raeissi, K.; Ashrafizadeh, F. Microstructural evolution and corrosion resistance of super-hydrophobic electrodeposited nickel films. Surf. Coat. Tech. 2015, 283, 337–346. [Google Scholar] [CrossRef]
- Younes-Metzler, O.; Zhu, L.; Gileadi, E. The anomalous codeposition of tungsten in the presence of nickel. Electrochim. Acta 2003, 48, 2551–2562. [Google Scholar] [CrossRef]
- Salehikahrizsangi, P.; Raeissi, K.; Karimzadeh, F.; Calabrese, L.; Proverbio, E. Highly hydrophobic Ni-W electrodeposited film with hierarchical structure. Surf. Coat. Tech. 2018, 344, 626–635. [Google Scholar] [CrossRef]
- Macdonald, D.D. The point defect model for the passive state. J. Electrochem. Soc. 1992, 139, 3434–3449. [Google Scholar] [CrossRef]
- Sikora, E.; Macdonald, D.D. Nature of the passive film on nickel. Electrochim. Acta 2002, 48, 69–77. [Google Scholar] [CrossRef]
- Wang, M. Electrodeposited free-crack NiW films under super gravity filed: Structure and excellent corrosion property. Mater. Chem. Phys. 2014, 148, 245–252. [Google Scholar] [CrossRef]
- Correia, A.; Barros, E.; Colares, R. Morphological, structural, microhardness and electrochemical characterisations of electrodeposited Cr and Ni–W coatings. Electrochim. Acta 2010, 55, 2078–2086. [Google Scholar]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Electrochemical behavior of hydrophobic silane–zeolite coatings for corrosion protection of aluminum substrate. J. Coat. Technol. Res. 2014, 11, 883–898. [Google Scholar] [CrossRef]
- Lyon, S.B. Corrosion of tungsten and its alloys. Shreir’s Corros. 2010, 3, 2151–2156. [Google Scholar]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Sebastian, D.; Yao, C.; Lian, I. Mechanical durability of engineered superhydrophobic surfaces for anti-corrosion. Coatings 2018, 8, 162. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.D.; Nakajima, A.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Photoinduced surface wettability conversion of ZnO and TiO2 thin films. J. Phys. Chem. B 2001, 105, 1984–1990. [Google Scholar] [CrossRef]
- Rahimi, E.; Rafsanjani-Abbasi, A.; Kiani-Rashid, A.; Jafari, H.; Davoodi, A. Morphology modification of electrodeposited superhydrophobic nickel coating for enhanced corrosion performance studied by AFM, SEM-EDS and electrochemical measurements. Colloids Surf. A Physicochem. Eng. Asp. 2018, 547, 81–94. [Google Scholar] [CrossRef]
- Khorsand, S.; Raeissi, K.; Ashrafizadeh, F.; Arenas, M.A. Super-hydrophobic nickel–cobalt alloy coating with micro-nano flower-like structure. Chem. Eng. J. 2015, 273, 638–646. [Google Scholar] [CrossRef] [Green Version]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]











| Parameter | Chloride | Sulfate |
|---|---|---|
| Ecorr (mV) | −285.8 | −227.5 |
| Rp (ohm/cm2) | 77.091 | 5774 |
| icorr (mA/cm2) | 3.4 × 10−4 | 4.5 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehikahrizsangi, P.; Raeissi, K.; Karimzadeh, F.; Calabrese, L.; Proverbio, E. Effects of Surface Morphology on Erosion–Corrosion and Corrosion Resistance of Highly Hydrophobic Nickel-Tungsten Electrodeposited Film. Coatings 2021, 11, 1084. https://doi.org/10.3390/coatings11091084
Salehikahrizsangi P, Raeissi K, Karimzadeh F, Calabrese L, Proverbio E. Effects of Surface Morphology on Erosion–Corrosion and Corrosion Resistance of Highly Hydrophobic Nickel-Tungsten Electrodeposited Film. Coatings. 2021; 11(9):1084. https://doi.org/10.3390/coatings11091084
Chicago/Turabian StyleSalehikahrizsangi, Parinaz, Keyvan Raeissi, Fathallah Karimzadeh, Luigi Calabrese, and Edoardo Proverbio. 2021. "Effects of Surface Morphology on Erosion–Corrosion and Corrosion Resistance of Highly Hydrophobic Nickel-Tungsten Electrodeposited Film" Coatings 11, no. 9: 1084. https://doi.org/10.3390/coatings11091084
APA StyleSalehikahrizsangi, P., Raeissi, K., Karimzadeh, F., Calabrese, L., & Proverbio, E. (2021). Effects of Surface Morphology on Erosion–Corrosion and Corrosion Resistance of Highly Hydrophobic Nickel-Tungsten Electrodeposited Film. Coatings, 11(9), 1084. https://doi.org/10.3390/coatings11091084