Abstract
In this work, the challenges associated with the formation of single and bilayer coatings based on Ce0.8Sm0.2O1.9 (SDC) and CuO modified BaCe0.5Zr0.3Y0.1Yb0.1O3−δ (BCZYYbO-CuO) solid state electrolytes on porous non-conducting NiO-SDC anode substrates by the method of electrophoretic deposition (EPD) are considered. Various approaches that had been selected after analysis of the literature data in order to carry out the EPD, are tested: direct deposition on a porous non-conductive anode substrate and multiple options for creating the conductivity of the anode substrate under EPD conditions such as the reduction of the NiO-SDC substrate and the creation of a surface conducting sublayer via synthesizing a polypyrrole (PPy) film. New effective method was proposed based on the deposition of a platinum layer on the front side of the substrate. It was ascertained that, during the direct EPD on the porous NiO-SDC substrate, the formation of a continuous coating did not occur, which may be due to insufficient porosity of the substrate used. It was shown that the use of reduced substrates leads to cracking and, in some cases, to the destruction of the entire SDC/NiO-SDC structure. The dependence of the electrolyte film sinterability on the substrate shrinkage was studied. In contrast to the literature data, the use of the substrates with a reduced pre-sintering temperature had no pronounced effect on the densification of the SDC electrolyte film. It was revealed that complete sintering of the SDC electrolyte layer with the formation of a developed grain structure is possible at a temperature of 1550 °C.
1. Introduction
Solid oxide fuel cells (SOFCs) are environment friendly electrochemical devices that convert chemical energy of continuously supplied hydrocarbon fuel into electricity with high efficiency (approximately 70%) [1,2]. The demand on decreasing SOFC operating temperatures has facilitated the development of the cell design with a thin-film solid state electrolyte on supporting substrates-anode, cathode, porous electrolyte and metallic carriers [3,4,5]. The recently developed technologies were extended to the deposition of two and multilayer electrolyte membranes prospective for the enhancement of the cell performance and durability [6,7,8,9,10]. To deposit thin dense electrolyte films various methods have been applied such as chemical and physical vapor deposition and plasma spaying [11,12], atomic layer deposition [13], pulsed laser deposition and magnetron sputtering [14,15,16] and some ceramic and colloidal techniques [17,18,19].
Among the technologies under intensive development, the method of electrophoretic deposition (EPD) is an industrially relevant process which is technologically simple, easily adaptable to the substrate shape and electrolyte content and less cost and time-consuming than many other colloidal and gas-phase processes [20]. Compared to the dipped or sprayed coatings, electrophoretically deposited coatings demonstrate superior adherence and high green density and thus ensure fabrication of uniform and gas-tight electrolyte films [21]. In comparison with casting methods, the EPD rate is less influenced by particle size and remains relatively fast when the coatings based on electrolyte nanopowders are fabricated [22,23]. As compared with tape casting, the EPD is also suitable for the formation of multilayered systems with easily controlled thickness of layers [24].
Since sufficient substrate conductivity is one of the main requirements for the successful EPD implementation, in this regard, for the deposition of electrolyte films, it would be preferable to use cathode substrates that possess a high conductivity level at room temperature. However, cathode polarization resistance is well known to be a key factor influencing the power density of thin-film electrolyte SOFCs, especially at reduced operating temperatures [25] due to complicated multistage process of oxygen reduction. Thus, thick cathode substrate would greatly contribute to reduction of the electrode kinetics and deterioration of the cell performance [26]. In this sense, anode-supported constructions are preferable to use. However, NiO-cermet anodes, traditionally used in SOFCs, are non-conductive under the EPD conditions. In Table 1 we summarized different approaches applied to deposition of thin electrolyte films on anode substrates.

Table 1.
Methods of the substrate preparation, EPD modes and sintering conditions of the electrolyte films fabricated on non-conductive anode substrates.
In a number of studies, the EPD of single or bilayer electrolyte films was performed by electrophoretic filtration on the porous non-conducting substrates of 50–60% porosity. To establish conductive path through the porous structure, the opposite side of the substrates was covered with carbon [27,28,29] or platinum [41]. In these studies, the anode substrates were pre-sintered at the temperatures of 900–1200 °C and their shrinkage during co-firing with the electrolyte at higher temperatures facilitated densification of the electrolyte film. The lowest sintering temperatures for the yttria stabilized zirconia (YSZ) films of 1200–1250 °C were reached when using for their deposition the anode substrates with intrinsic microporosity fabricated by ceramic injection molding from the mixture of NiO-YSZ and polymer binders (PEG and PVB resin) without any pre-sintering step [30] or when using for EPD nano-sized YSZ electrolyte material subjected special treatment to destroy agglomerates [23].
In another approach, electrolyte layers are deposited on highly conducting reduced anode substrates [28,33,42]. The main problem of this method is related to the selection of a proper layer’s thickness ratio and sintering conditions which could maximally match the sintering behavior of the reduced substrate and electrolyte films.
The substrate conductivity can be enhanced by introducing a conducting powder into green anode substrates [32,37] or creating a conductive layer on the front size of the substrate by means of deposition of polypyrrole (PPy) [34,35], graphite [29,37,39], or silver [43]. Comparison of the effectiveness of the methods of electrophoretic filtration and using a conductive surface layer in EPD on non-conductive substrates was performed by Talebi et al. [40]. In the first method, the NiO-YSZ highly porous substrate (~64% porosity) was placed in front of a stainless-steel electrode; in the second method, the substrate surface conductivity was facilitated by deposition of a thin graphite layer on the top of the substrate. It was shown that the deposition weight, thickness and quality of coatings formed on the graphite layer were significantly higher than those deposited directly on the non-conductive substrate. In the second case there were a lot of cracks on the film surface after sintering. In the study of Matsuda et al. [29], contrary, the direct deposition of the YSZ film on a non-conductive NiO-YSZ substrate (~57% porosity) was found to be more preferable that that on a graphite layer. Comparative studies make it possible to find an optimal solution for a specific task of forming SOFCs with a certain set of materials used for functional layers.
Another important issue to achieve good quality of the electrolyte film using the EPD is the preparation of stable suspension. It is a challenging task especially when using submicron powders due to their strong agglomeration tendency. Therefore, the choice of appropriate solvent and dispersant, as well as special suspension treatment is crucial for the suspensions’ stabilization.
The experimental part of this paper presents epy initial investigative steps for EPD of thin solid-state electrolyte films on non-conductive substrates undertaken by our group. We represent a full cycle of the necessary preparations and process implementation. Special attention is paid to the preparation of stable suspensions for EPD based on electrolytes of different dispersity, as well as an electrolyte with a sintering additive. To reduce particle aggregation in the suspensions, ultrasonic treatment is applied. Optimal deposition modes are established through the deposition on the model substrates. A comparative experimental analysis on the EPD process on non-conductive substrates is carried out using known approaches considered in the works of other scientific groups. The difficulties that arose when implementing the different methods are discussed. An alternative method for creating solid electrolyte films is proposed based of the surface modification of a non-conductive anode substrate by the deposition of a platinum sublayer on its front surface. The implementation of this approach makes it possible to carry out multiple deposition-sintering cycles while maintaining the conductivity of the substrate under EPD conditions.
2. Materials and Methods
2.1. Synthesis and Characterization of the Electrolytes
Ce0.8Sm0.2O1.9 (SDC) electrolyte powder was synthesized by a solution combustion synthesis (SCS) using a combined organic fuel-a mixture of glycine (gl) and citric acid (cit). The intensity of the combustion reaction was regulated by varying the gl:cit ratio to prevent the removal of the synthesis product from the reaction vessel. For the synthesis of SDC powder, the following initial reagents were used: Ce(NO3)3·6H2O (99.9 wt.%), Sm(NO3)3·6H2O (99.0 wt.%), glycine and citric acid. Cerium and samarium nitrate salts were dissolved in distilled water; the resulting solutions were mixed, and then the calculated amount of glycine and citric acid (1.4 mol of the combined fuel (0.6gl:0.8cit) per 1 mole of the mixed oxide) was added and stirred until complete dissolution. The reaction solution was heated until the formation of the xerogel and its subsequent combustion. The resulting powder was calcined in air at 900 °C for 5 h. The specific surface area of the SDC powder, determined by the brunauer, emmett and teller (BET) method, SBET, was 12 m2/g.
BaCe0.5Zr0.3Y0.1Yb0.1O3−δ (BCZYYbO) electrolyte powder was synthesized by a citrate-nitrate method using citric acid as a chelating agent and organic fuel in the pyrolysis of the reacting mixture. BaCO3 (99.0 wt.%), Y2O3 (99.9 wt.%), Yb2O3 (99.5 wt.%), Ce(NO3)3·6H2O (99.9 wt.%), Zr(OH)2CO3 (98.4 wt.%) were used as starting reagents. To remove adsorbed moisture and gases, BaCO3, Yb2O3 and Y2O3 materials were preliminarily calcined for 10 h at a temperature of 600, 1000, and 1100 °C, respectively. The starting powders were dissolved in distilled water, and the resulting solutions were mixed and heated for 10–20 min. After that an ammonia solution (10 vol.%) was added drop by drop until a slightly acidic medium was established (pH = 6–7). Citric acid was added to the solution in an amount calculated to the yield product. Then the solution was evaporated. The obtained powder was annealed in air sequentially in several 5-h stages at 1050 and 1150 °C with intermediate grinding in ethanol. The final stage of synthesis was performed at a temperature of 1150 °C for 10 h. The BCZYYbO specific surface area determined by the BET method was 1.3 m2/g. To enhance sintering of the BCZYYbO coatings, CuO as a sintering additive in the amount of 5 wt.% was introduced into the BCZYYbO electrolyte powder by adding an alcohol solution of Cu(NO3)2·3H2O (99.0 wt.%). The mixture was stirred, dried, and decomposed at 400 °C for 1 h.
The X-ray diffraction (XRD) analysis of the obtained samples was carried out on a XRD-7000 diffractometer (Shimadzu, Kyoto, Japan) in a CuKα radiation and in an angle range of 2θ = 20°–80°. Data processing and phase identification were performed by comparing the XRD patterns obtained with those in the PDF-4 database (ICDD, Newtown, PA, USA, Release 2018). The specific surface area of the powders was determined by a BET volumetric method for low-temperature equilibrium sorption of nitrogen vapor from mixtures with helium using a TriStar 3000 vacuum sorption unit (Micromeritics, Norcross, Germany). Dilatometric measurements were carried out on the samples of materials compacted by a magnetic-pulse method, as described elsewhere [44], using a DIL 402 C dilatometer (NETZSCH, Selb, Germany) in the heating mode up to 1500 °C and under subsequent cooling at a constant rate of 5 °C/min. The experiments were carried out in an air flow of 100 mL/min.
2.2. Fabrication of the Anode Substrates and Their Characterization
For the fabrication of anode substrates, the SDC electrolyte powder was calcined at 1100 °C for 5 h to decrease its specific surface area down to 1.2 m2/g. NiO (98.4 wt.%) and the SDC electrolyte powders were mixed in a ratio of 56/44 wt.% with the addition of a polyvinyl butyral binder and compacted by uniaxial semi-dry pressing at a pressure of |6 MPa, and then sintered at a temperature of 1400 °C for 2 h in air (Nabertherm LHT-04/18 furnace, Lilienthal, Germany). Pre-sintered anode substrates were polished using a diamond grinding disc and then treated in an ultrasonic bath in isopropyl alcohol to clean the surface and annealed at 900 °C for 1 h. The density of the anode substrates was determined by weighing and measuring the geometric dimensions and amounted approximately 72–74%. The gas permeability of the anode substrates was measured on a specialized installation (IEP UB RAS, Yekaterinburg, Russia) by a decay method. This method is based on a Darcy’s law [45]. The gas permeability coefficient was determined by a law of time-dependent pressure that is decreased due to gas permeation through the porous substrate. The average gas permeability coefficient measured for the anode substrates pre-sintered at 1400 °C (experiments with four samples) was 1.6 × 10−3 μm2.
To investigate the influence of the shrinkage of the anode substrate on the electrolyte film densification during co-sintering, some of the NiO-SDC anode substrates fabricated without/with a pore former (graphite, 13 m2/g taken in amount of 20 wt.%) were sintered at 1200 °C, 2 h. The density of the anode substrates sintered at decreased temperatures was in a range of 53–60% of the theoretical value. The conductive layer on the surface of the anode substrates was created by synthesizing a polypyrrole (PPy) film. PPy was synthesized by chemical polymerization [35,46].
2.3. Preparation of the Suspensions Based on the Electrolyte Materials and Their Characterization
For the preparation of suspensions based on the electrolyte materials, a mixed dispersion medium of isopropanol/acetylacetone in a ratio of 70/30 vol.% was chosen. Suspensions based on SDC and BCZYYbO-CuO with a concentration of 10 and 15 g/L, respectively, were prepared from an accurate weighed portion of the powders and sonicated using an UZV-13/150-TH ultrasonic bath (Reltec, Yekaterinburg, Russia)with power of the generator of 210 W at the operating frequency of 22 kHz for 5–125 min (at 25 °C). The temperature was controlled with a thermometer and maintained at a given level of water exchange in an ultrasonic bath. Suspensions of SDC and BCZYYbO-CuO with a concentration of 1 g/L were prepared to study the dispersed composition by the method of dynamic light scattering (DLS). Disperse composition and particle size distribution in suspensions was studied using a ZetaPlus particle size analyzer (Brookhaven Instruments Corporation, Holtsville, NY, USA). The electrokinetic zeta potential and pH in suspensions were measured by the electroacoustic method using a DT-300 analyzer (Dispersion Technology, Bedford Hills, NY, USA).
2.4. Electrophoretic Deposition of the Thin-Film Electrolyte Layers
Electrophoretic deposition of SDC and BCZYYbO-CuO electrolyte layers was performed using a specialized software-controlled installation (IEP UB RAS, Yekaterinburg, Russia) in a constant voltage mode. The current was measured using an Intelligent Digital Multimeter UNI-T UT71E (Uni-Trend Technology, Hong Kong, China). The electrodes of the EPD cell with a diameter of 12 mm were placed at a distance of 10 mm. For the deposition of the electrolyte layer an anode substrate was placed on the cathode of the EPD cell. A stainless-steel disk was used as a counter electrode of the EPD cell.
Examination of the powders’ morphology and microstructure of the deposited films were performed using a JSM-6390 LA scanning electron microscope (SEM, JEOL, Tokyo, Japan) equipped with a system of energy-dispersion X-ray microanalysis (EDX). SEM images in the BSE (back-scattered electrons) and SE (secondary electrons) modes were obtained at a high voltage of 10 kV. For the EDX analysis the high voltage of 10 kV was used as well. The morphology of thin-film layers at the stages of deposition was investigated using an ST-VS-520 optical microscope (IEP UB RAS, Yekaterinburg, Russia). The thickness of the deposited green coatings was estimated from the weight of the film, the surface area of the substrate to be coated with the film, and the theoretical density of the deposited material. The thickness of the sintered coating was determined from the cross-section SEM images.
3. Results and Discussion
3.1. Characteristics of Electrolyte Powder Materials
The XRD patterns of the resulting SDC and BCZYYbO powders are shown in Figure 1 (Supplementary Materials Figure S1). According to the XRD data, the sample of the microsized SDC powder was single-phase that possessed a cubic type structure of Fm-3m (225) space group with a lattice parameter of a = 5.4305(1) Å. The BCZYYbO powder sample was single-phase and had a rhombohedral crystal lattice with an R-3c (167) space group and the lattice parameters a = 6.1330(3) Å, b = 6.1330(3) Å, c = 14.9910(1) Å. The BCZYYbO-CuO powder was subjected to the XRD analysis after sintering at 1500 °C. It was found that CuO modified BCZYYbO preserved a rhombohedral structure with an R-3c space group, however the lattice parameters were lower due to partial copper dissolution in the structure (a = 6.0972(3) Å, b = 6.0972(3) Å, c = 14.9370(1) Å). Such a decrease agrees well with that observed in [47] for BaCe0.3Zr0.55Y0.15O3−δ modified by different sintering additives including CuO. In addition to the main BCZYYbO phase, the presence of secondary phases of CuO, BaO and was registered on the XRD of the sintered BCZYYbO-CuO powder (Figure 1c).
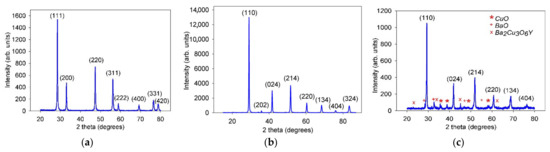
Figure 1.
XRD patterns obtained for the electrolyte powders: (a) as-prepared SDC powder; (b) as-prepared BCZYYbO powder; (c) BCZYYbO-CuO powder (sintered at 1500 °C).
The powders morphology investigated using SEM method is shown in Figure 2. It can be seen that the powders have a similar size of irregular aggregates ~2–4 μm, consisting of tightly linked smaller particles. However, SDC powder (Figure 2a) has a more developed surface of particle aggregates which is confirmed by the values of the specific surface area. The average particle radius, assuming the presence of loosely packed aggregates characteristic of SCS powders, can be calculated as follows [48]:
where ρth is the theoretical material density calculated from the XRD data. The calculated RBET values for the SDC and BCZYYbO powders were 35 and 366 nm, respectively. SBET is the specific surface area of the powders defined using the BET method.
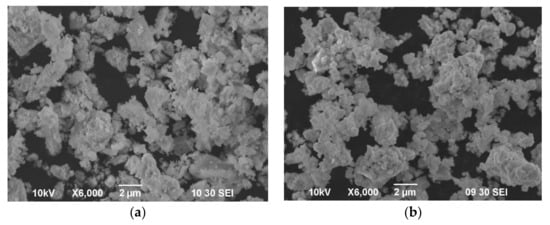
Figure 2.
Morphology of as-prepared powders: (a) SDC (Tcalc = 900 °C); (b) BCZYYbO (Tcalc = 1100 °C).
3.2. Fractional Composition and Electrokinetic Potential of the Suspensions of the Powder Electrolyte Materials
The disperse composition was studied by the DLS method in the suspensions of the BCZYYbO-CuO and SDC powders with a concentration of 1 g/L in the isopropanol/acetylacetone (70/30 vol.%) medium. Figure 3 shows the dependence of the effective hydrodynamic diameter of the aggregates (deff) for the suspensions in dependence on the time of the ultrasonic treatment (UST) under continuous cooling of the suspension. The initial deff values for the BCZYYbO-CuO and SDC suspensions were determined to be equal to 1150 and 1503 nm, respectively. After 25 min of UST, deff increased for both BCZYYbO-CuO and SDC suspensions to 1275 and 1687 nm, respectively. However, with further increasing the UST time, the size of aggregates decreased, and this tendency was more pronounced for the SDC suspension. Thus, for the BCZYYbO-CuO and SDC suspensions treated ultrasonically for 125 min deff was reduced to the values of 1043 and 732 nm, respectively.
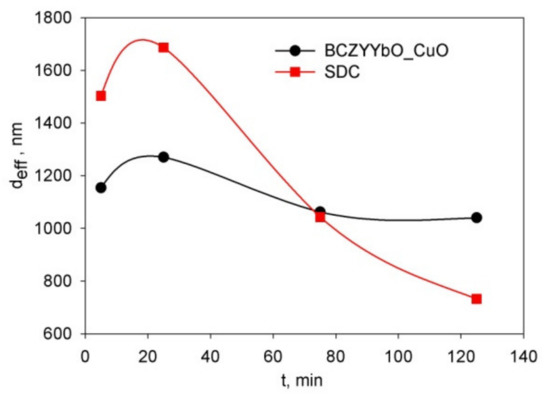
Figure 3.
Schemes follow the same formatting. Dependence of the effective hydrodynamic diameter of aggregates, deff, in the suspensions of the BCZYYbO-CuO and SDC powders on the time of the ultrasonic treatment carried out under continuous cooling.
Figure 4 demonstrates the unimodal distributions of particles by the scattering intensity of the BCZYYbO-CuO (Figure 4a) and SDC (Figure 4b) suspensions after sonication for 5, 25, 75, and 125 min. For both suspensions, after 25 min of the UST, the distributions broadened; with a further increase in the UST time, the distributions narrowed, which is characterized by the values of the geometric standard deviation (GSD). For the BCZYYbO-CuO suspension after UST for 5, 25, 75, and 125 min the GSD values were 1.44 ± 0.07, 1.57 ± 0.07, 1.54 ± 0.07, and 1.52 ± 0.07, respectively. While for the SDC suspension after UST for 5, 25, 75, and 125 min, the GSD values were 1.68 ± 0.07, 1.71 ± 0.07, 1.57 ± 0.07, and 1.53 ± 0.07, respectively.
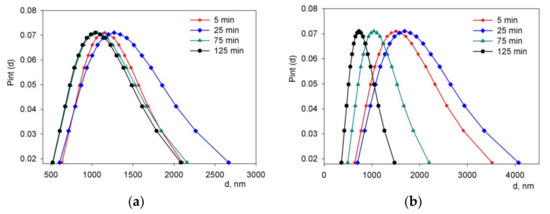
Figure 4.
Unimodal distributions of the aggregate size in suspensions after ultrasonic treatment for 5, 25, 75, and 125 min: (a) BCZYYbO-CuO; (b) SDC. Pint(d) is a probability density of unimodal distribution of aggregates by size.
The results of studying the fractional composition for the BCZYYbO-CuO (Figure 5a) and SDC (Figure 5b) suspensions revealed that the initial suspensions (after UST for 5 min) BCZYYbO-CuO and SDC contained one fraction of aggregates with a size of 1325 and 755 nm, respectively. After UST for 125 min, the distribution of aggregates in both suspensions changed, namely, they became bimodal. A fine fraction of aggregates with a size of 745 nm (74%) and a larger fraction with a size of 1960 nm (26%) appeared in the BCZYYbO-CuO suspension. In the SDC suspension, the fractional composition was characterized by the presence of 492 nm (47%) and 1662 nm (53%) fractions.
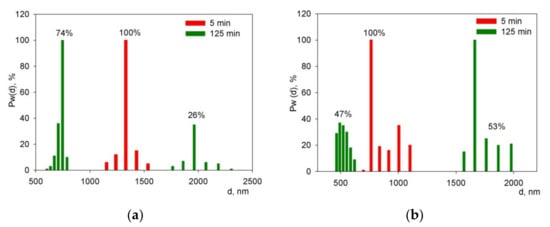
Figure 5.
Fractional composition after the ultrasonic treatment for 5 and 125 min in suspensions: (a) the BCZYYbO-CuO; (b) SDC. Pw(d) is a weight fraction (%) of aggregates with a diameter d (nm).
The ζ-potential and pH values measured in this study in the BCZYYbO-CuO and SDC suspensions were equal to +11 mV (6.1 pH) and +13 mV (2.9 pH), respectively. Despite the relatively low values of the ζ-potential, the suspensions are characterized by aggregative stability which made it possible to carry out the EPD process [49].
3.3. Investigation of EPD of Electrolyte Materials on a Model Substrate (Ni-Foil)
Preliminary studies of the EPD process from the stabilized suspensions of the BCZYYbO-CuO and SDC powders were carried out on a Ni-foil model substrate. Current-voltage characteristics (VAC) under increasing and decreasing voltage and kinetics of the change in the current strength for 15 g/L BCZYYbO-CuO and 10 g/L SDC suspensions were studied. VAC of the SDC suspension is characterized by slight nonlinearity and hysteresis with increasing and decreasing the cell voltage. No nonlinearity and hysteresis of VAC was registered in the study of the BCZYYbO-CuO suspension, which corresponds to the absence of a dependence of the electrophoretic mobility of particles in the suspension on the electric field strength.
For the EPD of the electrolyte layer from the suspensions of BCZYYbO-CuO (cell voltage 80 V) and SDC (cell voltage 60 V), the dependences of the current strength on time are shown in Figure 6. For the BCZYYbO-CuO suspension, there is a clear tendency to a decrease in the current strength with time, while the SDC suspension is characterized by an alternating change in the current with time-in the initial period of the process (up to 2 min) the current sharply increases and then gradually decreases with time (Figure 6).
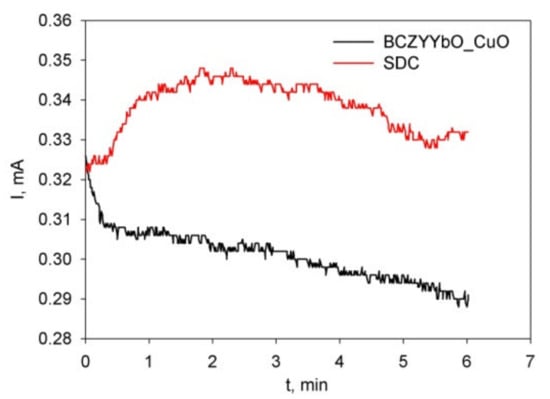
Figure 6.
Dependence of the current strength on time for EPD from a suspension of BCZYYbO-CuO (15 g/L) (cell voltage 80 V) and 10 g/L SDC (cell voltage 60 V).
During the deposition of the BCZYYbO-CuO and SDC coatings the deposition voltage was varied from 10 to 100 V at a constant deposition time for 1 min (Figure 7). The dependences of the deposit weight for both BCZYYbO-CuO and SDC suspensions with increasing voltage had a nonlinear character of increase, close to S-shaped.
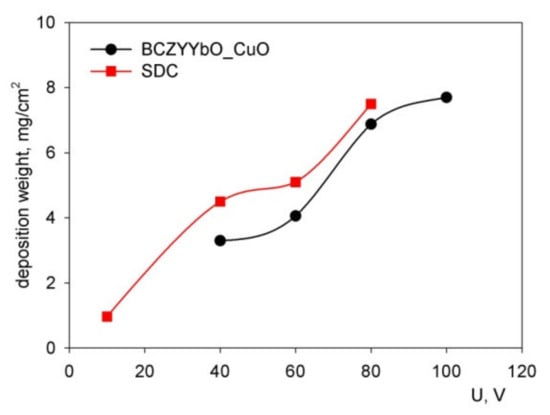
Figure 7.
Dependence of the deposit weight of the BCZYYbO-CuO and SDC coatings on the deposition voltage at a constant EPD time (1 min).
3.4. Formation of an Electrolyte Layer on Porous Anode Substrates by the EPD Method
3.4.1. Direct EPD on Non-Conductive Porous Substrates
Based on the results of EPD on non-conductive porous anodes presented in the study of Besra et al. [50], we carried out a series of experiments in which a porous non-conductive substrate (cermet anode) was placed on a metal electrode (cathode) of the EPD cell and a stainless-steel plate served as a counter electrode. As noted in [50], the deposition mechanism in this case is the presence of continuous pores in the substrate structure assuring conductive paths between the particles in the suspension with the electrode. The presence of such through conductive paths is a key factor that determines the implementation of deposition, and the value of porosity significantly affects the deposit weight. For instance, at EPD with a voltage of 100 V for 3 min, the authors obtained an (ZrO2)0.92(Y2O3)0.08(YSZ) electrolyte coating of approximately 27 mg/cm2 (40 µm) on a NiO-YSZ cermet substrate with porosity of 72.55%. In this study the porosity of the anode substrates did not exceed 35%; therefore, we increased the deposition voltage and carried out experiments on EPD at a voltage across the electrodes of 250 V and a deposition time of 3 min.
Figure 8 presents optical photographs of the EPD deposits obtained from the suspensions of the BCZYYbO-CuO (Figure 8a) and SDC (Figure 8b) powders. The deposit weight was 1.5 mg/cm2 (BCZYYbO-CuO) and 2.3 mg/cm2 (SDC). It can be seen that for both suspensions there was no formation of continuous deposited layers. Probably, this is due to the appearance of deposition instability when the levelling of the thickness of the deposited coating does not occur, and an island structure of the deposit is realized. Possible factors affecting the unevenness of the coating during deposition are uneven distribution of porous conducting channels of the non-conductive substrate, fluctuations in the electric field strength near the substrate surface, and the occurrence of electrohydrodynamic flows [51,52].
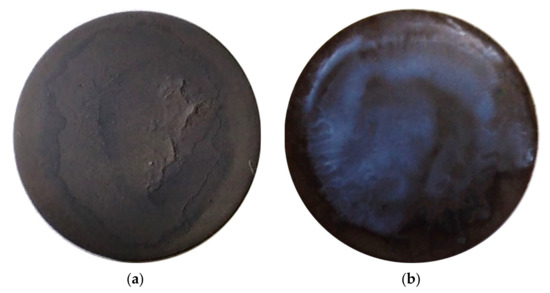
Figure 8.
Optical photographs of EPD deposits obtained by direct deposition on porous NiO-SDC cermet substrates from the suspensions based on powders: (a) BCZYYbO-CuO; (b) SDC.
Since we identified the problem of the continuity of the deposited layer on a non-conductive anode substrate, we identified as a key task the creation of uniform conductivity over the entire surface of the substrate and providing electrical connection with an external voltage source.
3.4.2. EPD on Reduced NiO-SDC Substrates
EPD of a Two-Layer BCZYYbO-CuO/SDC Electrolyte on a Reduced NiO-SDC Anode Substrate
As one of the methods for creating the conductivity across the entire NiO-SDC substrate, its reduction in a H2-containing atmosphere was chosen. The reduction was performed during 80 min in a H2 (5 vol.%)/Ar mixture at a temperature of 650 °C. EPD of the layer of the proton-conducting electrolyte BCZYYbO-CuO was carried out at 80 V for 1 min. The obtained coating of 4.4 mg/cm2 with a thickness of 7.8 μm was sintered in air at a temperature of 1300 °C for 2 h. Then, the anode-substrate was re-reduced under the conditions described above and the SDC layer was deposited at 100 V for 2 min. The deposited weight of the SDC layer was 3.4 mg/cm2 and the thickness was 4.7 μm. Thus, the presence of the pre-sintered BCZYYbO-CuO possessing relatively low conductivity at room temperature resulted in a decrease in the deposition rate of the SDC electrolyte layer. Nevertheless, the deposition was performed successfully due to conducting properties of the reduced substrate. The obtained two-layer coating was dried in air at a room temperature for 24 h. According to the observation using the optical microscope, as-prepared two-layer coating was uniform without any pores and cracks (Figure 9a). Sintering of the two-layer electrolyte structure was carried out at a temperature of 1400 °C for 5 h. After the sintering, multiple breaks occurred in the electrolyte layer (Figure 9b) and a significant curvature of the substrate was also observed. The control of the change in the weight and linear dimensions of the substrates during the reduction-oxidative sintering cycles was carried out on the test sample. Reduction of the anodes led to a decrease in their weight by 14%. The subsequent oxidative sintering led to an increase in their weight by 14% as well. The change in linear dimensions of the test sample was of the order of 10%. It can be concluded that the cycles of reduction-oxidative sintering are accompanied by a significant change in the specific volume of nickel oxide in the composition of the anode which is in agreement with the data presented in [53]. Due to repetitive redox cycles, the appearance of bending mechanical stresses in the substrate caused by different redox behavior of the NiO and SDC is also possible [54]. When the anode-substrate was reduced, the gas permeability coefficient of the anode substrate increased from 1.6 × 10−3 to 7.9 × 10−3 µm2. After oxidative sintering at 1400 °C for 5 h the gas permeability coefficient decreased to 2.5 × 10−3 µm2.
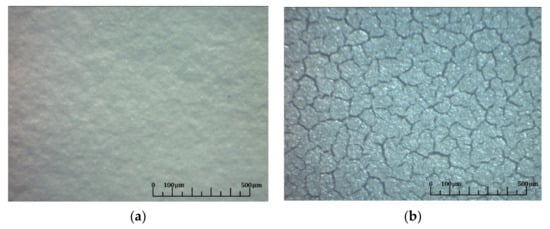
Figure 9.
Optical photographs of the surface of the deposited two-layer electrolyte BCZYYbO-CuO/SDC: (a) after deposition and drying of the SDC layer; (b) and after sintering in air at a temperature of 1400 °C for 5 h.
EPD of a Single-Layer SDC Electrolyte on a Reduced NiO-SDC Anode Substrate
To elucidate factors that determine the continuity of the sintered coating, we excluded the BCZYYbO-CuO barrier layer, and carried out the formation of a single-layer SDC electrolyte directly on the reduced anode substrate, followed by the oxidative sintering. The SDC layer was deposited at 100 V for 5 min. The deposited weight was 6.3 mg/cm2 and the coating thickness was 8.7 μm. During sintering at a temperature of 1400 °C for 5 h, the substrate broke into several fragments. On the separate fragments of the substrate, the SDC electrolyte layer with multiple breaks in it was observed, as in the sample with the two-layer film (Figure 9b). Thus, it can be concluded that the main factor preventing the formation of a continuous electrolyte layer during its sintering, in this case, is the reduction of the anode-substrate and its subsequent oxidative sintering, which causes significant mechanical stresses in the substrate-electrolyte structure up to its complete destruction.
Figure 10 shows dilatometric curves for initial powder materials (Figure 10a,b) and anode substrates (Figure 10c). The material BCZYYbO-CuO (Figure 10a) is characterized by complex behavior during heating and subsequent cooling. When heated to a temperature of about 900 °C, thermal expansion of the material occurred, which was followed by short-term compaction in the temperature range of 900–1000 °C. Then a sharp expansion of the material occurred and continued until the end of heating up to 1500 °C, which may be associated with ongoing transformations in the material probably connected with incorporation of Cu into the lattice. Upon subsequent cooling, a slight shrinkage was followed by a slight expansion at a temperature of about 900 °C. The size of the BCZYYbO-CuO sample increased during the heating-cooling temperature cycle and the dL/Lo value was about +0.3. A sample of powder material SDC (Figure 10b) showed a different behavior: primary thermal expansion was followed by sintering and compaction of the material at temperatures of 800–1450 °C. Upon cooling, thermal contraction of the material occurred. There was a decrease in the size of the SDC sample during the heating-cooling temperature cycle and the dL/Lo value was about −0.12. For unreduced anode substrates (Figure 10c), compaction was observed in the temperature range from 1160 to 1300 °C. Cooling was accompanied by thermal contraction of the material. The reduced anode substrate (Figure 10c) experienced a sharp expansion, exceeding thermal expansion in the temperature range of 500–845 °C, which was followed by shrinkage when heated to a temperature of 1300 °C. Upon cooling, a uniform compression of the material occurred, and there was no total change in the dimensions during the temperature cycle. The revealed features of the behavior of the applied powder materials during heating and cooling can be a determining factor affecting the sintering process of the formed EPD coatings.
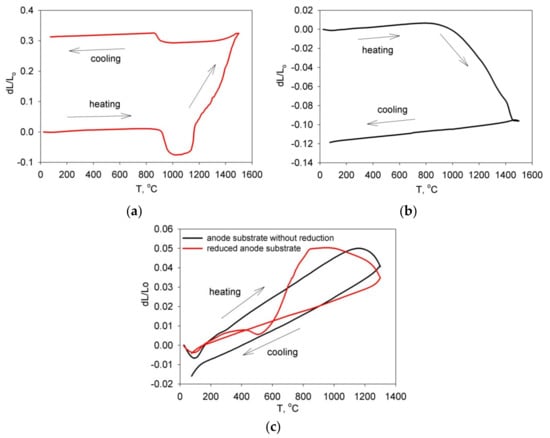
Figure 10.
Dilatometric curves: (a) for the compacted BCZYYbO-CuO powder; (b) for the compacted SDC powder; (c) for as-prepared and reduced anode substrates.
3.4.3. EPD on Porous Anodes with a Conductive Pt Sublayer
Further experiments on the formation of an SDC electrolyte layer by EPD were conducted on the NiO-SDC anode substrates with a conductive coating on its surface. A new method of surface modification was proposed by the deposition of a platinum layer on the front surface of the NiO-SDC substrates from a suspension of Pt particles in isopropanol followed by drying and annealing at a temperature of 900 °C for 1 h was performed. The platinum layer weight was 13.3 mg/cm2. Then, EPD of the SDC electrolyte layer was performed on the surface of the anode substrate with the applied platinum sublayer (at the EPD mode: voltage 100 V, time 2 min) by sequentially carrying out four deposition-sintering cycles with an increase in the total electrolyte thickness up to 32.7 μm during final sintering at a temperature of 1500 °C, 5 h (Table 2).

Table 2.
Cycles of deposition-sintering of the SDC electrolyte layer on the NiO-SDC anode substrate with a platinum sublayer: thickness, sintering modes and the electrolyte film properties.
At the third stage, after sintering at 1450 °C, for 5 h, SEM examination of the surface of the SDC electrolyte film was performed (Figure 11).
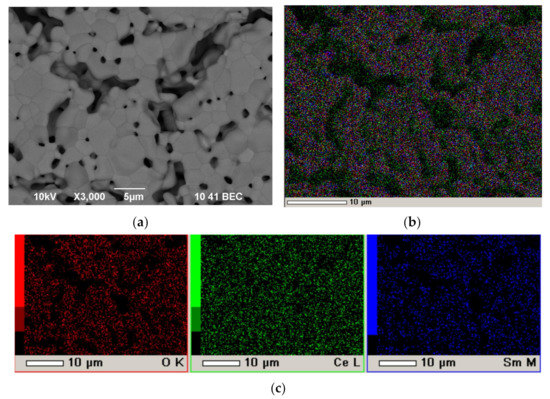
Figure 11.
The surface of the SDC electrolyte layer after intermediate sintering at a temperature of 1450 °C for 5 h on the porous NiO-SDC anode substrate with a platinum sublayer: (a) SEM image; (b) integral map of the elements’ distribution; (c) individual element maps.
It can be seen that there was no complete sintering of the SDC electrolyte layer at a temperature of 1450 °C for 5 h, namely, there were sintered areas of the coating about 10 μm in size with grains of about 3 μm in size, separated by pores and their clusters about 5–10 μm in size (Figure 11a). According to the EDX analysis, no platinum was detected on the surface of the sintered layer of SDC electrolyte (Figure 11b,c). There are only SDC electrolyte elements in an amount close to the nominal one: Ce-28 at.%, Sm-7 at.%, O-65 at.%. It can be concluded that the deposition of a single conducting Pt layer at the first stage allows the deposition of a multilayer coating containing pre-sintered low conducting layers.
Figure 12 shows a cross-section of the SDC/Pt/NiO-SDC structure after final sintering at a temperature of 1500 °C for 5 h. It can be seen that the SDC electrolyte layer has a thickness of about 40 μm and is characterized by closed porosity (Figure 12a). The elemental composition of the SDC electrolyte is presented in a nominal ratio (Figure 12b,c). The platinum layer deposited on the NiO-SDC substrate was porous and had a thickness of about 20 μm. Platinum was not found in the composition of the electrolyte, and the deposited Pt sublayer had no tendency to diffusion smearing during sintering (Figure S2).
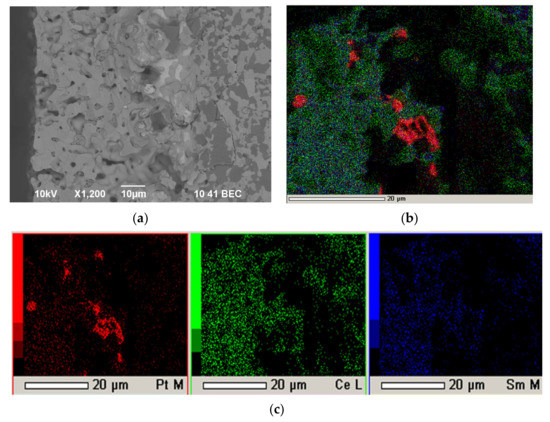
Figure 12.
Cross-section of the SDC electrolyte layer after final sintering at a temperature of 1500 °C for 5 h on a porous anode substrate with a platinum sublayer: (a) SEM image; (b) integral map of the elements’ distribution; (c) individual element maps.
3.4.4. EPD of the Single Layer SDC Electrolyte Film on the NiO-SDC Porous Substrates Pre-Sintered at Decreased Temperatures
In order to determine the possibility of reducing the sintering temperature of the SDC layer, experiments on EPD were carried out on the NiO-SDC substrates with/without the pore former pre-sintered at 1200 °C for the subsequent final co-sintering with the electrolyte layer, that, as we assumed, can contribute to its densification [27,28,29,41]. EPD of the SDC electrolyte layer was carried out on the anode substrates with deposited conductive layers (PPy, Pt) in a constant voltage mode of 80 V, and the deposition time was 2 min. The experimental results are presented in Table 3. It can be seen that the use of pre-sintered at lower temperature anode substrates with different porosity level had no a visible positive effect on the densification of the SDC electrolyte layer during its final co-sintering with the substrate at temperatures of 1450 or 1500 °C. Moreover, the anode substrate formed with addition of the pore former created conditions that led to the formation of numerous cracks in the SDC electrolyte layer during co-sintering. The use of the pore-former-free substrate resulted in better quality of the SDC film sintered 1450 °C, with only few single defects on its surface. An increase in the final sintering temperature from 1450 to 1500 °C eliminated surface defects, however, complete sintering of the film was not achieved. We also repeated the experiment on the pore-former-free substrate using Pt conductive coating and obtained the defect-free film at 1450 °C. The use of a platinum sublayer has an advantage over the coating of the substrate with polypyrrole, since such a factor as the burnout of the conductive Ppy coating during sintering was eliminated. Thus, the SDC electrolyte film sintered at 1450 °C on the platinum sublayer possessed a denser microstructure as compared to the film obtained on Ppy. However, the effect of the type of conductive coating (Ppy, Pt) on the complete densification of the SDC layer was not observed.

Table 3.
Features of co-sintering of the SDC layer deposited on the anode porous NiO-SDC substrates pre-sintered at decreased temperatures and with different conducting layer.
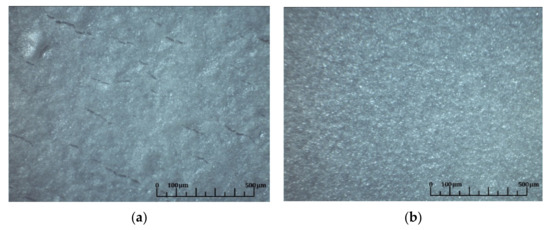
Figure 13.
Optical images of the surface of the SDC films obtained after the final co-sintering: (a) at 1450 °C, 5 h with the NiO-SDC substrate formed with the addition of 20 wt.% of graphite powder; (b) at 1500 °C, 5 h with the NiO-SDC substrate without the pore former.
Table 3.
Features of co-sintering of the SDC layer deposited on the anode porous NiO-SDC substrates pre-sintered at decreased temperatures and with different conducting layer.
| Anode Substrate | Conducting Layer | SDC Film Thickness, μm | SDC Film Sintering Mode | Characteristics of the SDC Film Surface |
|---|---|---|---|---|
| NiO-SDC + 20% graphite powder | Ppy | 8 | 1450 °C, 5 h | Unsintered film with numerous edge cracks (Figure 13a) |
| NiO-SDC | Ppy | 7 | 1450 °C, 5 h | Unsintered film with few cracks |
| NiO-SDC | Ppy | 7 | 1500 °C, 5 h | Unsintered film, no cracks (Figure 13b) |
| NiO-SDC | Pt | 8 | 1450 °C, 5 h | Unsintered film, no cracks |
Thus, it can be summarized that, despite the larger shrinkage of the anode substrates pre-sintered at 1200 °C, the temperature of 1500 °C is insufficient for sintering the SDC electrolyte layer. It should be noted that anode substrates with/without the pore former probably have different kinetics of changes in internal stresses during the final co-sintering with the SDC electrolyte layer, which influences the process of formation and development of cracks in the film during sintering. However, when fabricating anode-supported half-cells for SOFCs it is necessary to keep the anode porosity quite high and the use of a pore former is preferable. Additional studies should be done to find an optimal pore former content allowing the film of good quality to be obtained on the porous anode substrate.
3.5. Microstructure of EPD Coatings Deposited on Model Ni Foil and Dense SDC Substrates
In order to determine the reasons for the appearance of porosity in the SDC coatings sintered at the temperatures higher that that established in the dilatometry study (Figure 10b) the experiments on the deposition of an SDC coating was performed on the model Ni foil and dense SDC substrates. The structure of the SDC coating deposited on Ni-foil and annealed at 600 °C for 1 h is presented in Figure 14. It can be seen that in the SDC layer there are no large pores and discontinuities, as well as ruptures and cracks. The coating is homogeneous and has a nominal elemental composition (see the EDX analysis data). As a result, it was found that the EPD process allows the formation of uniform continuous coatings of good quality, and the formation of pores in the coating takes place in the subsequent process of the coating sintering.
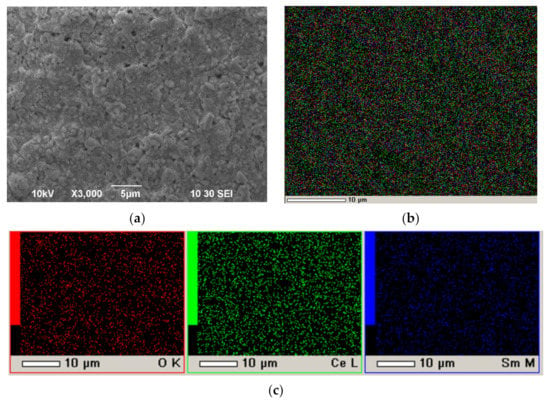
Figure 14.
The surface of the layer of unsintered SDC electrolyte on a Ni-foil after annealing at a temperature of 600 °C for 1 h: (a) SEM image, magnification ×3000; (b) integral map of the elements’ distribution; (c) individual element maps.
Additional experiments on the sintering of SDC electrolyte films were carried out on a model dense SDC substrate sintered at 1600 °C for 5 h, which was selected to determine the features of sintering and compaction of the deposited EPD layer in the absence of the substrate shrinkage. As far as the SDC conductivity is low at the room temperature, for the successful EPD similar metallization its surface was carried out by applying a Pt sublayer on the front surface of a non-conducting SDC substrate, as this method, developed in this study, gives the advantage of allowing a sequential deposition of layers with intermediate sintering. The experiment made it possible to determine the required temperature for the formation of a dense non-porous electrolyte microstructure and revealed the need for additional deposition of the coating material (SDC) after intermediate deposition and sintering (1470 °C for 5 h) to fill the formed pores. The thickness of the SDC layer (at the voltage of 100 V for 6 min) before intermediate sintering was 14.5 µm; upon sequential deposition of SDC, the coating thickness was increased to 24.3 µm. As can be seen in SEM images presented in Figure 15, that after the final sintering at 1550 °C for 5 h a dense, pore-free electrolyte film was formed with the average grain size of about 25 μm. According to the EDX analysis, the composition of the electrolyte film corresponded to the nominal one. As a result, it was shown that the used SDC powder requires sufficiently high temperatures for final sintering into a dense electrolyte layer, as well as additional EPD of the powder material to fill the pores formed during intermediate sintering due to compaction and shrinkage of the coating material. The results obtained showed that the formation of a dense film electrolyte is not achieved only by increasing the temperature, but also requires additional introducing the material into the coating at the intermediate EPD stages.
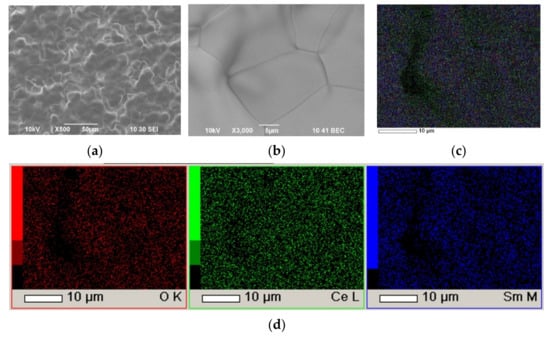
Figure 15.
The surface of the SDC electrolyte layer after final sintering at a temperature of 1550 °C for 5 h on a model dense SDC substrate with a platinum sublayer: (a) SEM image, magnification ×500; (b) SEM image, magnification ×3000; (c) integral map of the distribution of elements; (d) individual element maps.
4. Conclusions
This work presents the results of studies on the formation of thin-film SDC electrolyte and bilayer BCZYYbO-CuO/SDC electrolyte on the porous non-conducting NiO-SDC anode substrates. The work was aimed at identifying key features and possible implementations of the full technological cycle: characterization of the powders used, preparation of the suspensions based on the SDC and BCZYYbO-CuO powder materials in a non-aqueous medium, establishment of optimal deposition modes on the model substrates, implementation of direct EPD on the porous non-conductive NiO-SDC anode substrates, EPD on the reduced Ni-SDC anode substrates, EPD on the NiO-SDC anode substrates with different conductive sublayer (PPy, Pt), and study of the sintering kinetic of the substrates and deposited layers.
The applied powders BCZYYbO and SDC were obtained by citrate-nitrate and glycine-citrate methods, respectively, which were characterized by aggregate sizes of the order of 2–4 μm. Analysis of the disperse, fractional composition and electrokinetic potential of the suspensions after ultrasonic treatment for 5–125 min was carried out. It was shown that the size of the aggregates in the suspension can be effectively decreased using the UST for 125 min down to 492 nm (47%) and 1662 nm (53%) for the SDC powder and 745 nm (74%) and 1960 nm (26%) for BCZYYbO-CuO powder, respectively.
The main difficulty in implementing EPD on non-conductive substrates is the need to create conductivity on their surface. We have investigated various possible methods to form continuous electrolyte coatings on the porous NiO-SDC substrates within the context of issues related to the subsequent sintering of the obtained electrolyte film/anode structures. It was revealed that direct EPD on the porous NiO-SDC substrate is possible; however, a more developed porosity of the substrate is required for the formation of a continuous layer. A variant of the EPD technology, which includes deposition BCZYYbO-CuO/SDC and SDC films on the reduced Ni-SDC substrates, could not be successfully employed under our conditions due to significant mechanical stresses caused by the reduction and subsequent oxidation of both nickel and SDC in the composition of the anode substrates. This resulted in the formation of a network of breaks in the bilayer BCZYYbO-CuO/SDC film and complete disintegration of the single SDC film during the oxidative co-sintering with the substrate.
We have demonstrated the viability of creating PPy and Pt conducting sublayers on the NiO-SDC substrate front surface for the successful SDC electrolyte film deposition. However, the Pt conducting sublayer on the front surface of a non-conducting substrate was shown to have the advantage of allowing a sequential deposition of layers with intermediate sintering with no visible diffusion into the deposited layers.
It was demonstrated that, to obtain a gas-tight defect-free SDC electrolyte film, a higher temperature for the final sintering is required than that established in the dilatometric study of the compacted SDC powder. Moreover, it was shown that increased shrinkage of the substrates with lowered pre-sintering temperatures had no effect on the densification of the SDC electrolyte film. To preserve the porous structure of the anode substrate, a necessity for the effective operation of the SOFC, future studies should consider the possibility of reducing the sintering temperature of the SDC electrolyte layer using sintering additives.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/coatings11070805/s1, Figure S1: XRD patterns (log-scale) obtained for the electrolyte powders: as-prepared SDC powder (a), as-prepared BCZYYbO powder (b), BCZYYbO-CuO powder (sintered at 1500 °C) (c). Figure S2: Cross-section of the SDC electrolyte layer after final sintering at a temperature of 1500 °C for 5 hours on a porous anode substrate with a platinum sublayer: (a)-SEM image; (b)-integral map of the elements’ distribution; (c)-individual element maps. The platinum layer seen from the left side of the film is a Pt electrode sintered at 1100 °C serving for electrical measurements (not presented in this study).
Author Contributions
Conceptualization, methodology, investigation, formal analysis, writing—original draft preparation, E.K.; conceptualization, supervision, writing—review and editing, E.P.; investigation, L.E.; investigation, sample preparation, N.B. All authors have read and agreed to the published version of the manuscript.
Funding
The work was financially supported by the Russian Foundation for Basic Research, grant № 20-03-00151. Investigation of the kinetic properties of the suspensions was performed within the framework of the state assignment of IEP UB RAS (EPD thin-layer coatings, No. AAAA-A19-119061090040-7). The study was in part carried out on the equipment of the Shared Access Center of “Composition of compounds” IHTE UB RAS and the Shared Access Centers of the IEP UB RAS and ISSC UB RAS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the head of the Laboratory of chemistry of compounds of rare-earth elements (ISSC UB RAS), V.D. Zhuravlev and E.A. Filonova (Ural Federal University) for the materials supply and S.M. Pikalov (Institute of Metallurgy UB RAS) for the XRD characterization.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Su, H.; Hu, Y.H. Progress in low-temperature solid oxide fuel cells with hydrocarbon fuels. Chem. Eng. J. 2020, 402, 126235. [Google Scholar] [CrossRef]
- Sreedhar, I.; Agarwal, B.; Goyal, P.; Singh, S.A. Recent advances in material and performance aspects of solid oxide fuel cells. J. Electroanal. Chem. 2019, 848, 113315. [Google Scholar] [CrossRef]
- Tucker, M.C. Progress in metal-supported solid oxide fuel cells: A review. J. Power Sources 2010, 195, 4570–4582. [Google Scholar] [CrossRef]
- Nguyen, X.V.; Chang, C.T.; Jung, G.B.; Chan, S.H.; Yeh, C.C.; Yu, J.W.; Lee, C.Y. Improvement on the design and fabrication of planar SOFCs with anode–supported cells based on modified button cells. Renew. Energy 2018, 129, 806–813. [Google Scholar] [CrossRef]
- Ishihara, T.; Shimose, K.; Kudo, T.; Nishiguchi, H.; Akbay, T.; Takita, Y. Preparation of yttria-stabilized zirconia thin films on strontium-doped LaMnO3 cathode substrates via electrophoretic deposition for solid oxide fuel cells. J. Am. Ceram. Soc. 2000, 83, 1921–1927. [Google Scholar] [CrossRef]
- Solov’ev, A.A.; Shipilova, A.V.; Ionov, I.; Kovalchuk, A.N.; Rabotkin, S.V.; Oskirko, V.O. Magnetron-sputtered YSZ and CGO electrolytes for SOFC. J. Electron. Mater. 2016, 45, 3921–3928. [Google Scholar] [CrossRef]
- Park, B.K.; Barnett, S.A. Boosting solid oxide fuel cell performance via electrolyte thickness reduction and cathode infiltration. J. Mater. Chem. A 2020, 8, 11626–11631. [Google Scholar] [CrossRef]
- Prakash, B.S.; Pavitra, R.; Kumar, S.S.; Aruna, S.T. Electrolyte bi-layering strategy to improve the performance of an intermediate temperature solid oxide fuel cell: A review. J. Power Sources 2018, 381, 136–155. [Google Scholar] [CrossRef]
- Hou, J.; Bi, L.; Qian, J.; Zhu, Z.; Zhang, J.; Liu, W. High performance ceria–bismuth bilayer electrolyte low temperature solid oxide fuel cells (LT-SOFCs) fabricated by combining co-pressing with drop-coating. J. Mater. Chem. A 2015, 3, 10219–10224. [Google Scholar] [CrossRef]
- Hu, S.; Li, W.; Yao, M.; Li, T.; Liu, X. Electrophoretic deposition of gadolinium-doped ceria as a barrier layer on yttrium-stabilized zirconia electrolyte for solid oxide fuel cells. Fuel Cells 2017, 17, 869–874. [Google Scholar] [CrossRef]
- Hui, R.; Wang, Z.; Kesler, O.; Rose, L.; Jankovic, J.; Yick, S.; Maric, R.; Ghosh, D. Thermal plasma spraying for SOFCs: Applications, potential advantages, and challenges. J. Power Sources 2007, 170, 308–323. [Google Scholar] [CrossRef]
- Wen, J.; Song, C.; Liu, T.; Deng, Z.; Niu, S.; Zhang, Y.; Liu, L.; Liu, M. Fabrication of dense gadolinia-doped ceria coatings via very-low-pressure plasma spray and plasma spray–physical vapor deposition process. Coatings 2019, 9, 717. [Google Scholar] [CrossRef]
- Oh, J.; Seo, G.; Kim, J.; Bae, S.; Park, J.W.; Hwang, J.H. Plasma-enhanced atomic layer deposition of zirconium oxide thin films and its application to solid oxide fuel cells. Coatings 2021, 11, 362. [Google Scholar] [CrossRef]
- Smolyanskiy, E.A.; Linnik, S.A.; Ionov, I.V.; Shipilova, A.V.; Semenov, V.A.; Lauk, A.L.; Solovyev, A.A. Magnetron sputtered LSC thin films for solid oxide fuel cell application. J. Phys. Conf. Ser. 2018, 1115, 032080. [Google Scholar] [CrossRef]
- Xu, M.; Yu, J.; Song, Y.; Ran, R.; Wang, W.; Shao, Z. Advances in ceramic thin films fabricated by pulsed laser deposition for intermediate-temperature solid oxide fuel cells. Energy Fuels 2020, 34, 10568–10582. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Cho, G.Y.; Yu, W.; Lee, Y.; Chang, I.; Baek, J.D.; Cha, S.W. Investigation of reducing in-plane resistance of nickel oxide-samaria-doped ceria anode in thin-film solid oxide fuel cells. Energies 2020, 13, 1989. [Google Scholar] [CrossRef]
- Dunyushkina, L.A.; Khaliullina, A.S.; Kuimov, V.M.; Osinkin, D.A.; Antonov, B.D.; Pankratov, A.A. Influence of modification of chemical solution deposition on morphology and conductivity of CaZr0.9Y0.1O3-δ films. Solid State Ion. 2019, 329, 1–7. [Google Scholar] [CrossRef]
- Kalinina, E.; Kolchugin, A.; Shubin, K.; Farlenkov, A.; Pikalova, E. Features of electrophoretic deposition of a Ba-containing thin-film proton-conducting electrolyte on a porous cathode substrate. Appl. Sci. 2020, 10, 6535. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Yoon, K.J.; Son, J.W.; Lee, J.H.; Lee, J.H.; Lee, H.W.; Ji, H.I. Solid oxide fuel cells with zirconia/ceria bilayer electrolytes via roll calendering process. J. Alloys Compd. 2020, 846, 156318. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Place of electrophoretic deposition among thin-film methods adapted to the solid oxide fuel cell technology: A short review. Int. J. Energy Prod. Manag. 2019, 4, 1–27. [Google Scholar] [CrossRef]
- Sakka, Y.; Uchikoshi, T. Forming and microstructure control of ceramics by electrophoretic deposition (EPD). KONA Powder Part. J. 2010, 28, 74–90. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y.; Menshikova, A.V.; Nikolaenko, I.V. Electrophoretic deposition of a self-stabilizing suspension based on a nanosized multi-component electrolyte powder prepared by the laser evaporation method. Solid State Ion. 2016, 288, 110–114. [Google Scholar] [CrossRef]
- Das, D.; Bagchi, B.; Basu, R.N. Nanostructured zirconia thin film fabricated by electrophoretic deposition technique. J. Alloys Compd. 2017, 693, 1220–1230. [Google Scholar] [CrossRef]
- Zehbe, R.; Mochales, C.; Radzik, D.; Müller, W.D.; Fleck, C. Electrophoretic deposition of multilayered (cubic and tetragonal stabilized) zirconia ceramics for adapted crack deflection. J. Eur. Ceram. Soc. 2016, 36, 357–364. [Google Scholar] [CrossRef]
- Istomin, S.Y.; Antipov, E.V. Cathode materials based on perovskite-like transition metal oxides for intermediate temperature solid oxide fuel cells. Russ. Chem. Rev. 2013, 82, 686–700. [Google Scholar] [CrossRef]
- Chelmehsara, M.E.; Mahmoudimehr, J. Techno-economic comparison of anode-supported, cathode-supported, and electrolyte-supported SOFCs. Int. J. Hydrogen Energy 2018, 43, 15521–15530. [Google Scholar] [CrossRef]
- Majhi, S.M.; Behura, S.K.; Bhattacharjee, S.; Singh, B.P.; Chongdar, T.K.; Gokhale, N.M.; Besra, L. Anode supported solid oxide fuel cells (SOFC) by electrophoretic deposition. Int. J. Hydrogen Energy 2011, 36, 14930–14935. [Google Scholar] [CrossRef]
- Will, J.; Hruschka, M.K.M.; Gubler, L.; Gauckler, L.J. Electrophoretic deposition of zirconia on porous anodic substrates. J. Am. Ceram. Soc. 2004, 84, 328–332. [Google Scholar] [CrossRef]
- Hosomi, T.; Matsuda, M.; Miyake, M. Electrophoretic deposition for fabrication of YSZ electrolyte film on non-conducting porous NiO–YSZ composite substrate for intermediate temperature SOFC. J. Eur. Ceram. Soc. 2007, 27, 173–178. [Google Scholar] [CrossRef]
- Matsuda, M.; Hosomi, T.; Murata, K.; Fukui, T.; Miyake, M. Fabrication of bilayered YSZ/SDC electrolyte film by electrophoretic deposition for reduced-temperature operating anode-supported SOFC. J. Power Sources 2007, 165, 102–107. [Google Scholar] [CrossRef]
- Chauoon, S.; Meepho, M.; Chuankrerkkul, N.; Chaianansutcharit, S.; Pornprasertsuk, R. Fabrication of yttria stabilized zirconia thin films on powder-injected anode substrates by electrophoretic deposition technique for solid oxide fuel cell application. Thin Solid Films 2018, 660, 741–748. [Google Scholar] [CrossRef]
- Bozza, F.; Polini, R.; Traversa, E. High performance anode-supported intermediate temperature solid oxide fuel cells (IT-SOFCs) with La0.8Sr0.2Ga0.8Mg0.2O3−δ electrolyte films prepared by electrophoretic deposition. Electrochem. Commun. 2009, 11, 1680–1683. [Google Scholar] [CrossRef]
- Kobayashi, K.; Takahashi, I.; Shiono, M.; Dokiya, M. Supported Zr(Sc)O2 SOFCs for reduced temperature prepared by electrophoretic deposition. Solid State Ion. 2002, 152–153, 591–596. [Google Scholar] [CrossRef]
- Das, D.; Basu, R.N. Electrophoretic deposition of zirconia thin film on nonconducting substrate for solid oxide fuel cell application. J. Am. Ceram. Soc. 2014, 97, 3452–3457. [Google Scholar] [CrossRef]
- Suzuki, H.T.; Uchikoshi, T.; Kobayashi, K.; Suzuki, T.S.; Sugiyama, T.; Furuya, K.; Matsuda, M.; Sakka, Y.; Munakata, F. Fabrication of GDC/LSGM/GDC tri-layers on polypyrrole-coated NiO-YSZ by electrophoretic deposition for anode-supported SOFC. J. Ceram. Soc. Jpn. 2009, 117, 1246–1248. [Google Scholar] [CrossRef]
- Zunic, M.; Chevallier, L.; Deganello, F.; D’Epifanio, A.; Licoccia, S.; Di Bartolomeo, E.; Traversa, E. Electrophoretic deposition of dense BaCe0.9Y0.1O3−x electrolyte thick-films on Ni-based anodes for intermediate temperature solid oxide fuel cells. J. Power Sources 2009, 190, 417–422. [Google Scholar] [CrossRef]
- Azarian Borojeni, I.; Raissi, B.; Maghsoudipour, A.; Kazemzad, M.; Talebi, T. Fabrication of solid oxide fuel cells (SOFCs) electrolytes by electrophoretic deposition (EPD) and optimizing the process. Key Eng. Mater. 2015, 654, 83–87. [Google Scholar] [CrossRef]
- Meepho, M.; Wattanasiriwech, D.; Aungkavattana, P.; Wattanasiriwech, S. Reduction of electrode polarization in Anode-Supported Solid Oxide Fuel Cell. Energy Procedia 2015, 79, 272–277. [Google Scholar] [CrossRef][Green Version]
- Talebi, T.; Haji, M.; Raissi, B. Effect of sintering temperature on the microstructure, roughness and electrochemical impedance of electrophoretically deposited YSZ electrolyte for SOFCs. Int. J. Hydrogen Energy 2010, 35, 9420–9426. [Google Scholar] [CrossRef]
- Talebi, T.; Raissi, B.; Haji, M.; Maghsoudipour, A. The role of electrical conductivity of substrate on the YSZ film formed by EPD for solid oxide fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9405–9410. [Google Scholar] [CrossRef]
- Ishihara, T.; Sato, K.; Takita, Y. Electrophoretic deposition of Y2O3-Stabilized ZrO2 electrolyte films in solid oxide fuel cells. J. Am. Ceram. Soc. 1996, 79, 913–919. [Google Scholar] [CrossRef]
- Oskouyi, O.E.; Shahmiri, M.; Maghsoudipour, A.; Hasheminiasari, M. Pulsed constant voltage electrophoretic deposition of YSZ electrolyte coating on conducting porous Ni-YSZ cermet for SOFCs applications. J. Alloys Compd. 2019, 785, 220–227. [Google Scholar] [CrossRef]
- Jamil, Z.; Ruiz-Trejo, E.; Brandon, N.P. Nickel electrodeposition on silver for the development of solid oxide fuel cell anodes and catalytic membranes. J. Electrochem. Soc. 2017, 164, D210–D217. [Google Scholar] [CrossRef]
- Bokov, A.A.; Boltachev, G.S.; Volkov, N.B.; Zayats, S.V.; Il’ina, A.M.; Nozdrin, A.A.; Paranin, S.N.; Olevskii, E.A. Uniaxial compaction of nanopowders on a magnetic-pulse press. Tech. Phys. 2013, 58, 1459–1468. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y.; Kolchugin, A.A.; Pikalov, S.M.; Kaigorodov, A.S. Cyclic electrophoretic deposition of electrolyte thin-films on the porous cathode substrate utilizing stable suspensions of nanopowders. Solid State Ion. 2017, 302, 126–132. [Google Scholar] [CrossRef]
- Uchikoshi, T.; Furumi, S.; Shirahata, N.; Suzuki, T.S.; Sakka, Y. Conductive polymer coating on nonconductive ceramic substrates for use in the electrophoretic deposition process. J. Am. Ceram. Soc. 2008, 91, 1674–1677. [Google Scholar] [CrossRef]
- Nasani, N.; Shakel, Z.; Loureiro, F.J.A.; Panigrahi, B.B.; Kale, B.B.; Fagg, D.P. Exploring the impact of sintering additives on the densification and conductivity of BaCe0.3Zr0.55Y0.15O3−δ electrolyte for protonic ceramic fuel cells. J. Alloys Compd. 2021, 862, 158640. [Google Scholar] [CrossRef]
- Kirillov, S.A. Surface area and pore volume of a system of particles as a function of their size and packing. Micropor. Mesopor. Mat. 2009, 122, 234–239. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Electrophoretic deposition in the solid oxide fuel cell technology: Fundamentals and recent advances. Renew. Sustain. Energy Rev. 2019, 116, 109440. [Google Scholar] [CrossRef]
- Besra, L.; Compson, C.; Liu, M. Electrophoretic deposition on non-conducting substrates: The case of YSZ film on NiO–YSZ composite substrates for solid oxide fuel cell application. J. Power Sources 2007, 173, 130–136. [Google Scholar] [CrossRef]
- Solomentsev, Y.; Böhmer, M.; Anderson, J.L. Particle clustering and pattern formation during electrophoretic deposition: A hydrodynamic model. Langmuir 1997, 13, 6058. [Google Scholar] [CrossRef]
- Ferrari, B.; Moreno, R. EPD kinetics: A review. J. Eur. Ceram. Soc. 2010, 30, 1069–1078. [Google Scholar] [CrossRef]
- Heenan, T.M.M.; Vamvakeros, A.; Tan, C.; Finegan, D.P.; Daemi, S.R.; Jacques, S.D.M.; Beale, A.M.; Michiel, M.D.; Brett, D.J.L.; Shearing, P.R. The Detection of monoclinic zirconia and non-uniform 3D crystallographic strain in a Re-oxidized Ni-YSZ solid oxide fuel cell anode. Crystals 2020, 10, 941. [Google Scholar] [CrossRef]
- Maher, R.C.; Shearing, P.R.; Brightman, E.; Brett, D.J.L.; Brandon, N.P.; Cohen, L.F. Reduction dynamics of doped ceria, nickel oxide, and cermet composites probed using in situ Raman spectroscopy. Adv. Sci. 2016, 3, 1500146. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).