Substituted Hydroxyapatite, Glass, and Glass-Ceramic Thin Films Deposited by Nanosecond Pulsed Laser Deposition (PLD) for Biomedical Applications: A Systematic Review
Abstract
:1. Introduction
- Crystallinity: The presence of amorphous phases, which exhibit higher solubility in an aqueous medium, is desirable to accelerate bone formation. However, a too high dissolution rate compromises stability and increases the risk of failure. Consequently, crystalline phases are necessary to ensure term stability of the implant in clinical use [18]. Therefore, to prepare implants with predictable properties, it is necessary to design and control the crystallinity and purity of the coatings.
- Surface topography: The denser the microstructure of the coating is, less is its cracking or degradation. However, rough, textured, and porous surfaces could stimulate cell attachment and formation of an extra-cellular matrix. In particular, the combination of sub-microscale surface roughness, with microscale and nanoscale features, can stimulate both the adhesion of proteins involved in the regulation of osteoblast proliferation, and the adhesion and differentiation of cells [19,20]. Optimum coating porosity and roughness are important for in-growth of bone cells; conversely, the accumulation of macropores at the substrate/coating interface leads to a weakening of the coating adhesion.
- Mechanical properties: Good adhesion, high hardness, and high toughness are necessary to decrease residues generated during the functional loadings, which can be dangerous for the organism, and to prevent mechanical failures under load conditions [21].
2. Nanosecond PLD: Laser Ablation and Film Growth
3. PLD of Ion Doped HA Films
3.1. Nanosecond Ablation and Deposition of HA Films
3.2. Substituted HA
- Mg
- F
- Sr
- Si
- Ag
- Mn
- Fe
- Other elements
4. PLD of Glass and Glass-Ceramic Coatings
| BG and BGC Systems | Laser Source | Deposition Conditions | Substrate | Properties | Ref. |
|---|---|---|---|---|---|
| SiO2–Na2O–CaO–P2O5 (45S5) 1 | 532 nm, 7 ns, 10 Hz | 0.5–14 J/cm2, RT, 10−4 Pa | Ti4Al | Film adhesion | [106] |
| 532 nm, 7 ns, 10 Hz | 0.5–14 J/cm2, RT, 10−4 Pa | Ti6Al4V | Film adhesion and bioactivity in SBF | [107] | |
| 532 nm, 7 ns, 10 Hz | 9 J/cm2, RT, 10−4 Pa | Ti6Al4V | Hardness | [112] | |
| 248 nm, 20 ns, 10 Hz | 4 J/cm2, 20, 200 and 500 °C, 8.5 × 10−5 Pa, 55 mm, 1 h | Ti6Al4V | Film adhesion | [142] | |
| 532 nm, 6 ns, 10 Hz | 2 × 10−5 mbar, RT and 200 °C, 1 h | Ti–6Al–4V | Bioactivity in SBF and biocompatibility with U2OS osteosarcoma cells to | [154] | |
| SiO2–Na2O–CaO–P2O5–(MgO) (45S5, Mg10) 2 | 248 nm, 20 ns, 10 Hz | 4 J/cm2, 200 °C, 8.5 × 10−5 Pa, 55 mm, 1 h | Ti6Al4V | Film adhesion and bioactivity in SBF | [166] |
| HA/45S5 | 248 nm, 20 ns, 5 Hz | 5 J/cm2, 200 and 600 °C, 3 × 10−5 Pa, 1 h | Ti–6Al–4V | Bioactivity in SBF and in vivo osteointegration | [153] |
| 248 nm, 20 ns, 5 Hz | 5 J/cm2, 600 °C, 3 × 10−5 Pa, 1 h | Ti–6Al–4V | Film adhesion strength, biocompatibility with L929 mouse fibroblast and in vivo osteointegration | [167] | |
| SiO2–Na2O–K2O–MgO–CaO–P2O5 (BG42) 3 | 193 nm, 10 Hz | 175 mJ, 200 °C | SiC | Bioactivity in SBF | [158] |
| 193 nm, 10 Hz | 0.2–0.6 J/cm2, 25–500 °C, 10−5 mbar, 35 mm | Si | Hardness and elastic modulus | [15] | |
| 193 nm | 4.17 J/cm2, 200 °C | Si, Ti, SiC | Bioactivity in SBF | [159] | |
| 193 nm, 25 ns, 10 Hz | 4.17 J/cm2, 200 °C | SiC | Bioactivity in SBF, biocompatibility with MG-63 osteoblast-like cells | [161] | |
| SiO2–Na2O–K2O–MgO–CaO–P2O5–(B2O3) (BG42, BG50, BG55, BG59) 4 | 193 nm, 10 Hz | 4.2 J/cm2, 200 °C | Ti6Al4V, SiC | Bioactivity in SBF, biocompatibility, cell attachment and proliferation of MG-63 osteoblast-like cells | [160] |
| 193 nm, 10 Hz | 4.2 J/cm2, 200 °C | Ti | Bioactivity in SBF, biocompatibility in muscle tissue by an in vivo test | [149] | |
| SiO2–Na2O–K2O–MgO–CaO–P2O5 (6P57) 5 | 248 nm, 7 ns, 2 Hz | 400–550 °C, 5–15 Pa O2, 4 cm | Ti | Biocompatibility and proliferation of Hek293 cells. | [104] |
| SiO2–Na2O–K2O–MgO–CaO–P2O5 (6P57, 6P61) 6 | 248 nm, 25 ns, | 5.7 J/cm2, 400 °C, 13 Pa O2, 4 cm | Ti | Bioactivi−ty in SBF and cell adhesion of human osteoblasts after SBF soaking | [138] |
| 248 nm, 25 ns, | 5.7 J/cm2, 400 °C, 13 Pa O2, 4 cm | Ti | Biocompatibility and proliferation of osteoblast cells | [127] | |
| 248 nm, ≥ 7 ns, 2 Hz | 2.8 J/cm2, 400 °C, 13 Pa O2 | Etched Ti gr4d | Bioactivity in SBF | [129] | |
| 248 nm, 25 ns, 5 Hz | 6.6 J/cm2, 400 °C, 13 Pa O2, 4 cm | Ti | Bioactivity in SBF and corrosion resistance | [139] | |
| SiO2–Na2O–K2O–CaO–P2O5 7 | 1064 nm, 10 ns, 10 Hz, | 10 J/cm2, RT, 10−6 mbar, 4 cm, | Ti | Bioactivity in SBF | [114] |
| SiO2–Na2O–K2O–CaO–MgO–P2O5–CaF2–La2O3–Ta2O5 (RKKP) 8 | 532 nm, 10 ns, 10 Hz | 12–44 J/cm2, RT-500 °C, 4 × 10−4 Pa, 2 cm, 2 h | Ti | Hardness | [111] |
| 532 nm, 10 ns, 10 Hz | 12 J/cm2, 500 °C, 4 × 10−4 Pa, 2 cm, 2 h | Ti | Adhesion, growth and osteogenic differentiation of CaCo-2 cells | [151] | |
| 532 nm, 10 ns, 10 Hz | 12 J/cm2, 400 °C, 4 × 10−4 Pa, 2 cm, 2 h | Mg–Ca | Corrosion resistance | [164] | |
| 532 nm, 10 ns, 10 Hz | 12 J/cm2, 500 °C, 1.5 × 10−4 Pa, 2 cm, 2 h | Ti | Biocompatibility, proliferation and osteogenic differentiation of hAMSCs grown | [152] | |
| RKKP + C60 (5 wt.%) | 532 nm, 10 ns, 10 Hz | 12 J/cm2, RT, 300 and 500 °C, 4 × 10−4 Pa, 2 cm, 4 h | Ti | Hardness | [148] |
| RKKP-Mn 9 | 532 nm, 10 ns, 10 Hz | 12 J/cm2, RT and 500 °C,4 × 10−4 Pa, 2 cm, 4 h | Ti | Bioactivity in SBF | [146] |
| CaO–MgO–P2O5–SiO2 10 | 1064 nm, 7 ns, 10 Hz | 200 mJ, 200 °C, 10−5 Pa, 40 mm, 60 min | Ti6Al4V | Film adhesion and bioactivity in SBF | [150] |
| 1064 nm, 7 ns, 10 Hz | 200 mJ, 200 °C, 10−5 Pa, 40 mm, 60 min | Ti6Al4V treated with micro-arc oxidation | Film adhesion corrosion restistance and bioactivity in SBF | [157] | |
| SiO2–CaO– P2O5–CaF2–MgO (HASi) 11 | 355 nm, 10 Hz | 1.6 J/cm2, 400 °C, 10−3 mbar O2, 35 mm, 1 h | Ti6Al4V | Film adhesion hardness and bioactivity in SBF | [126] |
| Ca2MgSi2O7 (AKT) | 5 Hz | 180 MJ, RT, 20 MPa O2, 15, 25 and 40 min | PSU and PDLLA films | Hardness and elastic modulus test, bioactivity in SBF, cell attachment and proliferation of MC3T3 cells, osteogenic and angiogenic ability | [133] |
| 5 Hz | 180 MJ, RT, 20 MPa O2, 40 min | PET sheets | Proliferation and osteogenic/angiogenic differ- entiation of the BMSCs, in vivo osseointegration | [120] | |
| 5 Hz | 180 MJ, RT, 20 MPa O2, 30 min | PDLLA/PCL electrospun scaffold | Proliferation of human umbilical vein endothelial cells (HUVECs) and in vivo wound healing | [134] | |
| SiO2–Na2O–K2O–MgO–CaO–P2O5 (BG57) 12 | 248 nm, 25 ns, 10 Hz | 400 °C, 13 Pa O2, 4 cm | stainless steel 316L | Corrosion resistance, bioactivity in SBF, biocompatibility with WJ-MSCs (Wharton’s Jelly-derived Mesenchymal Stromal Cells) | [140] |
| SiO2–Na2O–K2O–MgO–CaO–P2O5 (BG61) 13 | 248 nm, 25 ns, 10 Hz | 3 J/cm2,RT, 1.5 × 10−3 Pa O2 | UHMWPE | Bioactivity in SBF | [137] |
| 248 nm, 25 ns, 10 Hz | 2.8 J/cm2, 400 °C, 13 Pa O2, 4 cm | Ti, stainless steel 316L | Corrosion resistance, bioactivity in SBF, biocompatibility with WJ-MSCs | [141] | |
| SiO2–CaO–P2O5–(CaF2) 14 | 355 nm, 5 ns, 10 Hz | 2.2–3.7 J/cm2, 400 °C, 10 mTorr O2, 4 cm | Ti | Biocompatibility with Human endothelial cells (EAhy926 cell line) | [132] |
| (Cu0.0x)(Ca0.25–0.0x)P0.05 Si0.75 (0Cu-BG, 2Cu-BG, 5Cu–BG) 15 | 5 Hz | 160 MJ, RT, 20 MPa O2, 40 min | Eggshell membrane | Hardness, attachment proliferation, and angiogenic expression of human umbilical vein endothelial cells (HUVECs), antibacterial activity and in vivo wound healing | [122] |
| (Cu0.0x)(Ca0.25–0.0x)P0.05 Si0.75 (0Cu–BG, 5Cu–BG) 15 | 5 Hz | 160 mJ, RT, 20 mPa O2, 40 min | PET sheet | Biocompatibility, adhesion and osteogenic/angiogenic differentiation of the rBMSCs, in vivo osteointegration | [121] |
| Ca0.25 P0.05 Si0.7 O5.2 | 5 Hz | 160 mJ, RT, 20 mPa O2, 40 min | PDLLA/PCL electrospun | Proliferation and attachment, angiogenic expression, in vivo wound healing | [123] |
| Ca2ZnSi2 O7 | 5Hz | 180 MJ, RT, 20 MPa O2 | BioGide® collagen membrane | Osteogenic differentiation of BMSCs, in vitro and in vivo osteoimmunomodulatory properties | [135] |
| P2O5–Nb2O5–CaO-(CaF2) (NbP–BG) | 355 nm, 6 ns, 10 Hz | 50–100 mJ, 3 × 10−6 mbar, 5 min, 30 mm | Etched Ti | Adhesion and proliferation of MC3T3 cells | [108] |
| SiO2–Na2O–K2O–MgO–CaO–P2O5 –CuO (Cu–BG) 16 | 532 nm, 10 ns, 10 Hz | 12 J/cm2, RT, 300 and 500 °C, 4 × 10−4 Pa, 2 cm, 4 h | Ti | Bioactivity in SBF and antibacterial activity | [144] |
| SiO2–CaO–P2O5–ZnO–MgO and SiO2–CaO–P2O5–ZnO–SrO 17 | 532 nm, 10 Hz | 73–74 mJ/pulse, 25–300 °C, 100 mTorr O2, 4 cm | Si | Bioactivity in SBF and cellular adhesion and proliferation of human fetal osteoblast cells | [119] |
| SiO2–Na2O–K2O–MgO–CaO–P2O5–(B2O3) (13–93, 19–93–B3) 18 | 532 nm, 7 ns, 10 Hz | 12 J/cm2, RT, 10−4 Pa, 2 cm, 5 h | Ti | Biocompatibility and osteogenic differentiation of Equine adipose tissue-derived mesenchymal stem cells (ADMSCs) | [155] |
| SiO2–P2O5–CaO–MgO–Na2O–CeO2 19 | 355 nm | 73–74 mJ/pulse, RT-300 °C, 100 mTorr O2, 4 cm | Si | Bioactivity in SBF, biocompatibility with human fibroblast BJ cells and antibacterial activity | [128] |
| SiO2–P2O5–CaO–MgO–Na2O 20 | 355 nm, 5 ns, 10 Hz | 1.5 J/cm2, RT-300 °C, 100 mTorr O2, 4 cm | Si | Bioactivity in SBF and biocompatibility with human fibroblast BJ cells | [130] |
| SiO2–P2O5–CaO–MgO–SrO–Na2O 21 | 355 nm | 73–74 mJ/pulse, RT-300 °C, 100 mTorr O2, 4 cm | - | Bioactivity in SBF and biocompatibility with human fibroblast BJ cells | [136] |
| CaO–SiO2 | - | 84 mJ/pulse, 400 °C, 100 mTorr O2, | Ti | Film adhesion strength, biocompatibility with endothelial cells | [125] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, Y.; Cui, S.; Luo, D.; Liu, Y. Novel inorganic nanomaterial-based therapy for bone tissue regeneration. Nanomaterials 2021, 11, 789. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite based materials for bone tissue engineering: A brief and comprehensive introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Panneerselvam, R.; Anandhan, N.; Gopu, G.; Amali Roselin, A.; Ganesan, K.P.; Marimuthu, T. Impact of different transition metal ions in the structural, mechanical, optical, chemico-physical and biological properties of nanohydroxyapatite. Appl. Surf. Sci. 2020, 506, 144802. [Google Scholar] [CrossRef]
- Sevencan, A.; Doyuk, E.K.; Köse, N. Silver ion doped hydroxyapatite-coated titanium pins prevent bacterial colonization. Jt. Dis. Relat. Surg. 2021, 32, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Kamonwannasit, S.; Futalan, C.M.; Khemthong, P.; Butburee, T.; Karaphun, A.; Phatai, P. Synthesis of copper-silver doped hydroxyapatite via ultrasonic coupled sol-gel techniques: Structural and antibacterial studies. J. Sol. Gel. Sci. Technol. 2020, 96, 452–463. [Google Scholar] [CrossRef]
- Wang, G.; Zreiqat, H. Functional coatings or films for hard-tissue applications. Materials 2010, 3, 3994–4050. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Dai, F.; Yan, T.; Xue, Y.; Zhang, L.; Han, Y. Magnetic Silicium Hydroxyapatite nanorods for enhancing osteoblast response in vitro and biointegration in vivo. ACS Biomater. Sci. Eng. 2019, 5, 2208–2221. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Brauer, D.S. Bioactive Glasses-Structure and Properties. Angew. Chem. Int. Ed. 2015, 54, 4160–4181. [Google Scholar] [CrossRef]
- Shelby, J.E. Introduction to Glass Science and Technology, 2nd ed.; Royal Society of Chemistry: Cambrige, UK, 2005. [Google Scholar] [CrossRef]
- FitzGerald, V.; Pickup, D.M.; Greenspan, D.; Sarkar, G.; Fitzgerald, J.J.; Wetherall, K.M.; Moss, R.M.; Jones, J.R.; Newport, R.J. A neutron and X-ray diffraction study of bioglass® with reverse monte carlo modelling. Adv. Funct. Mater. 2007, 17, 3746–3753. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Du, J. Effect of strontium substitution on the structure of 45S5 bioglasses. Chem. Mater. 2011, 23, 2703–2717. [Google Scholar] [CrossRef]
- Pedone, A.; Malavasi, G.; Menziani, C.M.; Segre, U.; Cormack, A.N. Role of magnesium in soda-lime glasses: Insight into structural, transport, and mechanical properties through computer simulations. J. Phys. Chem. C 2008, 112, 11034–11041. [Google Scholar] [CrossRef]
- Serra, J.; Liste, S.; González, P.; Serra, C.; Borrajo, J.P.; Chiussi, S.; León, B.; Pérez-Amor, M. The role of the temperature and laser fluence on the properties of PLD bioactive glass films. Appl. Phys. A Mater. Sci. Process 2004, 79, 983–986. [Google Scholar] [CrossRef]
- Ravaglioli, A.; Krajewski, A.; Baldi, G.; Tateo, F.; Peruzzo, L.; Piancastelli, A. Glass–ceramic scaffolds for tissue engineering. Adv. Appl. Ceram. 2008, 107, 268–273. [Google Scholar] [CrossRef]
- El-Meliegy, E.; van Noort, R. Glasses an Glass Ceramics for Medical Applications; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S.; Bandyopadhyay, A. Hydroxyapatite coatings for metallic implants. In Hydroxyapatite (HAp) for Biomedical Applications; Mucalo, M., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 7, pp. 143–157. [Google Scholar] [CrossRef]
- Gittens, R.A.; Mclachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [Green Version]
- Im, B.J.; Lee, S.W.; Oh, N.; Lee, M.H.; Kang, J.H.; Leesungbok Ri Lee SCAhn, S.J.; Park, J.S. Texture direction of combined microgrooves and submicroscale topographies of titanium substrata influence adhesion, proliferation, and differentiation in human primary cells. Arch. Oral Biol. 2011, 57, 898–905. [Google Scholar] [CrossRef]
- Cabañas, M.V. Bioceramic Coatings for Medical Implants. In Bio-Ceramics with Clinical Applications, 1st ed.; Vallet-Regí, M., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2014; Volume 9, pp. 249–289. [Google Scholar] [CrossRef]
- Choi, G.; Choi, A.H.; Evans, L.A.; Akyol, S.; Ben-Nissan, B. A review: Recent advances in sol-gel-derived hydroxyapatite nanocoatings for clinical applications. J. Am. Ceram. Soc. 2020, 103, 5442–5453. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-gel derived hydroxyapatite coatings for titanium implants: A review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Pandey, S.K.; Arunan, E.; Srivastava, C. A review on hydroxyapatite coatings for the biomedical applications: Experimental and theoretical perspectives. J. Mater. Chem. B 2021, 9, 228–249. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Hassan, M.A.; Ghani, S.A.C.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol-gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef] [PubMed]
- Ratha, I.; Datta, P.; Balla, V.K.; Nandi, S.K.; Kundu, B. Effect of doping in hydroxyapatite as coating material on biomedical implants by plasma spraying method: A review. Ceram. Int. 2021, 47, 4426–4445. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process—An alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Durham, J.W.; Allen, M.J.; Rabiei, A. Preparation, characterization and in vitro response of bioactive coatings on polyether ether ketone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 560–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, F.Z.; Luo, Z.S. Biomaterials modification by ion-beam processing. Surf. Coatings Technol. 1999, 112, 278–285. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R. Comparative investigation on the adhesion of hydroxyapatite coating on Ti-6Al-4V implant: A review paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
- Safavi, M.S.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. RF-magnetron sputter deposited hydroxyapatite-based composite & multilayer coatings: A systematic review from mechanical, corrosion, and biological points of view. Ceram. Int. 2021, 47, 3031–3053. [Google Scholar] [CrossRef]
- Kozelskaya, A.I.; Kulkova, S.E.; Fedotkin, A.Y.; Bolbasov, E.N.; Zhukov, Y.M.; Stipniece, L.; Bakulin AVUseinov, A.S.; Shesterikov, E.V.; Locs, J.; et al. Radio frequency magnetron sputtering of Sr- and Mg-substituted β-tricalcium phosphate: Analysis of the physicochemical properties and deposition rate of coatings. Appl. Surf. Sci. 2020, 509, 144763. [Google Scholar] [CrossRef]
- Surmenev, R.A. A review of plasma-assisted methods for calcium phosphate-based coatings fabrication. Surf. Coat. Technol. 2012, 206, 2035–2056. [Google Scholar] [CrossRef]
- Nelea, V.; Morosanu, C.; Iliescu, M.; Mihailescu, I.N. Hydroxyapatite thin films grown by pulsed laser deposition and radio-frequency magnetron sputtering: Comparative study. Appl. Surf. Sci. 2004, 228, 346–356. [Google Scholar] [CrossRef]
- Solla, E.L.; González, P.; Serra, J.; Chiussi, S.; León, B.; López, J.G. Pulsed laser deposition of silicon substituted hydroxyapatite coatings from synthetical and biological sources. Appl. Surf. Sci. 2007, 254, 1189–1193. [Google Scholar] [CrossRef]
- Rau, J.V.; Cacciotti, I.; De Bonis, A.; Fosca, M.; Komlev, V.S.; Latini, A.; Santagata, A.; Teghil, R. Fe-doped hydroxyapatite coatings for orthopedic and dental implant applications. Appl. Surf. Sci. 2014, 307, 301–305. [Google Scholar] [CrossRef]
- Rodríguez-Valencia, C.; Lopez-Álvarez, M.; Cochón-Cores, B.; Pereiro, I.; Serra, J.; González, P. Novel selenium-doped hydroxyapatite coatings for biomedical applications. .J Biomed. Mater. Res. Part A 2013, 101A, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Motoc, M.M.; Axente, E.; Popescu, C.; Sima, L.E.; Petrescu, S.M.; Mihailescu, I.N.; Gyorgy, E. Active protein and calcium hydroxyapatite bilayers grown by laser techniques for therapeutic applications. J. Biomed. Mater. Res. Part A 2013, 101A, 2706–2711. [Google Scholar] [CrossRef] [PubMed]
- Popescu-Pelin, G.; Sima, F.; Sima, L.E.; Mihailescu, C.N.; Luculescu, C.; Iordache, I.; Socol, M.; Socol, G.; Mihailescu, I.N. Hydroxyapatite thin films grown by pulsed laser deposition and matrix assisted pulsed laser evaporation: Comparative study. Appl. Surf. Sci. 2017, 418, 580–588. [Google Scholar] [CrossRef]
- Duta, L. In vivo assessment of synthetic and biological-derived calcium phosphate-based coatings fabricated by pulsed laser deposition: A review. Coatings 2021, 11, 99. [Google Scholar] [CrossRef]
- De Bonis, A.; Teghil, R. Ultra-short pulsed laser deposition of oxides, borides and carbides of transition elements. Coatings 2020, 10, 501. [Google Scholar] [CrossRef]
- Schneider, C.W.; Lippert, T. Laser Ablation and Thin Film Deposition. In Laser Processing of Materials: Fundamentals, Applications and Developments; Schaaf, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 5, pp. 89–112. [Google Scholar]
- Fähler, S.; Krebs, H.U. Calculations and experiments of material removal and kinetic energy during pulsed laser ablation of metals. Appl. Surf. Sci. 1996, 96–98, 61–65. [Google Scholar] [CrossRef]
- Kelly, R.; Miotello, A.; Mele, A.; Giardini Guidoni, A. Plume Formation and Characterization in Laser-Surface Interactions. In Experimental Methods in the Physical Sciences; Miller, J.C., Haglund, R.F., Eds.; Academic Press by Elsevier: Cambridge, MA, USA, 1997; Volume 30, pp. 225–289. [Google Scholar] [CrossRef]
- Miotello, A.; Kelly, R. Laser-induced phase explosion: New physical problems when a condensed phase approaches the thermodynamic critical temperature. Appl. Phys. A Mater. Sci. Process. 1999, 69, 67–73. [Google Scholar] [CrossRef]
- Perez, D.; Lewis, L.J.; Lorazo, P.; Meunier, M. Ablation of molecular solids under nanosecond laser pulses: The role of inertial confinement. Appl. Phys. Lett. 2006, 89, 141907. [Google Scholar] [CrossRef]
- Willmott, P.R.; Huber, J.R. Pulsed laser vaporization and deposition. Rev. Mod. Phys. 2000, 2, 315–328. [Google Scholar] [CrossRef]
- Anisimov, S.I.; Luk’yanchuk, B.S.; Luches, A. An analytical model for three-dimensional laser plume expansion into vacuum in hydrodynamic regime. Appl. Surf. Sci. 1996, 96–98, 24–32. [Google Scholar] [CrossRef]
- Lowndes, D.H. Growth and Doping of Compound Semiconductor Films by Pulsed Laser Ablation. In Experimental Methods in the Physical Sciences; Miller, J.C., Haglund, R.F., Eds.; Academic Press by Elsevier: Cambridge, MA, USA, 1997; Volume 30, pp. 475–571. [Google Scholar] [CrossRef]
- Cotell, C.M.; Grabowski, K.S. Novel Materials Applications of Pulsed Laser Deposition. MRS Bull 1992, 17, 44–53. [Google Scholar] [CrossRef]
- Cotell, C.M. Pulsed Laser Deposition and Processing of Biocompatible Hydroxylapatite Thin Films. Appl. Surf. Sci. 1993, 69, 140–148. [Google Scholar] [CrossRef]
- Singh, R.K.; Qian, F.; Nagabushnam, V.; Damodaran, R.; Moudgil, B.M. Excimer laser deposition of hydroxyapatite thin films. Biomaterials 1994, 15, 522–528. [Google Scholar] [CrossRef]
- Arias, J.L.; Garcıá-Sanz, F.J.; Mayor, M.B.; Chiussi, S.; Pou, J.; León, B.; Pérez-Amor, M. Physicochemical properties of calcium phosphate coatings produced by pulsed laser deposition at different water vapour pressures. Biomaterials 1998, 19, 883–888. [Google Scholar] [CrossRef]
- Solla, E.L.; Borrajo, J.P.; González, P.; Serra, J.; Liste, S.; Chiussi, S.; León, B.; Pérez-Amor, M. Plasma assisted pulsed laser deposition of hydroxylapatite thin films. Appl. Surf. Sci. 2005, 248, 360–364. [Google Scholar] [CrossRef]
- Nelea, V.; Ristoscu, C.; Chiritescu, C.; Ghica, C.; Mihailescu, I.N.; Pelletier, H.; Mille, P.; Cornet, A. Pulsed laser deposition of hydroxyapatite thin films on Ti-5Al-2.5Fe substrates with and without buffer layers. Appl. Surf. Sci. 2000, 168, 127–131. [Google Scholar] [CrossRef]
- Arias, J.L.; Mayor, M.B.; Pou, J.; León, B.; Pérez-Amor, M. Stoichiometric transfer in pulsed laser deposition of hydroxylapatite. Appl. Surf. Sci. 2000, 154–155, 434–438. [Google Scholar] [CrossRef]
- Baeri, P.; Torrisi, L.; Marino, N.; Foti, G. Ablation of hydroxyapatite by pulsed laser irradiation. Appl. Surf. Sci. 1992, 54, 210–214. [Google Scholar] [CrossRef]
- Serra, P.; Palau, J.; Varela, A.; Esteve, J.; Morenza, J.L. Characterization of hydroxyapatite laser ablation plumes by fast intensified CCD-imaging. J. Mater. Res. 1995, 10, 473–478. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Lacefield, W.R.; Mirov, S. Structural and morphological study of pulsed laser deposited calcium phosphate bioceramic coatings: Influence of deposition conditions, laser parameters, and target properties. J. Biomed. Mater. Res. 2000, 50, 248–258. [Google Scholar] [CrossRef]
- Serra, P.; Clèries, L.; Morenza, J.L. Analysis of the expansion of hydroxyapatite laser ablation plumes. Appl. Surf. Sci. 1996, 96–98, 216–221. [Google Scholar] [CrossRef]
- Serra, P.; Fernandez-Pradas, J.M.; Sardin, G.; Morenzá. JLInteraction Effects of an Excimer Laser Beam with Hydroxyapatite Targets. Appl. Surf. Sci. 1997, 109, 384–388. [Google Scholar] [CrossRef]
- Serra, P.; Morenza, J.L. Fluence dependence of hydroxyapatite laser ablation plumes. Thin Solid Films 1998, 335, 43–48. [Google Scholar] [CrossRef]
- Agop, M.; Cimpoesu, N.; Gurlui, S.; Irimiciuc, S.A. Investigations of transient plasma generated by laser ablation of hydroxyapatite during the pulsed laser deposition process. Symmetry 2020, 12, 132. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, H.; Hasegawa, T.; Miyake, A.; Tashiro, Y.; Hashimoto, Y.; Blank, D.H.A.; Rijnders, G. Relationship between the Ca/P ratio of hydroxyapatite thin films and the spatial energy distribution of the ablation laser in pulsed laser deposition. Mater. Lett. 2016, 165, 95–98. [Google Scholar] [CrossRef]
- Tri, L.Q.; Chua, D.H.C. An investigation into the effects of high laser fluence on hydroxyapatite/calcium phosphate films deposited by pulsed laser deposition. Appl. Surf. Sci. 2009, 256, 76–80. [Google Scholar] [CrossRef]
- Gomes, G.C.; Borghi, F.F.; Ospina, R.O.; López, E.O.; Borges, F.O.; Mello, A. Nd:YAG (532 nm) pulsed laser deposition produces crystalline hydroxyapatite thin coatings at room temperature. Surf. Coat. Technol. 2017, 329, 174–183. [Google Scholar] [CrossRef]
- Checca, N.R.; Borghi, F.F.; Rossi, A.M.; Mello, A.; Rossi, A.L. Nanostructure of calcium phosphate films synthesized by pulsed laser deposition under 1 Torr: Effect of wavelength and laser energy. Appl. Surf. Sci. 2021, 545, 148880. [Google Scholar] [CrossRef]
- Bao, Q.; Chen, C.; Wang, D.; Liu, J. The influences of target properties and deposition times on pulsed laser deposited hydroxyapatite films. Appl. Surf. Sci. 2008, 255, 619–621. [Google Scholar] [CrossRef]
- Guillot-Noël, O.; Gomez-San Roman, R.; Perrière, J.; Hermann, J.; Craciun, V.; Boulmer-Leborgne, C.; Barboux, P. Growth of apatite films by laser ablation: Reduction of the droplet areal density. J. Appl. Phys. 1996, 80, 1803–1808. [Google Scholar] [CrossRef]
- György, E.; Toricelli, P.; Socol, G.; Iliescu, M.; Mayer, I.; Mihailescu, I.N.; Bigi, A.; Werckman, J. Biocompatible Mn2+-doped carbonated hydroxyapatite thin films grown by pulsed laser deposition. J. Biomed. Mater. Res. Part A 2004, 71, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Generosi, A.; Laureti, S.; Komlev Vladimir, S.; Ferro, D.; Cesaro, S.N.; Paci, B.; Rossi Albertini, V.; Agostinelli, E.; Barinov, S.M. Physicochemical investigation of pulsed laser deposited carbonated hydroxyapatite films on titanium. ACS Appl. Mater. Interfaces. 2009, 1, 1813–1820. [Google Scholar] [CrossRef]
- Okazaiki, M. Crystallographic behaviour of fluoridated hydroxyapatites containing Mg2+ and CO32- ions. Biomaterials 1991, 12, 831–835. [Google Scholar] [CrossRef]
- Mróz, W.; Jedyński, M.; Prokopiuk, A.; Ślósarczyk, A.; Paszkiewicz, Z. Characterization of calcium phosphate coatings doped with Mg, deposited by pulsed laser deposition technique using ArF excimer laser. Micron 2009, 40, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Mróz, W.; Bombalska, A.; Burdyńska, S.; Jedyński, M.; Prokopiuk, A.; Budner, B.; Ślósarczyk, A.; Zima, A.; Menaszek, E.; Ścisłowska-Czarnecka, A.; et al. Structural studies of magnesium doped hydroxyapatite coatings after osteoblast culture. J. Mol. Struct. 2010, 977, 145–152. [Google Scholar] [CrossRef]
- Mróz, W.; Budner, B.; Syroka, R.; Niedzielski, K.; Golański, G.; Ślósarczyk, A.; Schwarze, D.; Douglas, T.E.L. In vivo implantation of porous titanium alloy implants coated with magnesium-doped octacalcium phosphate and hydroxyapatite thin films using pulsed laser depostion. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Vandrovcova, M.; Douglas, T.E.L.; Mróz, W.; Musial, O.; Schaubroeck, D.; Budner, B.; Syroka, R.; Dubruel, P.; Bacakova, L. Pulsed laser deposition of magnesium-doped calcium phosphate coatings on porous polycaprolactone scaffolds produced by rapid prototyping. Mater. Lett. 2015, 148, 178–183. [Google Scholar] [CrossRef]
- Graziani, G.; Bianchi, M.; Sassoni, E.; Russo, A.; Marcacci, M. Ion-substituted calcium phosphate coatings deposited by plasma-assisted techniques: A review. Mater. Sci. Eng. C 2017, 74, 219–229. [Google Scholar] [CrossRef]
- Ferro, D.; Barinov, S.M.; Rau, J.V.; Teghil, R.; Latini, A. Calcium phosphate and fluorinated calcium phosphate coatings on titanium deposited by Nd:YAG laser at a high fluence. Biomaterials 2005, 26, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Smirnov, V.V.; Laureti, S.; Generosi, A.; Varvaro, G.; Fosca, M.; Ferro, D.; Cesaro, N.; Rossi Albertini, V.; Barinov, S.M. Properties of pulsed laser deposited fluorinated hydroxyapatite films on titanium. Mater. Res. Bull. 2010, 45, 1304–1310. [Google Scholar] [CrossRef]
- Cao, J.; Lian, R.; Jiang, X.; Liu, X. Formation of Porous Apatite Layer after Immersion in SBF of Fluorine-Hydroxyapatite Coatings by Pulsed Laser Deposition Improved in Vitro Cell Proliferation. ACS Appl. Bio. Mater. 2020, 3, 3698–3706. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Ueda, M.; Kohiga, Y.; Imura, K.; Hontsu, S. Application of fluoridated hydroxyapatite thin film coatings using KrF pulsed laser deposition. Dent. Mater. J. 2018, 37, 408–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Lian, R.; Jiang, X. Magnesium and fluoride doped hydroxyapatite coatings grown by pulsed laser deposition for promoting titanium implant cytocompatibility. Appl. Surf. Sci. 2020, 515, 146069. [Google Scholar] [CrossRef]
- Montesi, M.; Panseri, S.; Dapporto, M.; Tampieri, A.; Sprio, S. Sr-substituted bone cements direct mesenchymal stem cells, osteoblasts and osteoclasts fate. PLoS ONE 2017, 12, e0172100. [Google Scholar] [CrossRef] [Green Version]
- Capuccini, C.; Torricelli, P.; Sima, F.; Boanini, E.; Ristoscu, C.; Bracci, B.; Socol, G.; Fini, M.; Mihailescu, I.N.; Bigi, A. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: In vitro osteoblast and osteoclast response. Acta Biomater. 2008, 4, 1885–1893. [Google Scholar] [CrossRef]
- Pereiro, I.; Rodríguez-Valencia, C.; Serra, C.; Solla, E.L.; Serra, J.; González, P. Pulsed laser deposition of strontium-substituted hydroxyapatite coatings. Appl. Surf. Sci. 2012, 258, 9192–9197. [Google Scholar] [CrossRef]
- De Bonis, A.; Uskoković, V.; Barbaro, K.; Fadeeva, I.; Curcio, M.; Imperatori, L.; Teghil, R.; Rau, J.V. Pulsed laser deposition temperature effects on strontium-substituted hydroxyapatite thin films for biomedical implants. Cell Biol. Toxicol. 2020, 36, 537–551. [Google Scholar] [CrossRef]
- Solla, E.L.; Borrajo, J.P.; González, P.; Serra, J.; Chiussi, S.; León, B.; García López, J. Study of the composition transfer in the pulsed laser deposition of silicon substituted hydroxyapatite thin films. Appl. Surf. Sci. 2007, 253, 8282–8286. [Google Scholar] [CrossRef]
- Solla, E.L.; Borrajo, J.P.; González, P.; Serra, J.; Chiussi, S.; Cerra, S.; Leon, B.; Perez-Amor, M. Pulsed laser deposition of silicon-substituted hydroxyapatite coatings. Vacuum 2008, 82, 1383–1385. [Google Scholar] [CrossRef]
- López-Álvarez, M.; Solla, E.L.; González, P.; Serra, J.; Chiussi, S.; Serra, C.; León, B.; Pérez-Amor, M. Silicon-hydroxyapatite bioactive coatings (Si-HA) from diatomaceous earth and silica. Study of adhesion and proliferation of osteoblast-like cells. J. Mater. Sci. Mater. Med. 2009, 20, 1131–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rau, J.V.; Fosca, M.; Cacciotti, I.; Laureti, S.; Bianco, A.; Teghil, R. Nanostructured Si-substituted hydroxyapatite coatings for biomedical applications. Thin Solid Films 2013, 543, 167–170. [Google Scholar] [CrossRef]
- Rau, J.V.; Cacciotti, I.; Laureti, S.; Fosca, M.; Varvaro, G.; Latini, A. Bioactive, nanostructured Si-substituted hydroxyapatite coatings on titanium prepared by pulsed laser deposition. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Valencia, C.; Pereiro, I.; Pirraco, R.P.; López-Álvarez, M.; Serra, J.; González PMarques, A.P.; Reis, R.L. Human mesenchymal stem cells response to multi-doped silicon-strontium calcium phosphate coatings. J. Biomater. Appl. 2014, 28, 1397–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, P.; Fang, J.; Fang, L.; Wang, K.; Lu, X.; Ren, F. Novel niobium and silver toughened hydroxyapatite nanocomposites with enhanced mechanical and biological properties for load-bearing bone implants. Appl. Mater. Today 2019, 15, 531–542. [Google Scholar] [CrossRef]
- Jelínek, M.; Kocourek, T.; Jurek, K.; Remsa, J.; Mikšovský, J.; Weiserová, M.; Strnad, J.; Luxbacher, T. Antibacterial properties of Ag-doped hydroxyapatite layers prepared by PLD method. Appl. Phys. A Mater. Sci. Process. 2010, 101, 615–620. [Google Scholar] [CrossRef]
- Sygnatowicz, M.; Keyshar, K.; Tiwari, A. Antimicrobial properties of silver-doped hydroxyapatite nano-powders and tin films. Biol. Biomed. Mater. 2010, 62, 65–70. [Google Scholar]
- Kotoka, R.; Yamoah, N.K.; Mensah-Darkwa, K.; Moses, T.; Kumar, D. Electrochemical corrosion behavior of silver doped tricalcium phosphate coatings on magnesium for biomedical application. Surf. Coat. Technol. 2016, 292, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Mihailescu, I.N.; Torricelli, P.; Bigi, A.; Mayer, I.; Iliescu, M.; Wreckmann, J.; Socol, G.; Miroiu, F.; Cuisinier, F.; Elkaim, R.; et al. Calcium phosphate thin films synthesized by pulsed laser deposition: Physico-chemical characterization and in vitro cell response. In Applied Surface Science; Elsevier: Amsterdam, The Netherlands, 2005; Volume 248, pp. 344–348. [Google Scholar] [CrossRef]
- Bigi, A.; Bracci, B.; Cuisinier, F.; Elkaim, R.; Fini, M.; Mayer, I.; Mihailescu, I.N.; Socol, G.; Sturba, L.; Torricelli, P. Human osteoblast response to pulsed laser deposited calcium phosphate coatings. Biomaterials 2005, 26, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Rau, J.V.; Santagata, A.; Teghil, R.; Laureti, S.; De Bonis, A. Laser synthesis of iron nanoparticle for Fe doped hydroxyapatite coatings. Mater. Chem. Phys. 2019, 225, 365–370. [Google Scholar] [CrossRef]
- Antoniac, I.V.; Filipescu, M.; Barbaro, K.; Bonciu, A.; Birjega, R.; Cotrut, C.M.; Galvano, E.; Fosca, M.; Fadeeva, I.V.; Vadalà, G.; et al. Iron ion-doped tricalcium phosphate coatings improve the properties of biodegradable magnesium alloys for biomedical implant application. Adv. Mater. Interfaces 2020, 7, 2000531. [Google Scholar] [CrossRef]
- Hidalgo-Robatto, B.M.; López-Álvarez, M.; Azevedo, A.S.; Dorado, J.; Serra, J.; Azevedo, N.F.; González, P. Pulsed laser deposition of copper and zinc doped hydroxyapatite coatings for biomedical applications. Surf. Coat. Technol. 2018, 333, 168–177. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Ramadan, R.; Afifi, M.; Menazea, A.A. Au-doped carbonated hydroxyapatite sputtered on alumina scaffolds via pulsed laser deposition for biomedical applications. J. Mater. Res. Technol. 2020, 9, 8854–8866. [Google Scholar] [CrossRef]
- Gyorgy, E.; Grigorescu, S.; Socol, G.; Mihailescu, I.N.; Janackovic, D.; Dindune, A.; Kanepe, Z.; Palcevskis, E.; Zdrentu, E.L.; Petrescu, S.M. Bioactive glass and hydroxyapatite thin films obtained by pulsed laser deposition. Appl. Surf. Sci. 2007, 253, 7981–7986. [Google Scholar] [CrossRef]
- Eraković, S.; Janković, A.; Ristoscu, C.; Duta, L.; Serban, N.; Visan, A.; Mihailescu, I.N.; Stan, G.E.; Socol, M.; Iordache, O.; et al. Antifungal activity of Ag:hydroxyapatite thin films synthesized by pulsed laser deposition on Ti and Ti modified by TiO2 nanotubes substrates. Appl. Surf. Sci. 2014, 293, 37–45. [Google Scholar] [CrossRef]
- D’Alessio, L.; Teghil, R.; Zaccagnino, M.; Zaccardo, I.; Ferro, D.; Marotta, V. Pulsed laser ablation and deposition of bioactive glass as coating material for biomedical applications. Appl. Surf. Sci. 1999, 138–139, 527–532. [Google Scholar] [CrossRef]
- D’Alessio, L.; Ferro, D.; Marotta, V.; Santagata, A.; Teghil, R.; Zaccagnino, M. Laser ablation and deposition of Bioglass® 45S5 thin films. Appl. Surf. Sci. 2001, 183, 10–17. [Google Scholar] [CrossRef]
- Sanz, C.K.; dos Santos, A.R.; da Silva, M.H.P.; Marçal, R.; Tute, E.M.; Meza, E.L.; Mello, A.; Borghi, F.F.; de Souza Camargo, S.A. Niobo-phosphate bioactive glass films produced by pulsed laser deposition on titanium surfaces for improved cell adhesion. Ceram. Int. 2019, 45, 18052–18058. [Google Scholar] [CrossRef]
- Floroian, L.; Savu, B.; Sima, F.; Mihailescu, I.N.; Tanaskovic, D.; Janackovic, D. Synthesis and characterization of bioglass thin films. Dig. J. Nanomater. Biostructures 2007, 2, 285–291. [Google Scholar]
- Liste, S.; González, P.; Serra, J.; Borrajo, J.P.; Chiussi, S.; Leon, B.; Perez-Amor, M.; Garcia-Lopez, J.; Ferrer, F.J.; Morilla, Y.; et al. Study of the stoichiometry transfer in pulsed laser deposition of bioactive silica-based glasses. Thin Solid Films 2004, 453–454, 219–223. [Google Scholar] [CrossRef]
- Rau, J.V.; Teghil, R.; Fosca, M.; De Bonis, A.; Cacciotti, I.; Bianco, A.; Rossi Albertini, V.; Caminiti, R.; Ravaglioli, A. Bioactive glass-ceramic coatings prepared by pulsed laser deposition from RKKP targets (sol-gel vs melt-processing route). Mater. Res. Bull. 2012, 47, 1130–1137. [Google Scholar] [CrossRef]
- Teghil, R.; Ferro, D.; Barinov, S.M. Hardness of bioactive glass film deposited on titanium alloy by pulsed laser ablation Certain glasses which contain specific amounts of SiO. J. Mater. Sci. Lett. 2002, 21, 379–382. [Google Scholar] [CrossRef]
- Borrajo, J.P.; González, P.; Liste, S.; Serra, J.; Chiussi, S.; León, B.; Pérez-Amor, M. Pulsed laser deposition of bioactive glass films in ammonia and disilane atmospheres. Appl. Surf. Sci 2005, 248, 369–375. [Google Scholar] [CrossRef]
- Kwiatkowska, J.; Suchanek, K.; Rajchel, B. Bioactive glass coatings synthesized by pulsed laserdeposition. Tech. Acta Phys. Pol. A 2012, 121, 502–505. [Google Scholar] [CrossRef]
- González, P.; Serra, J.; Liste, S.; Chiussi, S.; León, B.; Pérez-Amor, M. Ageing of pulsed-laser-deposited bioactive glass films. Vacuum 2002, 67, 647–651. [Google Scholar] [CrossRef]
- Liste, S.; Serra, J.; González, P.; Borrajo, J.P.; Chiussi, S.; León BPérez-Amor, M. The role of the reactive atmosphere in pulsed laser deposition of bioactive glass films. Thin Solid Films 2004, 453–454, 224–228. [Google Scholar] [CrossRef]
- Solla, E.L.; Borrajo, J.P.; González, P.; Serra, J.; Liste, S.; Chiussi, S.; León, B.; Pérez-Amor, M. Nuevas tecnologías en el procesamiento de recubrimientos de cerámicas bioactivas. Bol. Soc. Esp. Ceram. V 2006, 45, 65–69. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, M.; Chen, C.; Liu, J. The role of the pressure in pulsed laser deposition of bioactive glass films. J. Non Cryst. Solids 2008, 354, 4000–4004. [Google Scholar] [CrossRef]
- Jinga, S.I.; Toma, V.P.; Constantinoiu, I.; Banciu, A.; Banciu, D.D.; Busuioc, C. Development of new Mg-or Sr-containing bioactive interfaces to stimulate osseointegration of metallic implants. Appl. Sci. 2020, 10, 6647. [Google Scholar] [CrossRef]
- Li, H.; Wu, C.; Chang, J.; Ge, Y.; Chen, S. Functional Polyethylene Terephthalate with Nanometer-Sized Bioactive Glass Coatings Stimulating in Vitro and in Vivo Osseointegration for Anterior Cruciate Ligament Reconstruction. Adv. Mater. Interfaces 2014, 1, 1400027. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Jiang, J.; Lv, F.; Chang, J.; Chen, S.; Wu, C. An osteogenesis/angiogenesis-stimulation artificial ligament for anterior cruciate ligament reconstruction. Acta Biomater. 2017, 54, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhai, D.; Lv, F.; Yu, Q.; Ma, H.; Yin, J.; Yi, Z.; Liu, M.; Chang, J.; Wu, C. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomater. 2016, 36, 254–266. [Google Scholar] [CrossRef]
- Li, J.; Lv, F.; Xu, H.; Zhang, Y.; Wang, J.; Yi, Z.; Yin, J.; Chang, J.; Wu, C. A patterned nanocomposite membrane for high-efficiency healing of diabetic wound. J. Mater. Chem. B 2017, 5, 1926–1934. [Google Scholar] [CrossRef]
- Mihailescu, N.; Stan, G.E.; Ristoscu, C.; Sopronyi, M.; Mihailescu, I.N. Bioactive glass thin films synthesized by advanced pulsed laser techniques. J. Phys. Conf. Ser. 2016, 764, 012020. [Google Scholar] [CrossRef]
- Miu, D.M.; Jinga, S.I.; Voicu, G.; Iordache, F. Characteristics of wollastonite ceramic coatings obtained by pulsed laser deposition. J. Inorg. Organomet. Polym. Mater. 2020, 4, 1601–1607. [Google Scholar] [CrossRef]
- Palangadan, R.; Sukumaran, A.; Fernandez, F.B.; John, A.; Varma, H. Pulsed laser deposition and in vitro characteristics of triphasic—HASi composition on titanium. J. Biomater. Appl. 2014, 28, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Popescu, A.C.; Sima, F.; Duta, L.; Popescu, C.; Mihailescu, I.N.; Capitanu, D.; Mustata, R.; Sima, L.E.; Petrescu, S.M.; Janackovic, D. Biocompatible and bioactive nanostructured glass coatings synthesized by pulsed laser deposition: In vitro biological tests. Appl. Surf. Sci. 2009, 255, 5486–5490. [Google Scholar] [CrossRef]
- Prefac, G.A.; Milea, M.L.; Vadureanu, A.M.; Muraru, S.; Dobrin, D.I.; Isopencu, G.O.; Jinga, S.I.; Raileanu, M.; Bacalum, M.; Busuioc, C. CeO2 containing thin films as bioactive coatings for orthopaedic implants. Coatings 2020, 10, 642. [Google Scholar] [CrossRef]
- Berbecaru, C.; Alexandru, H.V.; Ianculescu, A.; Popescu, A.; Socol, G.; Sima, F. Mihailescu, Ion Bioglass thin films for biomimetic implants. Appl. Surf. Sci. 2009, 255, 5476–5479. [Google Scholar] [CrossRef]
- Schitea, R.I.; Nitu, A.; Ciobota, A.A.; Munteanu, A.L.; David, I.M.; Miu, D.; Raileanu, M.; Bacalum, M.; Busuioc, C. Pulsed laser deposition derived bioactive glass-ceramic coatings for enhancing the biocompatibility of scaffolding materials. Materials 2020, 13, 2615. [Google Scholar] [CrossRef]
- Tanaskovic, D.; Jokic, B.; Socol, G.; Popescu, A.; Mihailescu, I.N.; Petrovic, R.; Janackovic, D. Synthesis of functionally graded bioactive glass-apatite multistructures on Ti substrates by pulsed laser deposition. Appl. Surf. Sci. 2007, 254, 1279–1282. [Google Scholar] [CrossRef]
- Voicu, G.; Miu, D.; Dogaru, I.; Jinga, S.I.; Busuioc, C. Vitroceramic interface deposited on titanium substrate by pulsed laser deposition method. Int. J. Pharm 2016, 510, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhai, D.; Ma, H.; Li, X.; Zhang, Y.; Zhou, Y.; Luo, Y.; Wang, Y.; Xiao, Y.; Chang, J. Stimulation of osteogenic and angiogenic ability of cells on polymers by pulsed laser deposition of uniform akermanite-glass nanolayer. Acta Biomater. 2014, 10, 3295–3306. [Google Scholar] [CrossRef]
- Xu, H.; Lv, F.; Zhang, Y.; Yi, Z.; Ke, Q.; Wu, C.; Liu, M.; Chang, J. Hierarchically micro-patterned nanofibrous scaffolds with a nanosized bio-glass surface for accelerating wound healing. Nanoscale 2015, 7, 18446–18452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Chen, L.; Liu, R.; Lin, Y.; Chen, S.; Lu, S.; Lin, Z.; Chen, Z.; Wu, C.; Xiao, Y. The osteoimmunomodulatory property of a barrier collagen membrane and its manipulation: Via coating nanometer-sized bioactive glass to improve guided bone regeneration. Biomater. Sci. 2018, 6, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Draghici, D.A.; Mihai, A.A.; Aioanei, M.O.; Negru, N.E.; Nicoara, A.I.; Jinga, S.I.; Miu, D.; Bacalum, M.; Busuioc, C. Strontium-Substituted Bioactive Glass-Ceramic Films for Tissue Engineering. Bol. Soc. Esp. Ceram. V 2020, in press. [Google Scholar] [CrossRef]
- Duta, L.; Popa, A.C.; Miculescu, F.; Mihailescu, I.N. Ultra high molecular weight polyethylene acetabular cups functionalized with bioactive glass coatings synthesized by pulsed laser deposition. Rom. Reports Phys. 2014, 66, 788–800. [Google Scholar]
- Floroian, L.; Savu, B.; Stanciu, G.; Popescu, A.C.; Sima, F.; Mihailescu, I.N.; Mustata, R.; Sima, L.E.; Petrescu, S.M.; Tanaskovic, D.; et al. Nanostructured bioglass thin films synthesized by pulsed laser deposition: CSLM, FTIR investigations and in vitro biotests. Appl. Surf. Sci. 2008, 255, 3056–3062. [Google Scholar] [CrossRef]
- Floroian, L.; Florescu, M.; Sima, F.; Popescu-Pelin, G.; Ristoscu, C.; Mihailescu, I.N. Synthesis of biomaterial thin films by pulsed laser technologies: Electrochemical evaluation of bioactive glass-based nanocomposite coatings for biomedical applications. Mater. Sci. Eng. C 2012, 32, 1152–1157. [Google Scholar] [CrossRef]
- Floroian, L.; Florescu, M.; Munteanu, D.; Badea, M.; Popescu-Pelin, G.; Ristoscu, C.; Sima, F.; Chifiriuc, M.C.; Mihailescu, I.N. A new concept of stainless steel medical implant based upon composite nanostructures coating. Dig. J. Nanomater. Biostructures 2014, 9, 1555–1568. [Google Scholar]
- Floroian, L.; Samoila, C.; Badea, M.; Munteanu, D.; Ristoscu, C.; Sima, F.; Negut, I.; Chifiriuc, M.C.; Mihailescu, I.N. Stainless steel surface biofunctionalization with PMMA-bioglass coatings: Compositional, electrochemical corrosion studies and microbiological assay. J. Mater. Sci Mater. Med. 2015, 26, 195. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, M.; Chen, C.; Liu, J. Effects of the substrate temperature on the bioglass films deposited by pulsed laser. Appl. Surf. Sci. 2008, 254, 6897–6901. [Google Scholar] [CrossRef]
- De Bonis, A.; Curcio, M.; Fosca, M.; Cacciotti, I.; Santagata, A.; Teghil, R.; Rau, J.V. RBP1 bioactive glass-ceramic films obtained by Pulsed Laser Deposition. Mater. Lett. 2016, 175, 195–198. [Google Scholar] [CrossRef]
- Rau, J.V.; Curcio, M.; Raucci, M.G.; Barbaro, K.; Fasolino, I.; Teghil, R.; Ambrosio, L.; De Bonis, A.; Boccaccini, A.R. Cu-Releasing Bioactive Glass Coatings and Their in Vitro Properties. ACS Appl. Mater. Interfaces 2019, 11, 5812–5820. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Miguez-Pacheco, V.; Boccaccini, A.R.; Vitale-Brovarone, C. Bioactive glasses: Special applications outside the skeletal system. J. Non Cryst. Solids 2016, 432, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Curcio, M.; De Stefanis, A.; De Bonis, A.; Teghil, R.; Rau, J.V. Pulsed laser deposited bioactive RKKP-Mn glass-ceramic coatings on titanium. Surf. Coatings Technol. 2019, 357, 122–128. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Curcio, M.; De Bonis, A.; Fosca, M.; Santagata, A.; Teghil, R.; Rau, J.V. Pulsed laser-deposited composite carbon–glass–ceramic films with improved hardness. J. Mater. Sci. 2017, 52, 9140–9150. [Google Scholar] [CrossRef]
- Borrajo, J.P.; Serra, J.; González, P.; León, B.; Muñoz, F.M.; López, M. In vivo evaluation of titanium implants coated with bioactive glass by pulsed laser deposition. J. Mater. Sci. Mater. Med. 2007, 18, 2371–2376. [Google Scholar] [CrossRef]
- Ma, J.; Chen, C.Z.; Wang, D.G.; Wang, C.Z.; Zhang, H.M. Preparation and apatite formation on CaO-MgO-P2O5-SiO2 glass film grown by pulsed laser deposition. J. Non Cryst. Solids 2013, 382, 5–10. [Google Scholar] [CrossRef]
- Ledda, M.; De Bonis, A.; Bertani, F.R.; Cacciotti, I.; Teghil, R.; Lolli, M.G.; Ravaglioli, A.; Lisi, A.; Rau, J.V. Interdisciplinary approach to cell-biomaterial interactions: Biocompatibility and cell friendly characteristics of RKKP glass-ceramic coatings on titanium. Biomed. Mater. 2015, 10, 035005. [Google Scholar] [CrossRef]
- Ledda, M.; Fosca, M.; De Bonis, A.; Curcio, M.; Teghil, R.; Lolli, M.G.; De Stefanis, A.; Marchese, R.; Rau, J.V.; Lisi, A. Placenta Derived Mesenchymal Stem Cells Hosted on RKKP Glass-Ceramic: A Tissue Engineering Strategy for Bone Regenerative Medicine Applications. Biomed. Res. Int. 2016, 2016, 3657906. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.G.; Chen, C.Z.; Ma, Q.S.; Jin, Q.P.; Li, H.C. A study on in vitro and in vivo bioactivity of HA/45S5 composite films by pulsed laser deposition. Appl. Surf. Sci. 2013, 270, 667–674. [Google Scholar] [CrossRef]
- Shaikh, S.; Kedia, S.; Majumdar, A.G.; Subramanian, M.; Sinha, S. 45S5 bioactive glass coating on Ti6Al4V alloy using pulsed laser deposition technique. Mater. Res. Express 2019, 6, 125428. [Google Scholar] [CrossRef]
- Rau, J.V.; De Bonis, A.; Curcio, M.; Schuhladen, K.; Barbaro, K.; De Bellis, G.; Teghil, R.; Boccaccini, A.R. Borate and silicate bioactive glass coatings prepared by nanosecond pulsed laser deposition. Coatings 2020, 10, 1105. [Google Scholar] [CrossRef]
- Nádai, L.; Katona, B.; Terdik, A.; Bognár, E. Chemical etching of titanium samples. Period. Polytech Mech Eng. 2013, 57, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Wang, C.Z.; Ban, C.L.; Chen, C.Z.; Zhang, H.M. Pulsed laser deposition of magnesium-containing bioactive glass film on porous Ti-6Al-4V substrate pretreated by micro-arc oxidation. Vacuum 2016, 125, 48–55. [Google Scholar] [CrossRef]
- González, P.; Serra, J.; Liste, S.; Chiussi, S.; León, B.; Pérez-Amor, M.; Martínez-Fernández, J.; De Arellano-López, A.R.; Varela-Feria, F.M. New biomorphic SiC ceramics coated with bioactive glass for biomedical applications. Biomaterials 2003, 24, 4827–4832. [Google Scholar] [CrossRef]
- Borrajo, J.P.; González, P.; Liste, S.; Serra, J.; Chiussi, S.; León, B.; Pérez-Amor, M. The role of the thickness and the substrate on the in vitro bioactivity of silica-based glass coatings. Mater. Sci. Eng. C 2005, 25, 187–193. [Google Scholar] [CrossRef]
- De Carlos, A.; Borrajo, J.P.; Serra, J.; González, P.; León, B. Behaviour of MG-63 osteoblast-like cells on wood-based biomorphic SiC ceramics coated with bioactive glass. J. Mater. Sci Mater. Med. 2006, 17, 523–529. [Google Scholar] [CrossRef]
- Borrajo, J.P.; González, P.; Serra, J.; Liste, S.; Chiussi, S.; León, B.; Carlos Villamarin, A.; Varela Feria, F.M.; Martinez Fernandez, J.; Ramirez de Arellano Lopez, A. Estudio de la citotoxicidad de cerámicas biomórficas de sic recubiertas con vidrio bioactivo. Bol. Soc. Esp. Ceram. V 2006, 45, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.N.; Li, S.S.; Li, X.M.; Fan, Y.B. Magnesium based degradable biomaterials: A review. Front. Mater. Sci 2014, 8, 200–218. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate coatings on magnesium and its biodegradable alloys. Acta Biomater. 2014, 10, 2919–2934. [Google Scholar] [CrossRef]
- Rau, J.V.; Antoniac, I.; Fosca, M.; De Bonis, A.; Blajan, A.I.; Cotrut, C.; Graziani, V.; Curcio, M.; Cricenti, A.; Niculescu, M.; et al. Glass-ceramic coated Mg-Ca alloys for biomedical implant applications. Mater. Sci. Eng. C 2016, 64, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Maçon, A.L.B.; Kim, T.B.; Valliant, E.M.; Goetschius, K.; Brow, R.K.; Day, D.E.; Hoppe, A.; Boccaccini, A.R.; Kim, I.Y.; Ohtsuki, C.; et al. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015, 26, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Song, M.; Liu, J. Characteristics of bioactive glass coatings obtained by pulsed laser deposition. Surf. Interface Anal. 2008, 40, 1463–1468. [Google Scholar] [CrossRef]
- Wang, D.G.; Chen, C.Z.; Yang, X.X.; Ming, X.C.; Zhang, W.L. Effect of bioglass addition on the properties of HA/BG composite films fabricated by pulsed laser deposition. Ceram. Int. 2018, 44, 14528–14533. [Google Scholar] [CrossRef]

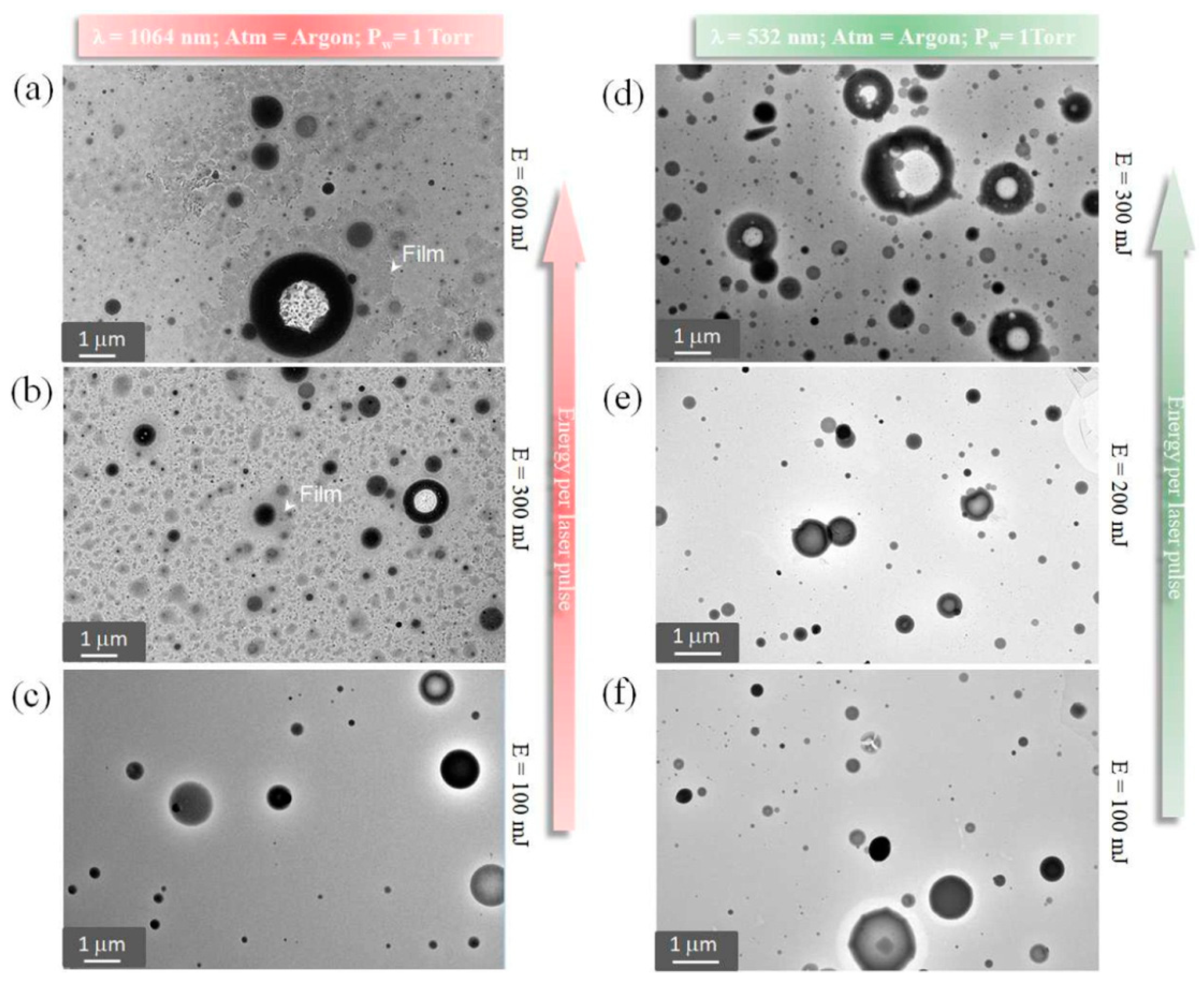
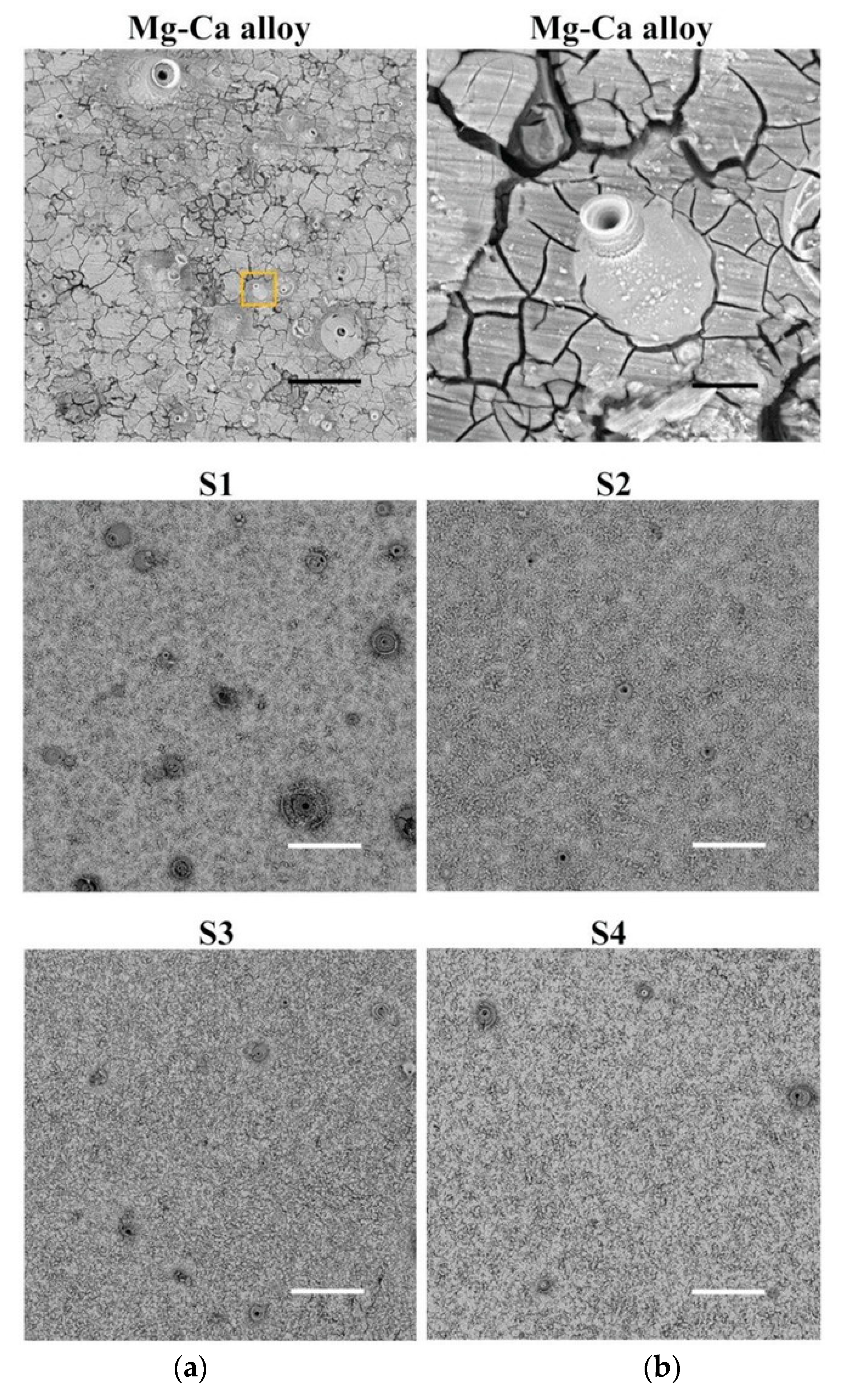
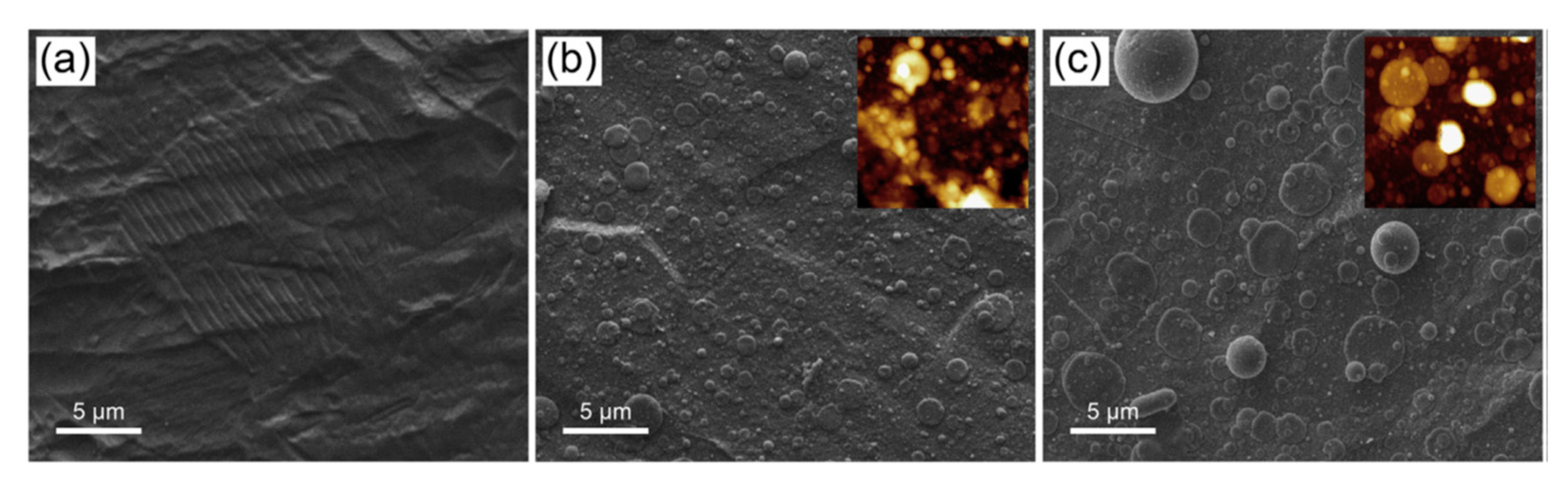
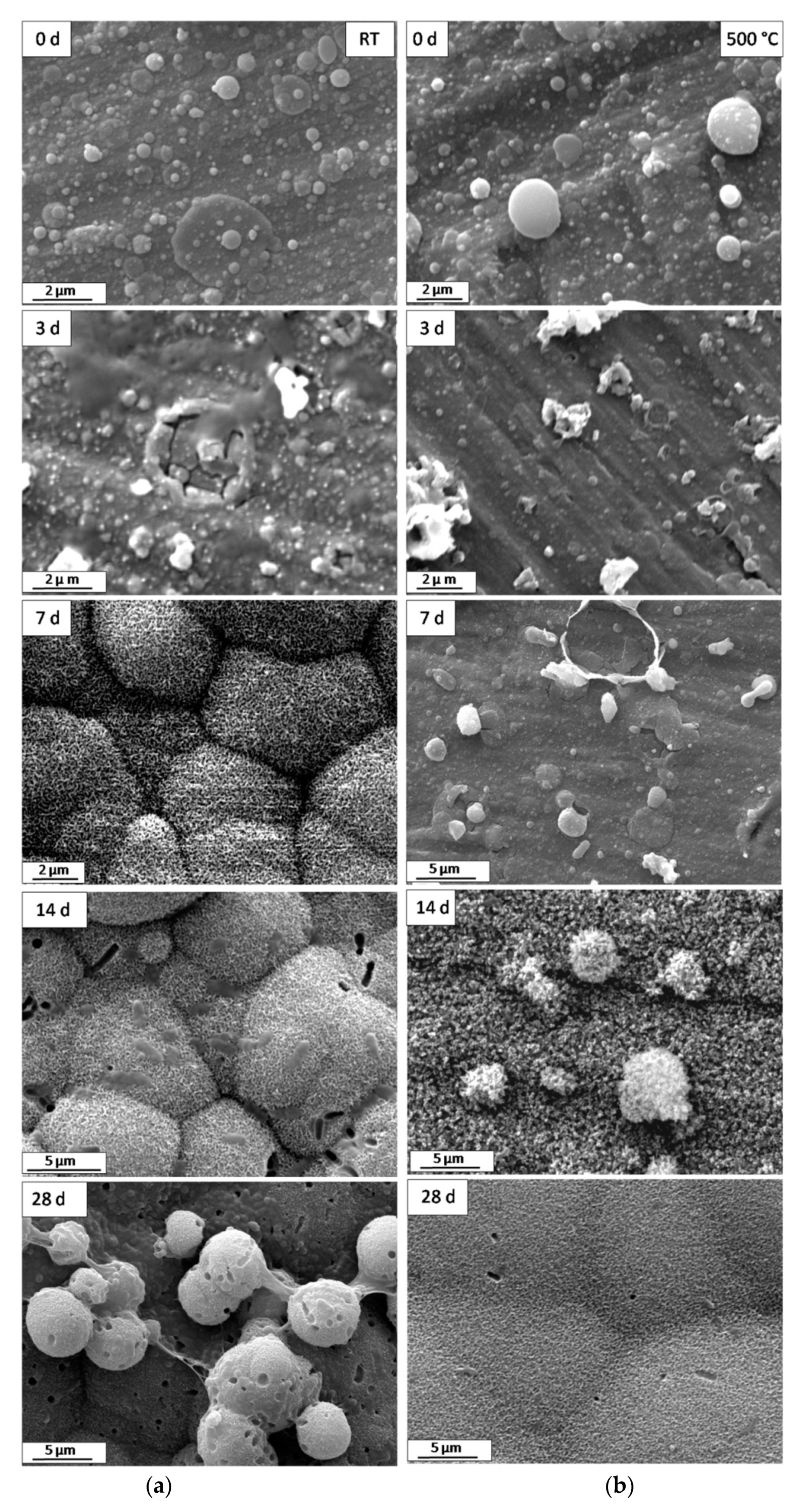

| Deposition Technique | Advantages | Drawbacks | Ref. |
|---|---|---|---|
| PS | Low cost, high deposition rate | Poor adhesion, tendency to form cracks and debris, hard control of phase composition and surface morphology, delamination during implantation | [3,26,27,28,34] |
| RF-MS | Uniform thickness, controllable surface roughness | Low deposition rate, expensive | [3,28,32,33,34] |
| IBAD | High adhesion, homogeneus coatings | Cracks, expensive | [3,28,29,30,31,34] |
| MAPLE | Deposition of hybrid and thermal sensitive materials, accurate control of thickness, uniform coatings | Line of sight technique, low deposition rate | [39,40] |
| PLD | Films with complex stoichiometry, good adhesion and mechanical properties, controllable crystallinity, surface roughness and thickness | Low deposition rate, possibility of splashing or particles deposition | [3,28,34,35,36,37,38] |
| Doping Element (s) | Laser Source | Deposition Conditions | Substrate | Properties | Ref. |
|---|---|---|---|---|---|
| Mg | 193 nm, 20 ns, 50 Hz | 7 J/cm2, 600 °C, 35 Pa air pressure | Ti6Al4V | Improved crystallinity | [74] |
| 193 nm, 20 ns | 7 J/cm2, 450 °C, 30 Pa water pressure | Ti6Al4V | Osteogenic differentiation | [75] | |
| 193 nm, 20 ns, 50 Hz | 2 J/cm2, RT, 3.2 × 10−2 mbar water pressure | PLC scaffold | Osteogenic differentiaition | [77] | |
| F | 532 nm, 10 ns, 10 Hz | 12 J/cm2, RT, 1 × 10−4 Pa | Ti | Hardness | [79] |
| 284 nm, 17 ns, 5 Hz | 2–7 J/cm2, 400–600 °C, 5 × 10−4 mbar N2 | Ti | Polycrystalline films with improved mechanical properties | [80] | |
| 248 nm, 20 ns, 10 Hz | 1 J/cm2, RT, 1 Pa water pressure, post annealing | Ti | Dissolution resistance, HMS cells adhesion | [82] | |
| 1024 nm, 18 ns | Post annealing | Ti | In vitrobioactivity, BMS cells adhesion | [81] | |
| Sr | 248 nm, 7 ns | 2 J/cm2, 400 °C, 50 Pa water pressure | Ti | Osteoblast cells adhesion and activity | [85] |
| 193 nm, 10 Hz | 3.2 J/cm2, 460 °C, 45 Pa water pressure | Ti, Si | Incorporation of Sr in the HA lattice | [86] | |
| 532 nm, 7 ns, 10 Hz | 12 J/cm2, Rt-500 °C, 1 × 10−4 Pa | Ti | DPS cells adhesion | [87] | |
| Si | 193 nm, 10 Hz | 460 °C, 0.45 mbar water pressure | Ti, Si | Diminution of films crystallinity with increasing of Si content | [36,88] |
| 193 nm, 10 Hz | 460 °C, 0.45 mbar water pressure | Ti | Adhesion and proliferation of osteoblast cells | [90] | |
| 248 nm, 17 ns, 5 Hz | 2 J/cm2, 600 °C, 5 × 10−2 mbar N2 | Ti | In vitrobioactivity | [91,92] | |
| Ag | 248 nm, 20 ns, 10 Hz | 2 J/cm2, RT-600 °C, 40 Pa water pressure | Ti, fused silica, Si | Antibacterial activity | [95] |
| 248 nm, 25 ns, 10 Hz | 2–3 J/cm2, 300 °C, 10−6 mbar, post annealing | Al | Amorphous and crystalline films with antibacterial activity | [96] | |
| 248 nm, 25 ns, 10 Hz | 4.5 J/cm2, 500 °C, 50 Pa water pressure | Ti, TiO2 nanotubes | Antifungal activity | [105] | |
| 248 nm, 20 ns, 10 Hz | 2 J/cm2, RT, 5 × 10−6 Torr | Mg | Corrosion resistance | [97] | |
| Mn | 248 nm, 20 ns, 2 Hz | 2J/cm2, 300–400 °C, 10 Pa O2 | Ti | Osteoblast proliferation | [71] |
| 248 nm, 30 ns, 2 Hz | 400 °C, 10 Pa O2 | Ti | Osteoblast differentiation on crystalline CHA | [99] | |
| Fe | 532 nm, 10 ns, 10 Hz | 90 J/cm2, RT-600 °C, 4 × 10−4 Pa | Ti | Crystalline films with improved hardness | [37] |
| 532 nm, 10 ns, 10 Hz | 37 J/cm2, RT-500 °C, 4 × 10−4 Pa | Ti | Magnetic properties | [100] | |
| 355 nm, 7 ns, 10 Hz | 2J/cm2, RT-300 °C, | Mg-Ca alloy | Corrosion resistance, reduction of degradation rate | [101] | |
| Se | 193 nm, 10 Hz | 3.2 J/cm2, 460 °C, 45 Pa water pressure | Ti, Si | Antibacterial activity | [38] |
| Cu, Zn | 193 nm, 10 Hz | 3.2 J/cm2, 460 °C, 0.45 mbar | Ti6Al4V | Osteoblast cells growth and proliferation, antibacterial activity | [102] |
| Au | 1064 nm, 8 ns, 10 Hz | 4.5 × 10−4 Pa | Alumina scaffold | HFB4 cells adhesion and proliferation | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teghil, R.; Curcio, M.; De Bonis, A. Substituted Hydroxyapatite, Glass, and Glass-Ceramic Thin Films Deposited by Nanosecond Pulsed Laser Deposition (PLD) for Biomedical Applications: A Systematic Review. Coatings 2021, 11, 811. https://doi.org/10.3390/coatings11070811
Teghil R, Curcio M, De Bonis A. Substituted Hydroxyapatite, Glass, and Glass-Ceramic Thin Films Deposited by Nanosecond Pulsed Laser Deposition (PLD) for Biomedical Applications: A Systematic Review. Coatings. 2021; 11(7):811. https://doi.org/10.3390/coatings11070811
Chicago/Turabian StyleTeghil, Roberto, Mariangela Curcio, and Angela De Bonis. 2021. "Substituted Hydroxyapatite, Glass, and Glass-Ceramic Thin Films Deposited by Nanosecond Pulsed Laser Deposition (PLD) for Biomedical Applications: A Systematic Review" Coatings 11, no. 7: 811. https://doi.org/10.3390/coatings11070811
APA StyleTeghil, R., Curcio, M., & De Bonis, A. (2021). Substituted Hydroxyapatite, Glass, and Glass-Ceramic Thin Films Deposited by Nanosecond Pulsed Laser Deposition (PLD) for Biomedical Applications: A Systematic Review. Coatings, 11(7), 811. https://doi.org/10.3390/coatings11070811








