Improving the CO and CH4 Gas Sensor Response at Room Temperature of α-Fe2O3(0001) Epitaxial Thin Films Grown on SrTiO3(111) Incorporating Au(111) Islands
Abstract
:1. Introduction
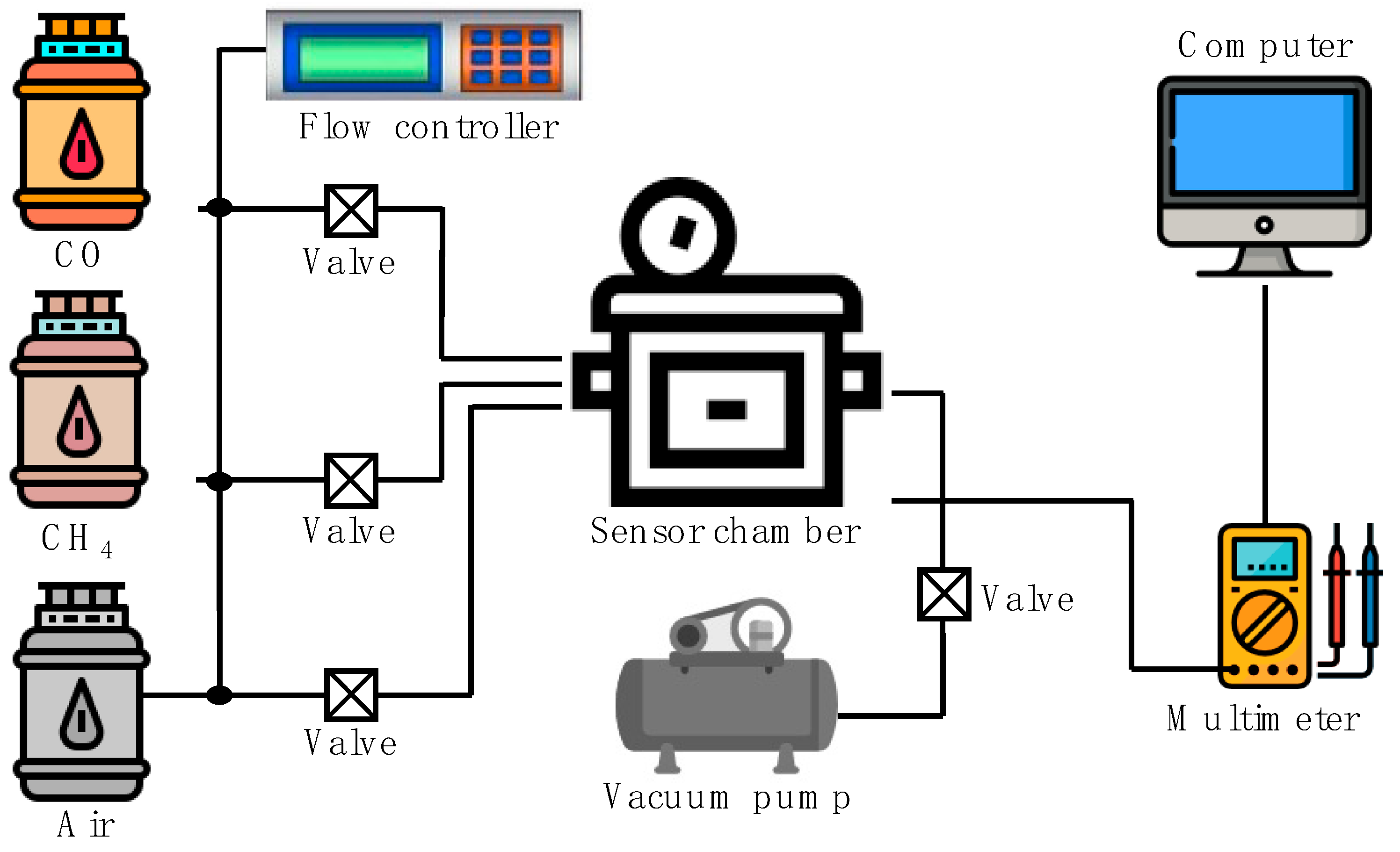
2. Materials and Methods
3. Results
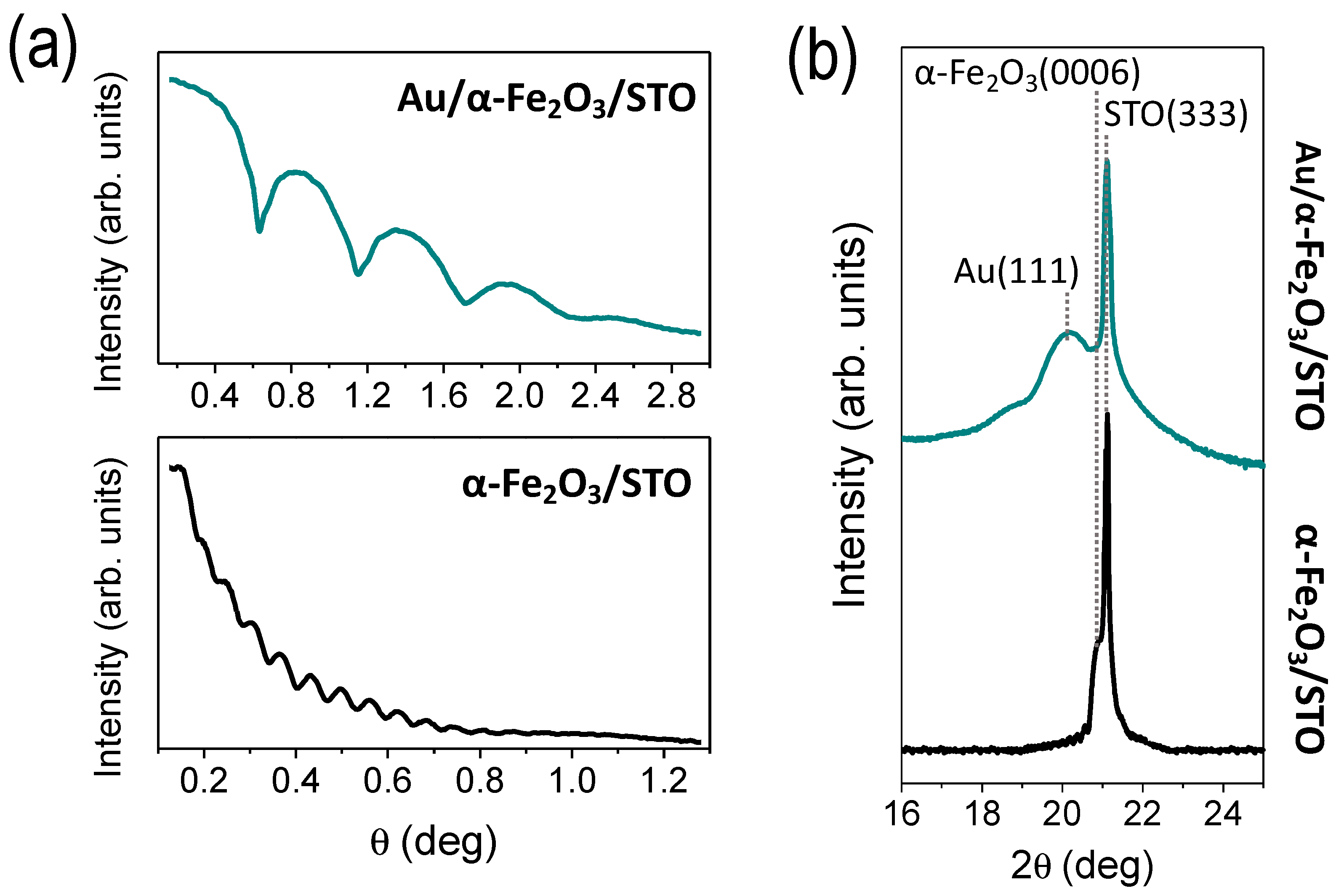
3.1. Morphological and Structural Characterization
3.2. Conductance Response
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Li, H.; Zhang, N.; Zhao, X.; Zhang, Z.; Wang, Y. Self-sacrificial templated formation of ZnO with decoration of catalysts for regulating CO and CH4 sensitive detection. Sens. Actuators B Chem. 2021, 330, 129286. [Google Scholar] [CrossRef]
- Qiu, X.; Wei, Y.; Li, J.; Zhang, E.; Li, N.; Li, C.; Wei, J. Early detection system for coal spontaneous combustion by laser dual-species sensor of CO and CH4. Opt. Laser Technol. 2020, 121, 105832. [Google Scholar] [CrossRef]
- Garcia-Osorio, D.; Hidalgo-Falla, P.; Peres, H.E.M.; Gonçalves, J.M.; Araki, K.; Garcia-Segura, S.; Picasso, G. Silver enhances hematite nanoparticles based ethanol sensor response and selectivity at room temperature. Sensors 2021, 21, 440. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Picasso, G.; Hidalgo, P.; Peres, H.E.M.; Sun Kou, R.; Gonçalves, J.M. Sensors based on Ag-loaded hematite (α-Fe2O3) nanoparticles for methyl mercaptan detection at room temperature. Anal. Chem. Res. 2017, 12, 74–81. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Mirzaei, A.; Hashemi, B.; Janghorban, K. α-Fe2O3 based nanomaterials as gas sensors. J. Mater. Sci. Mater. Electron. 2016, 27, 3109–3144. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [Green Version]
- Khatavkar, S.N.; Sartale, S.D. α-Fe2O3 thin films by liquid phase deposition: Low-cost option for supercapacitor. J. Solid State Electrochem. 2017, 21, 2555–2566. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses, 2nd ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Liang, H.; Jiang, X.; Qi, Z.; Chen, W.; Wu, Z.; Xu, B.; Wang, Z.; Mi, J.; Li, Q. Hematite concave nanocubes and their superior catalytic activity for low temperature CO oxidation. Nanoscale 2014, 6, 7199. [Google Scholar] [CrossRef]
- Yan, S.; Wu, Q. A novel structure for enhancing the sensitivity of gas sensors-α-Fe2O3 nanoropes containing a large amount of grain boundaries and their excellent ethanol sensing performance. J. Mater. Chem. A 2015, 3, 5982–5990. [Google Scholar] [CrossRef]
- Marti, X.; Fina, I.; Jungwirth, T. Prospect for antiferromagnetic spintronics. IEEE Trans. Magn. 2015, 51, 18–21. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Li, K.; Wang, H.; Yu, H.; Zhu, X.; Wei, Y.; Ning, P.; Shi, C.; Luo, Y. CO Oxidation on Au/α-Fe2O3-Hollow Catalysts: General Synthesis and Structural Dependence. J. Phys. Chem. C 2017, 121, 12696–12710. [Google Scholar] [CrossRef]
- Shen, S.; Lindley, S.A.; Chen, X.; Zhang, J.Z. Hematite heterostructures for photoelectrochemical water splitting: Rational materials design and charge carrier dynamics. Energy Environ. Sci. 2016, 9, 2744–2775. [Google Scholar] [CrossRef]
- Schultz, A.M.; Salvador, P.A.; Rohrer, G.S. Enhanced photochemical activity of α-Fe2O3 films supported on SrTiO3 substrates under visible light illumination. Chem. Commun. 2012, 48, 2012–2014. [Google Scholar] [CrossRef] [Green Version]
- Aronniemi, M.; Lahtinen, J.; Hautojärvi, P. Characterization of iron oxide thin films. Surf. Interface Anal. 2004, 36, 1004–1006. [Google Scholar] [CrossRef]
- Mirzaeian, M.; Ogwu, A.A.; Jirandehi, H.F.; Aidarova, S.; Ospanova, Z.; Tsendzughul, N. Surface characteristics of silver oxide thin film electrodes for supercapacitor applications. Colloids Surf. A Physicochem. Eng. Asp. 2017, 519, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lou, Z.; Deng, J.; Zhang, R.; Zhang, T. ethanol gas detection using a Yolk-Shell (Core-Shell) α-Fe2O3 nanospheres as sensing material. ACS Appl. Mater. Interfaces 2015, 7, 13098–13104. [Google Scholar] [CrossRef] [PubMed]
- Cuong, N.D.; Hoa, T.T.; Khieu, D.Q.; Hoa, N.D.; Van Hieu, N. Gas sensor based on nanoporous hematite nanoparticles: Effect of synthesis pathways on morphology and gas sensing properties. Curr. Appl. Phys. 2012, 12, 1355–1360. [Google Scholar] [CrossRef]
- Li, X.; Wei, W.; Wang, S.; Kuai, L.; Geng, B. Single-crystalline α-Fe2O3 oblique nanoparallelepipeds: High-yield synthesis, growth mechanism and structure enhanced gas-sensing properties. Nanoscale 2011, 3, 718–724. [Google Scholar] [CrossRef]
- Hung, C.M.; Hoa, N.D.; Van Duy, N.; Van Toan, N.; Le, D.T.T.; Van Hieu, N. Synthesis and gas-sensing characteristics of α-Fe2O3 hollow balls. J. Sci. Adv. Mater. Devices 2016, 1, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, L.; Hu, H.; Truong, N.; Zhang, Y.; Schmuki, P.; Bi, Y. Plasmon-Induced hole-depletion layer on hematite nanoflake photoanodes for highly efficient solar water splitting nano energy plasmon-induced hole-depletion layer on hematite nano flake photoanodes for highly efficient solar water splitting. Nano Energy 2017, 35, 171–178. [Google Scholar] [CrossRef]
- Gao, H.; Liu, C.; Jeong, H.E.; Yang, P. Plasmon-enhanced photocatalytic activity of iron oxide on gold nanopillars. ACS Nano 2012, 6, 234–240. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Wang, L.; Yang, T.; Guo, X.; Wu, S.; Wang, S.; Zhang, S. Au-functionalized hematite hybrid nanospindles: General synthesis, gas sensing and catalytic properties. J. Phys. Chem. C 2011, 115, 5352–5357. [Google Scholar] [CrossRef]
- Thompson, C. V Solid-state dewetting of thin films. Annu. Rev. Mater. Res. 2012, 42, 399–434. [Google Scholar] [CrossRef]
- Leroy, F.; Borowik, Ł.; Cheynis, F.; Almadori, Y.; Curiotto, S.; Trautmann, M.; Barbe, J.C.; Müller, P. How to control solid state dewetting: A short review. Surf. Sci. Rep. 2016, 71, 391–409. [Google Scholar] [CrossRef]
- Serrano, A.; De La Fuente, O.R.; García, M.A. Extended and localized surface plasmons in annealed Au films on glass substrates. J. Appl. Phys. 2010, 108, 074303. [Google Scholar] [CrossRef] [Green Version]
- Serrano, A.; Fernandez, J.F.; Rodriguez de la Fuente, O.; García, M.A. A novel route to obtain metal and oxide nanoparticles co-existing on a substrate. Mater. Today Chem. 2017, 4, 64–72. [Google Scholar] [CrossRef]
- Gatel, C.; Snoeck, E. Epitaxial growth of Au and Pt on Fe3O4 (111) surface. Surf. Sci. 2007, 601, 1031–1039. [Google Scholar] [CrossRef]
- Lachebi, I.; Fedala, A.; Djenizian, T.; Hadjersi, T.; Kechouane, M. Morphological and optical properties of aluminum nanoparticles deposited by thermal evaporation on heated substrates. Surf. Coat. Technol. 2018, 343, 160–165. [Google Scholar] [CrossRef]
- Serrano, A.; Rubio-Zuazo, J.; López-Sánchez, J.; Enríquez, E.; Salas-Cólera, E.; Castro, G.R. Nanostructured Au(111)/Oxide epitaxial heterostructures with tailoring plasmonic response by a one-step strategy. J. Phys. Chem. C 2019, 123, 25294–25302. [Google Scholar] [CrossRef]
- Serrano, A.; Llorca-Hernando, O.; Del Campo, A.; Rubio-Marcos, F.; Rodríguez de La Fuente, O.; Fernández, J.F.; García, M.A. Ag–AgO nanostructures on glass substrates by solid-state dewetting: From extended to localized surface plasmons. J. Appl. Phys. 2018, 124, 133103. [Google Scholar] [CrossRef]
- Rubio-zuazo, J.; Ferrer, P.; López, A.; Gutiérrez-león, A.; Silva, I.; Castro, G.R. Nuclear instruments and methods in physics research a the multipurpose X-ray diffraction end-station of the BM25B-SpLine synchrotron beamline at the ESRF. Nucl. Inst. Methods Phys. Res. A 2013, 716, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Newville, M.; Ravel, B.; Haskel, D.; Rehra, J.J.; Stern, E.A.; Yacoby, Y. Analysis of multiple-scattering XAFS data using theoretical standards. Phys. B Condens. Matter 1995, 208, 154–156. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.K.; Spitz, J. Hillock formation, hole growth and agglomeration in thin silver films. Thin Solid Films 1980, 65, 339–350. [Google Scholar] [CrossRef]
- Serrano Rubio, A. Modified Au-Based Nanomaterials Studied by Surface Plasmon Resonance Spectroscopy; Springer: Berlin, Germany, 2015. [Google Scholar]
- Serrano, A.; Rubio-Zuazo, J.; López-Sánchez, J.; Arnay, I.; Salas-Colera, E.; Castro, G.R. Stabilization of epitaxial α-Fe2O3 thin films grown by pulsed laser deposition on oxide substrates. J. Phys. Chem. C 2018, 122, 16042–16047. [Google Scholar] [CrossRef]
- Rubio-Zuazo, J.; Onandia, L.; Salas-Colera, E.; Muñoz-Noval, A.; Castro, G.R. Incommensurate growth of thin and ultrathin films of single-phase Fe3O4(001) on SrTiO3(001). J. Phys. Chem. C 2015, 119, 1108–1112. [Google Scholar] [CrossRef]
- Malferrari, D.; Castellini, E.; Bernini, F.; Rubio, A.S.; Castro, G.R.; Sainz-Díaz, C.I.; Caleffi, M.; Brigatti, M.F.; Borsari, M. Chemical trapping of gaseous H2S at high and low partial pressures by an iron complex immobilized inside the montmorillonite interlayer. Microporous Mesoporous Mater. 2018, 265, 8–17. [Google Scholar] [CrossRef]
- Sanson, A.; Mathon, O.; Pascarelli, S. Local vibrational dynamics of hematite (α-Fe₂O₃) studied by extended X-ray absorption fine structure and molecular dynamics. J. Chem. Phys. 2014, 140, 224504. [Google Scholar] [CrossRef] [PubMed]
- Oku, M.; Wagatsuma, K.; Matsuta, H. Background subtraction from transition metal 2p XPS by deconvolution using ligand atom XPS: Study on first transition metal cyanide complexes. J. Electron Spectrosc. Relat. Phenom. 1997, 83, 31–39. [Google Scholar] [CrossRef]
- Mills, P.; Sullivan, J.L. A study of the core level electrons in iron and its three oxides by means of X-ray photoelectron spectroscopy. J. Phys. D Appl. Phys. 1983, 16, 723–732. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Kruse, N.; Chenakin, S. XPS characterization of Au/TiO2 catalysts: Binding energy assessment and irradiation effects. Appl. Catal. A Gen. 2011, 391, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Strohmeier, B.R. Copper/silver/gold alloy by XPS. Surf. Sci. Spectra 1994, 3, 175–181. [Google Scholar] [CrossRef]
- Široký, K.; Jirešová, J.; Hudec, L.L.; Sirok, K.; Jire, J. Iron oxide thin film gas sensor. Thin Solid Films 1994, 245, 211–214. [Google Scholar] [CrossRef]
- Vuong, N.M.; Kim, D.; Kim, H. Surface gas sensing kinetics of a WO3 nanowire sensor: Part 1—Oxidizing gases. Sens. Actuators B Chem. 2015, 220, 932–941. [Google Scholar] [CrossRef]
- Vuong, N.M.; Kim, D.; Kim, H. Surface gas sensing kinetics of a WO3 nanowire sensor: Part 2—Reducing gases. Sens. Actuators B Chem. 2016, 224, 425–433. [Google Scholar] [CrossRef]



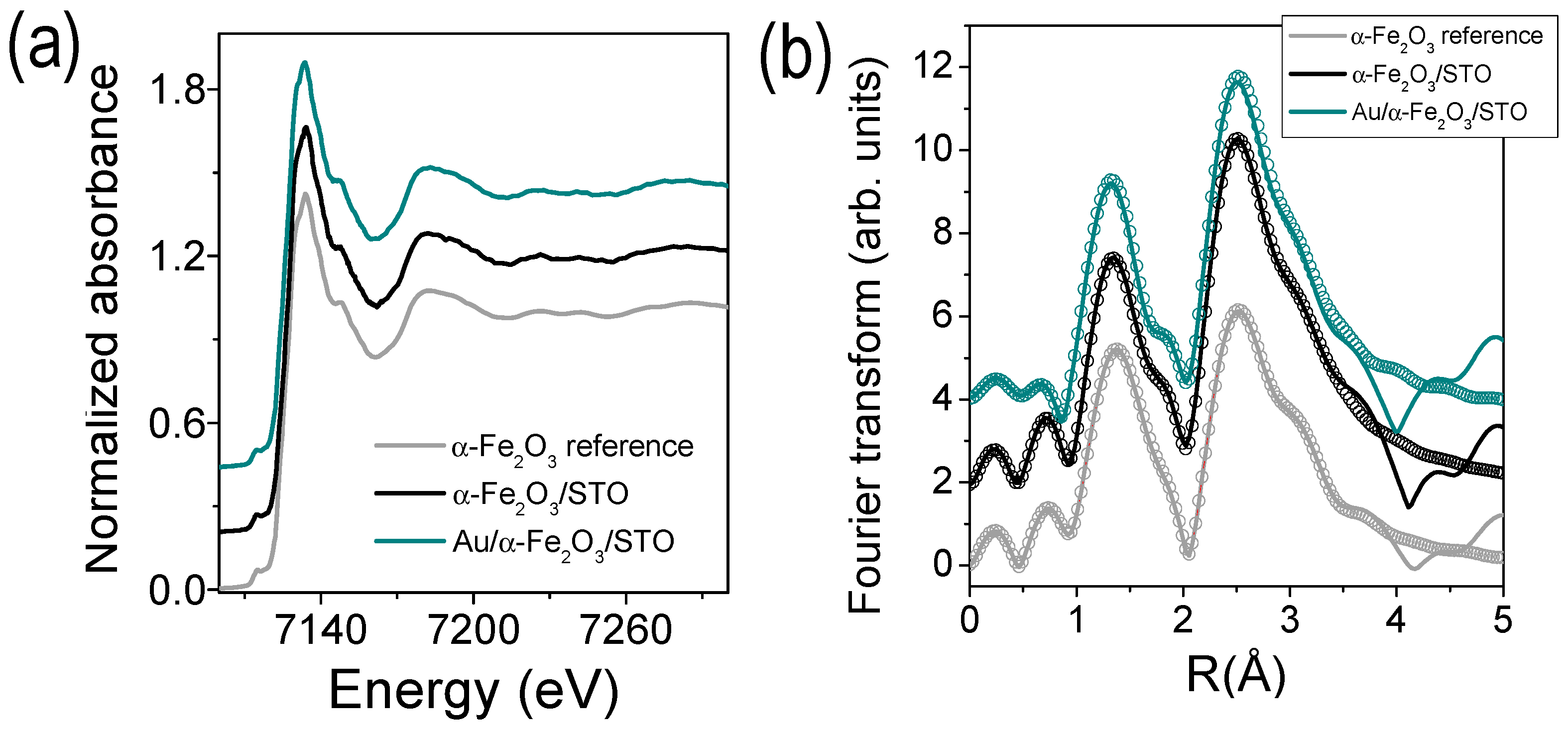


| Sample | Shell | N | R (Å) | DW(Å2) |
|---|---|---|---|---|
| α-Fe2O3 reference | Fe–O1 | 3 | 1.967(4) | 0.006(1) |
| Fe–O2 | 3 | 2.13(1) | 0.017(2) | |
| Fe–Fe1 | 1 | 2.977(1) | 0.006(1) | |
| Fe–Fe2 | 3 | 3.06(1) | 0.007(1) | |
| Fe–Fe3 | 3 | 3.454(1) | 0.017(1) | |
| α-Fe2O3/STO | Fe–O1 | 3.4(2) | 1.981(8) | 0.003(1) |
| Fe–O2 | 3.4(2) | 2.188(3) | 0.014(1) | |
| Fe–Fe1 | 1.2(1) | 2.90(4) | 0.032(9) | |
| Fe–Fe2 | 3.6(3) | 2.983(2) | 0.003(1) | |
| Fe–Fe3 | 3.6(3) | 3.380(2) | 0.005(1) | |
| Au/α-Fe2O3/STO | Fe–O1 | 3.7(2) | 1.993(6) | 0.003(1) |
| Fe–O2 | 3.7(2) | 2.230(5) | 0.013(2) | |
| Fe–Fe1 | 1.2(1) | 2.82(6) | 0.032(9) | |
| Fe–Fe2 | 3.6(3) | 3.001(3) | 0.004(2) | |
| Fe–Fe3 | 3.6(3) | 3.394(4) | 0.005(1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, A.; López-Sánchez, J.; Arnay, I.; Cid, R.; Vila, M.; Salas-Cólera, E.; Castro, G.R.; Rubio-Zuazo, J. Improving the CO and CH4 Gas Sensor Response at Room Temperature of α-Fe2O3(0001) Epitaxial Thin Films Grown on SrTiO3(111) Incorporating Au(111) Islands. Coatings 2021, 11, 848. https://doi.org/10.3390/coatings11070848
Serrano A, López-Sánchez J, Arnay I, Cid R, Vila M, Salas-Cólera E, Castro GR, Rubio-Zuazo J. Improving the CO and CH4 Gas Sensor Response at Room Temperature of α-Fe2O3(0001) Epitaxial Thin Films Grown on SrTiO3(111) Incorporating Au(111) Islands. Coatings. 2021; 11(7):848. https://doi.org/10.3390/coatings11070848
Chicago/Turabian StyleSerrano, Aída, Jesús López-Sánchez, Iciar Arnay, Rosalía Cid, María Vila, Eduardo Salas-Cólera, Germán R. Castro, and Juan Rubio-Zuazo. 2021. "Improving the CO and CH4 Gas Sensor Response at Room Temperature of α-Fe2O3(0001) Epitaxial Thin Films Grown on SrTiO3(111) Incorporating Au(111) Islands" Coatings 11, no. 7: 848. https://doi.org/10.3390/coatings11070848







